Establishing the Physiological Values of Minimally Invasive Biomarkers in Gilthead Sea Bream (Sparus aurata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish Sampling
2.3. Blood Sampling
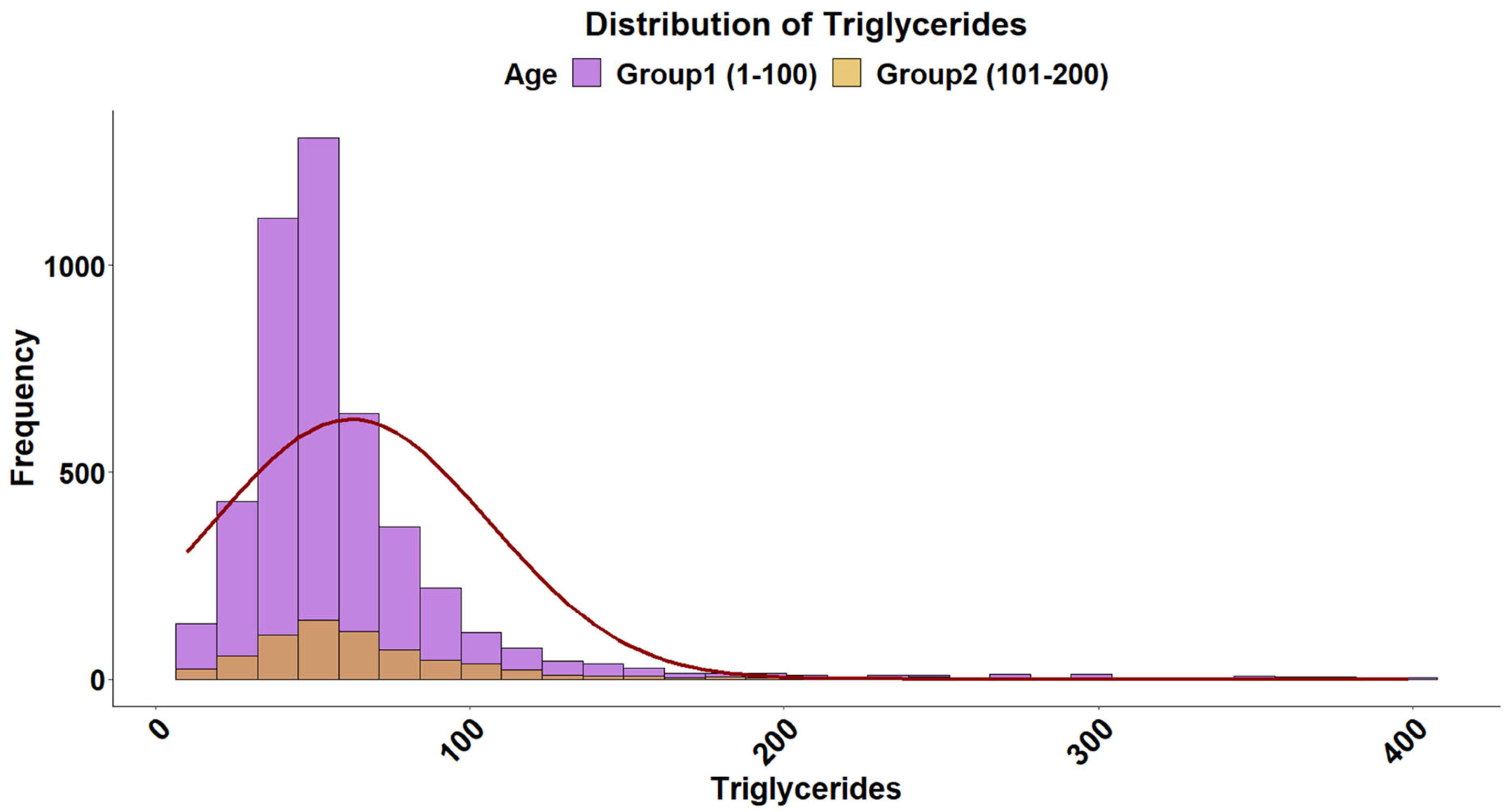
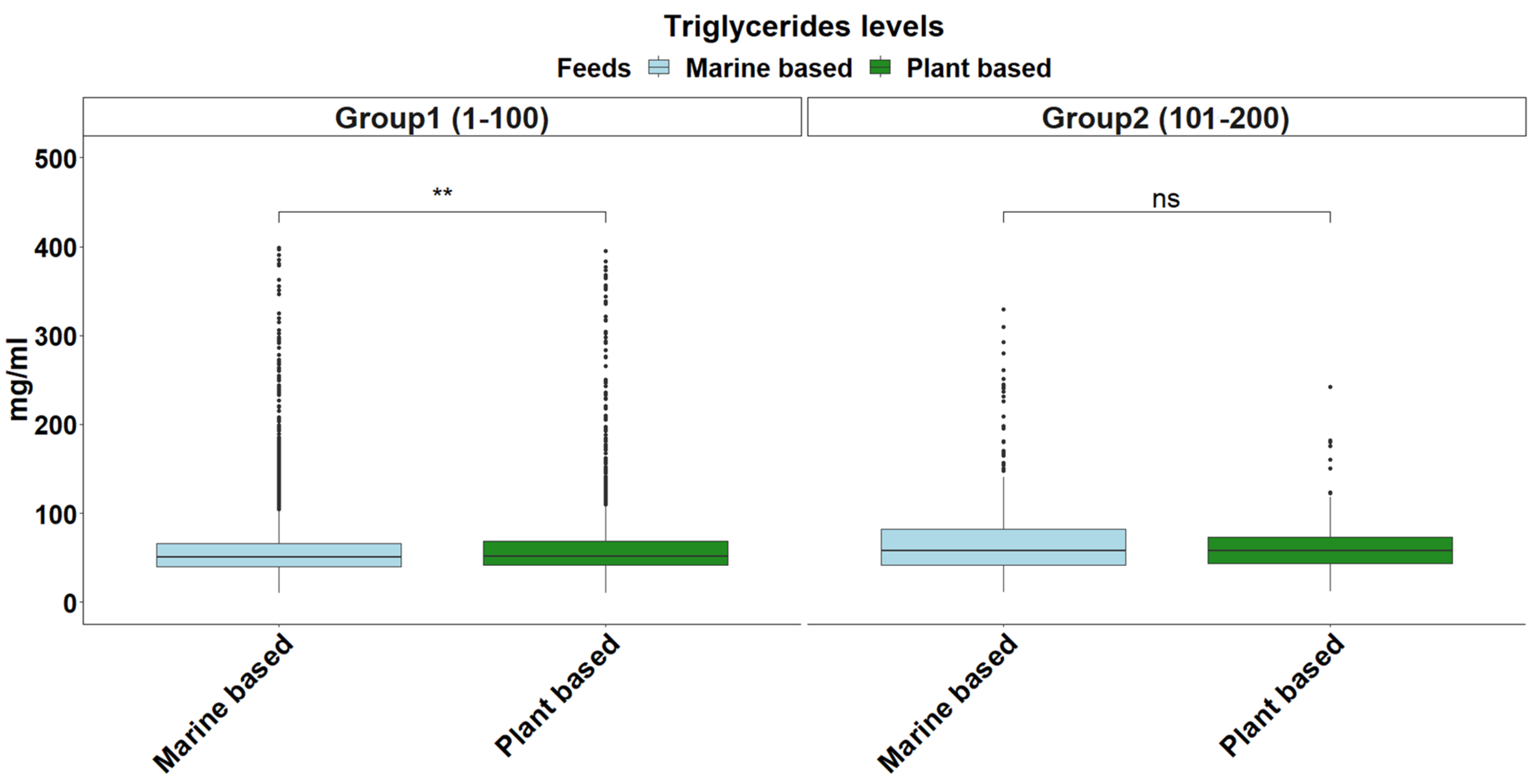
2.4. Triglyceride Levels
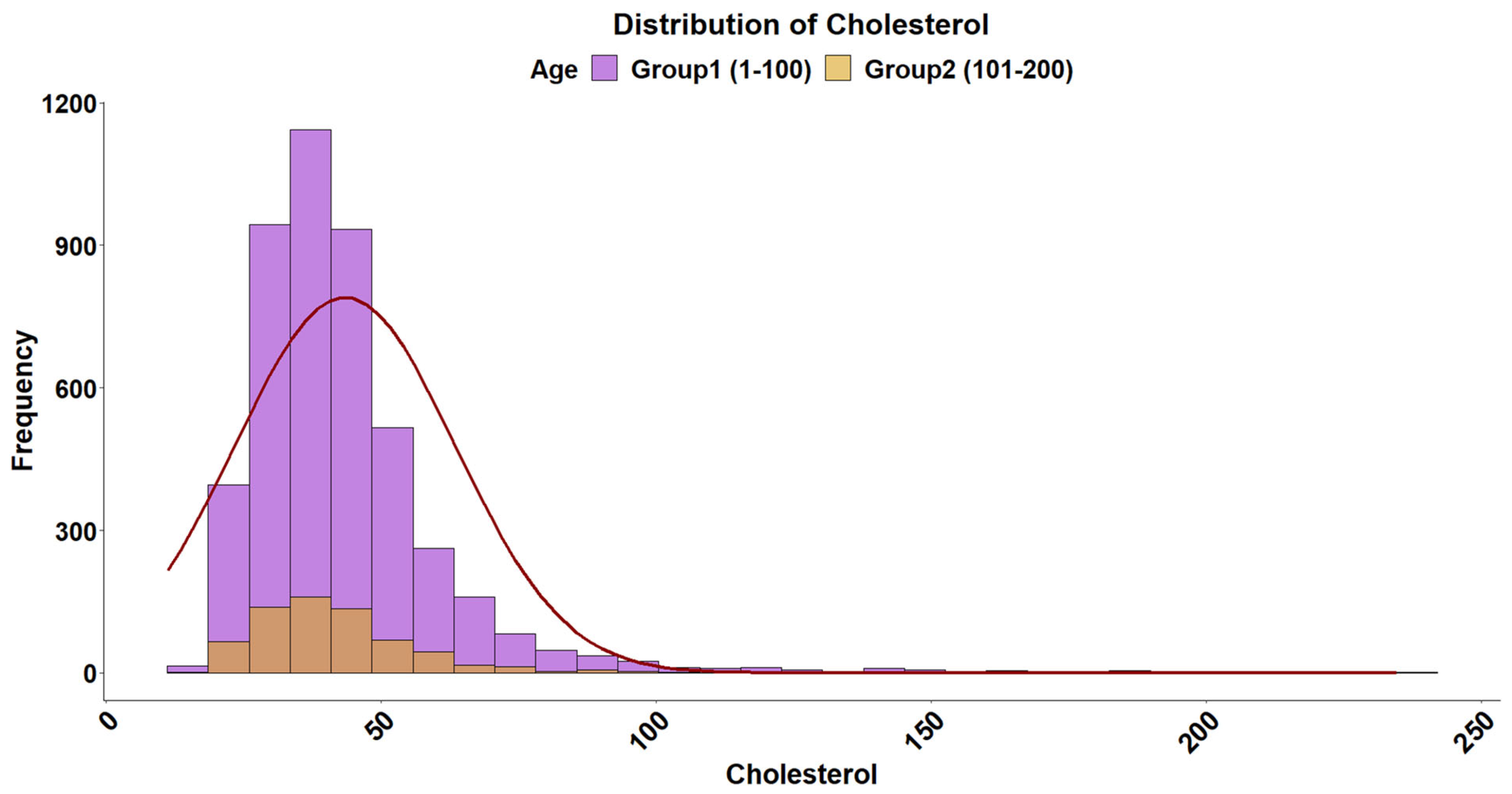
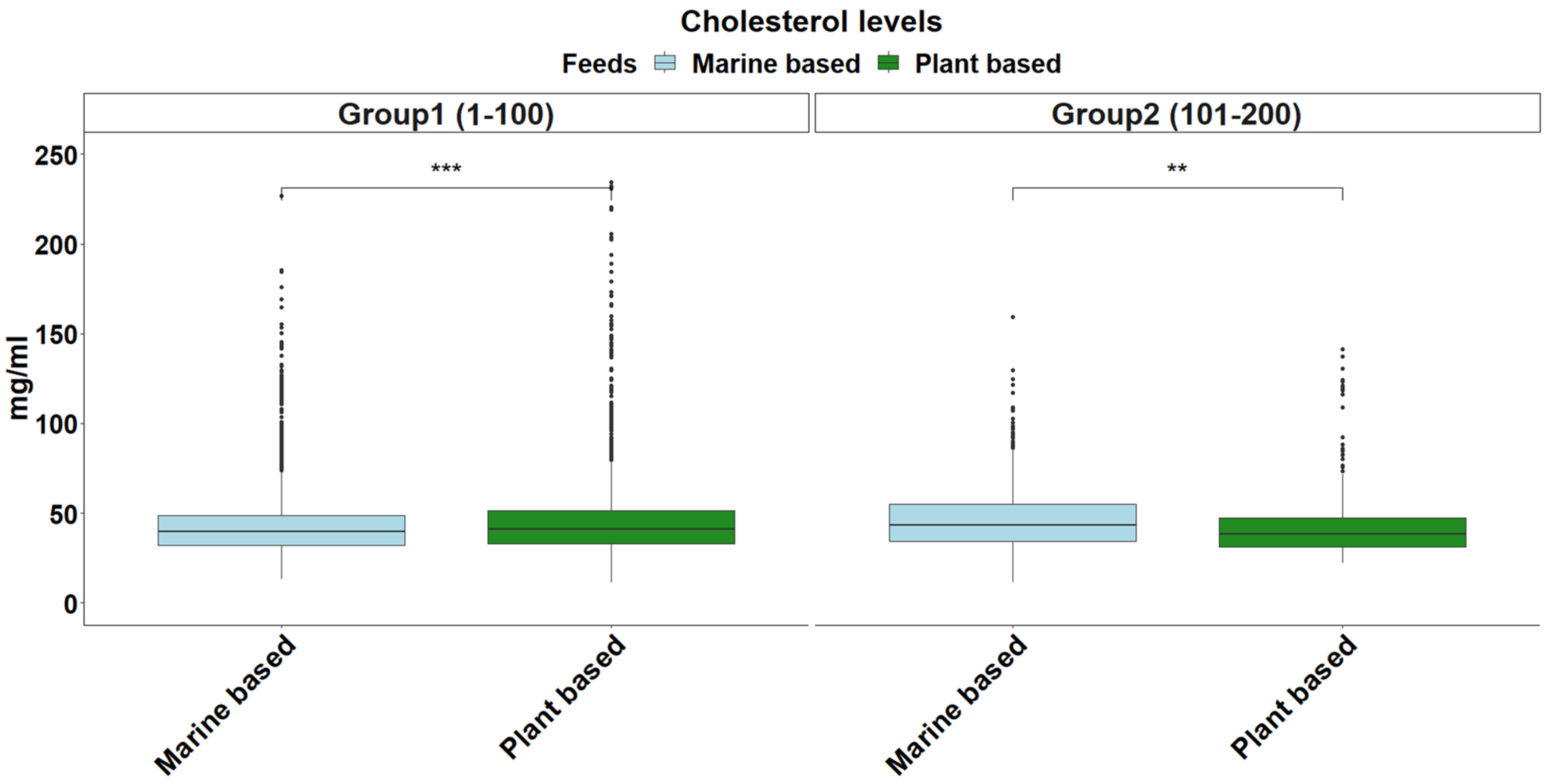
2.5. Cholesterol Levels
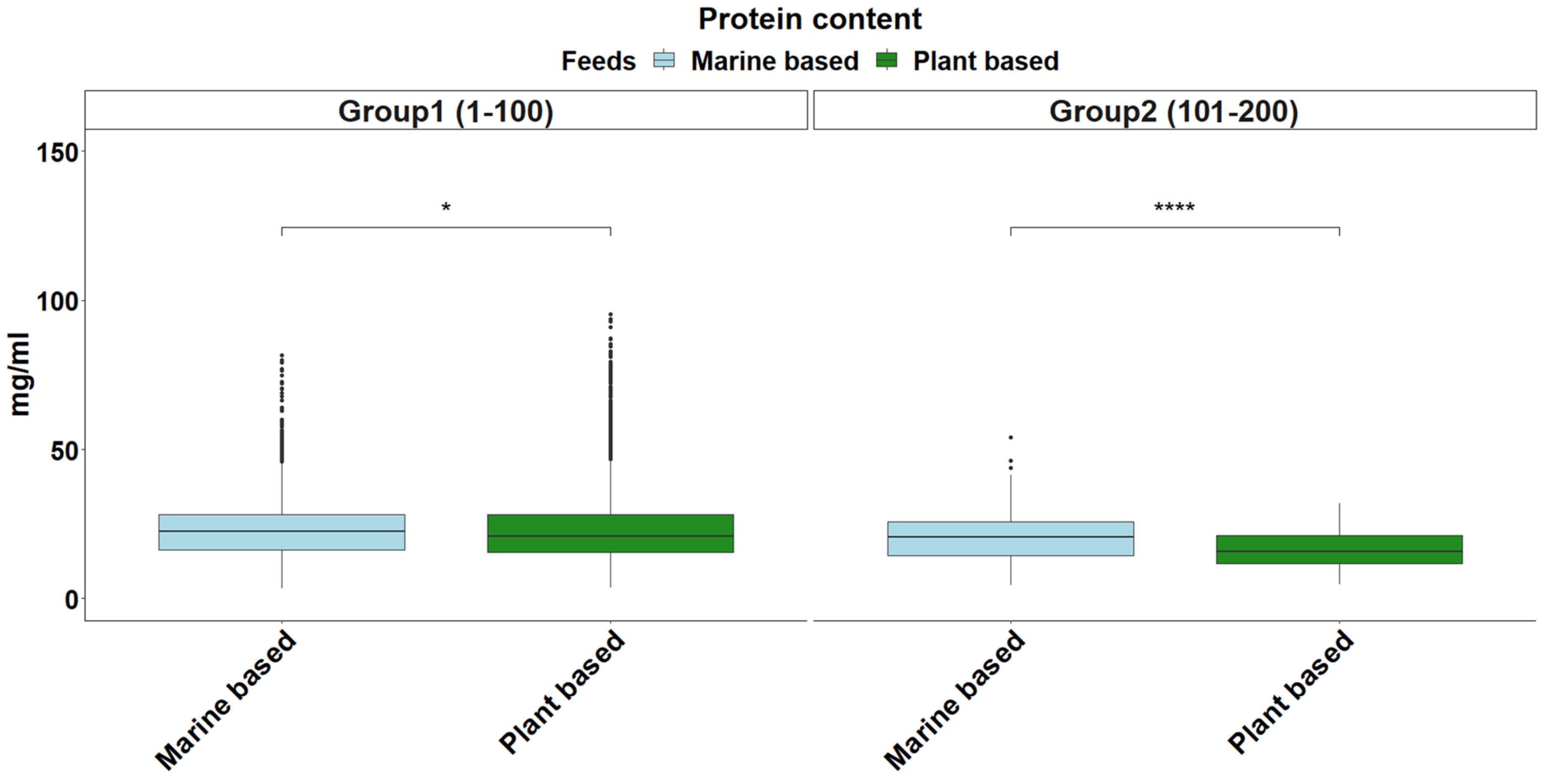
2.6. Total Protein Content
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FFEAP. European Aquaculture Production Report; FEAP: Brussels, Belgium, 2024; Available online: https://feap.info (accessed on 14 November 2024).
- Federation of Greek Mariculture. Aquaculture in Greece 2020. Annual Report; Federation of Greek Mariculture: Ilioupoli, Greece, 2020. [Google Scholar]
- Mhalhel, K.; Levanti, M.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Porcino, C.; Briglia, M.; Germanà, A.; Montalbano, G. Review on Gilthead Seabream (Sparus aurata) Aquaculture: Life Cycle, Growth, Aquaculture Practices and Challenges. J. Mar. Sci. Eng. 2023, 11, 2008. [Google Scholar] [CrossRef]
- Habibu, B.; Dzenda, T.; Ayo, J.O.; Yaqub, L.S.; Kawu, M.U. Haematological Changes and Plasma Fluid Dynamics in Livestock during Thermal Stress, and Response to Mitigative Measures. Livest. Sci. 2018, 214, 189–201. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a Diagnostic Tool in Bovine Medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F. Fish Hematology Analysis as an Important Tool of Aquaculture: A Review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Coutinho, F.; Moutinho, S.; Sousa, S.; Delerue-Matos, C.; Domingues, V.F.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Effect of Dietary ARA/EPA/DHA Ratios on Growth Performance and Intermediary Metabolism of Gilthead Sea Bream (Sparus aurata) Juveniles. Aquaculture 2020, 516, 734644. [Google Scholar] [CrossRef]
- Basto-Silva, C.; Enes, P.; Oliva-Teles, A.; Capilla, E.; Guerreiro, I. Dietary Protein/Carbohydrate Ratio and Feeding Frequency Affect Feed Utilization, Intermediary Metabolism, and Economic Efficiency of Gilthead Seabream (Sparus aurata) Juveniles. Aquaculture 2022, 554, 738182. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The Influence of the Endogenous and Exogenous Factors on Hematological Parameters in Different Fish Species: A Review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Fazio, F.; Ferrantelli, V.; Fortino, G.; Arfuso, F.; Giangrosso, G.; Faggio, C. The Influence of Acute Handling Stress on Some Blood Parameters in Cultured Sea Bream (Sparus aurata Linnaeus, 1758). Ital. J. Food Saf. 2015, 4, 4–6. [Google Scholar] [CrossRef]
- Fazio, F.; Ferrantelli, V.; Saoca, C.; Giangrosso, G.; Piccione, G. Stability of Haematological Parameters in Stored Blood Samples of Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). Vet. Med. 2017, 62, 401–405. [Google Scholar] [CrossRef]
- Puppel, K.; Kuczyńska, B. Metabolic Profiles of Cow’s Blood; A Review. J. Sci. Food Agric. 2016, 96, 4321–4328. [Google Scholar] [CrossRef]
- Dias, J.; Alvarez, M.J.; Arzel, J.; Corraze, G.; Diez, A.; Bautista, J.M.; Kaushik, S.J. Dietary Protein Source Affects Lipid Metabolism in the European Seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Řehulka, J.; Minařík, B. Cholesterolaemia and Triacylglycerolaemia in Farmed Rainbow Trout, Oncorhynchus mykiss. Aquac. Res. 2012, 43, 1651–1659. [Google Scholar] [CrossRef]
- Albassam, N.H.; Al Habib, F.M.; Hassan, S.M. Studying Cholesterol and Triglyceride Levels in Some Types of Fish in Salah Al-Din Governorate. Al-Qadisiyah J. Pure Sci. 2021, 26, 95–99. [Google Scholar] [CrossRef]
- Esmaeili, N. Blood Performance: A New Formula for Fish Growth and Health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Sallam, E.A.; Matter, A.F.; Mohammed, L.S.; Azam, A.E.; Shehab, A.; Mohamed Soliman, M. Replacing Fish Meal with Rapeseed Meal: Potential Impact on the Growth Performance, Profitability Measures, Serum Biomarkers, Antioxidant Status, Intestinal Morphometric Analysis, and Water Quality of Oreochromis niloticus and Sarotherodon galilaeus Finge. Vet. Res. Commun. 2021, 45, 223–241. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; do Carmo, B.; Santos, P.; Simões, M.; Afonso, C.; et al. Effect on Health Status and Pathogen Resistance of Gilthead Seabream (Sparus aurata) Fed with Diets Supplemented with Gracilaria Gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The Importance of Selective Breeding in Aquaculture to Meet Future Demands for Animal Protein: A Review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 13 November 2024).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 13 November 2024).
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Kruskal-Wallis Test. In The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008. [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.M.P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; Wagner, H.B.M.; et al. Vegan: Community Ecology Package, Version 2.6-4; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). Wiley Stats Ref. Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Zapala, M.A.; Schork, N.J. Multivariate Regression Analysis of Distance Matrices for Testing Associations between Gene Expression Patterns and Related Variables. Proc. Natl. Acad. Sci. USA 2006, 103, 19430. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.6.0. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 14 November 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kumar, P.; Ahmed, M.A.; Abubakar, A.A.; Hayat, M.N.; Kaka, U.; Ajat, M.; Goh, Y.M.; Sazili, A.Q. Improving Animal Welfare Status and Meat Quality through Assessment of Stress Biomarkers: A Critical Review. Meat Sci. 2023, 197, 109048. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Simó-Mirabet, P.; Shin, H.S.; Rosell-Moll, E.; Naya-Catalá, F.; de las Heras, V.; Martos-Sitcha, J.A.; Karalazos, V.; Armero, E.; Arizcun, M.; et al. Selection for Growth Is Associated in Gilthead Sea Bream (Sparus aurata) with Diet Flexibility, Changes in Growth Patterns and Higher Intestine Plasticity. Aquaculture 2019, 507, 349–360. [Google Scholar] [CrossRef]
- Tocher, D.R. Reviews in Fisheries Science Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2010, 11, 37–41. [Google Scholar]
- Jerez-Cepa, I.; Gorissen, M.; Mancera, J.M.; Ruiz-Jarabo, I. What Can We Learn from Glucocorticoid Administration in Fish? Effects of Cortisol and Dexamethasone on Intermediary Metabolism of Gilthead Seabream (Sparus aurata L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 231, 1–10. [Google Scholar] [CrossRef]
- Blanco, A.M.; Antomagesh, F.; Comesaña, S.; Soengas, J.L.; Vijayan, M.M. Chronic Cortisol Stimulation Enhances Hypothalamus-Specific Enrichment of Metabolites in the Rainbow Trout Brain. Am. J. Physiol.—Endocrinol. Metab. 2024, 326, E382–E397. [Google Scholar] [CrossRef]
- Kaplan, Ç.; Erdoğan, F. Effect of Dietary Propolis on Growth, Body Composition, and Serum Biochemistry of Juvenile Sea Bream (Sparus aurata). Aquac. Int. 2021, 29, 553–563. [Google Scholar] [CrossRef]
- Campos-Sánchez, J.C.; Guardiola, F.A.; Esteban, M.Á. Serum Proteinogram of Gilthead Seabream (Sparus Aurata) and European Sea Bass (Dicentrarchus labrax) as a New Useful Approach for Detecting Loss of Haemostasis. Fish Shellfish Immunol. 2024, 151, 109699. [Google Scholar] [CrossRef]
- Han, Y.K.; Xu, Y.C.; Luo, Z.; Zhao, T.; Zheng, H.; Tan, X.Y. Fish Meal Replacement by Mixed Plant Protein in the Diets for Juvenile Yellow Catfish Pelteobagrus fulvidraco: Effects on Growth Performance and Health Status. Aquac. Nutr. 2022, 2022, 2677885. [Google Scholar] [CrossRef]
- Donadelli, V.; Di Marco, P.; Mandich, A.; Finoia, M.G.; Cardinaletti, G.; Petochi, T.; Longobardi, A.; Tibaldi, E.; Marino, G. Effects of Dietary Plant Protein Replacement with Insect and Poultry By-Product Meals on the Liver Health and Serum Metabolites of Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Animals 2024, 14, 241. [Google Scholar] [CrossRef]
- Mabe, L.T.; Su, S.; Tang, D.; Zhu, W.; Wang, S.; Dong, Z. The Effect of Dietary Bamboo Charcoal Supplementation on Growth and Serum Biochemical Parameters of Juvenile Common Carp (Cyprinus carpio L.). Aquac. Res. 2018, 49, 1142–1152. [Google Scholar] [CrossRef]
- Cardona, E.; Baranek, E.; Vigor, C.; Gros, V.; Reversat, G.; Surget, A.; Larroquet, L.; Maunas, P.; Turronet, N.; Oger, C.; et al. A Two-Year Plant-Based Diet Alters the Fatty Acid Profile and Enzymatic and Non-Enzymatic Lipid Metabolites, in the Eggs and Fry of Female Rainbow Trout. Aquaculture 2025, 595, 741602. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, M.; Gupta, G.; Pathak, N. A Review on Replacing Fish Meal in Aqua Feeds Using Plant and Animal Protein Sources. Int. J. Fish. Aquat. Stud. 2019, 6, 164–179. [Google Scholar]
- Kortner, T.M.; Björkhem, I.; Krasnov, A.; Timmerhaus, G.; Krogdahl, Å. Dietary Cholesterol Supplementation to a Plant-Based Diet Suppresses the Complete Pathway of Cholesterol Synthesis and Induces Bile Acid Production in Atlantic Salmon (Salmo salar L.). Br. J. Nutr. 2014, 111, 2089–2103. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Ananthan, G.; Kumar, D.S.; Jagadeesan, L. Haematology and Biochemical Parameters of Different Feeding Behaviour of Teleost Fishes from Vellar Estuary, India. Comp. Clin. Path. 2012, 21, 1187–1191. [Google Scholar] [CrossRef]
- Ripa, R.; Ballhysa, E.; Steiner, J.D.; Laboy, R.; Annibal, A.; Hochhard, N.; Latza, C.; Dolfi, L.; Calabrese, C.; Meyer, A.M.; et al. Refeeding-Associated AMPKγ1 Complex Activity Is a Hallmark of Health and Longevity. Nat. Aging 2023, 3, 1544–1560. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- Michail, G.; Berillis, P.; Nakas, C.; Henry, M.; Mente, E. Haematology Reference Values for Dicentrarchus labrax and Sparus aurata: A Systematic Review and Meta-Analysis. J. Fish Dis. 2022, 45, 1549–1570. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological Methods in Fish—Not Only for Beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Barreto, M.O.; Rey Planellas, S.; Yang, Y.; Phillips, C.; Descovich, K. Emerging Indicators of Fish Welfare in Aquaculture. Rev. Aquac. 2022, 14, 343–361. [Google Scholar] [CrossRef]

| Farm No. | Geographic Coordinates | Farming System | Breeding Practice | No. of Families | Size Class | Type of Diet |
|---|---|---|---|---|---|---|
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 20 | 1–100 g | Marine-based |
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 20 | 1–100 g | Plant-based |
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 97 | 1–100 g | Marine-based |
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 97 | 1–100 g | Plant-based |
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 97 | 101–200 g | Marine-based |
| 1 | 38°45′47.5″ N 20°46′56.2″ E | Sea Cages | Family-based | 97 | 101–200 g | Plant-based |
| 2 | 38°22′03.4″ N 22°06′41.2″ E | Sea Cages | Family-based | 3 | 101–200 g | Marine-based |
| 2 | 38°22′03.4″ N 22°06′41.2″ E | Sea Cages | Family-based | 3 | 101–200 g | Plant-based |
| 3 | 38°21′57.4″ N 24°03′02.9″ E | Sea Cages | Family-based | 3 | 101–200 g | Marine-based |
| 3 | 38°21′57.4″ N 24°03′02.9″ E | Sea Cages | Family-based | 3 | 101–200 g | Plant-based |
| 4 | 38°36′12.5″ N 23°20′12.6″ E | Sea Cages | Mass spawning | - | 1–100 g | Marine-based |
| 4 | 38°36′12.5″ N 23°20′12.6″ E | Sea Cages | Mass spawning | - | 1–100 g | Plant-based |
| 5 | 39°40′19.9″ N 20°04′26.3″ E | Sea Cages | Mass spawning | - | 1–100 g | Marine-based |
| 5 | 39°40′19.9″ N 20°04′26.3″ E | Sea Cages | Mass spawning | - | 1–100 g | Plant-based |
| 5 | 39°40′19.9″ N 20°04′26.3″ E | Sea Cages | Mass spawning | - | 101–200 g | Marine-based |
| 5 | 39°40′19.9″ N 20°04′26.3″ E | Sea Cages | Mass spawning | - | 101–200 g | Plant-based |
| 6 | 38°33′31.8″ N 23°36′35.0″ E | Close circuit | Family-based | 4 | 1–100 g | Marine-based |
| 6 | 38°33′31.8″ N 23°36′35.0″ E | Close circuit | Family-based | 4 | 1–100 g | Plant-based |
| 6 | 38°33′31.8″ N 23°36′35.0″ E | Close circuit | Family-based | 4 | 101–200 g | Marine-based |
| 6 | 38°33′31.8″ N 23°36′35.0″ E | Close circuit | Family-based | 4 | 101–200 g | Plant-based |
| Parameter | Distribution | AIC | BIC | Kolmogorov–Smirnov Statistic |
|---|---|---|---|---|
| Total Protein | Normal | 43,675.72 | 43,688.88 | 0.1493 |
| Exponential | 44,614.44 | 44,621.02 | 0.247 | |
| Gamma | 41,463.86 | 41,477.02 | 0.0817 | |
| Cholesterol | Normal | 46,943.03 | 46,956.19 | 0.1462 |
| Exponential | 50,762.33 | 50,768.91 | 0.3754 | |
| Gamma | 44,236.26 | 44,249.41 | 0.082 | |
| Triglycerides | Normal | 55,284.88 | 55,298.04 | 0.1904 |
| Exponential | 54,587.85 | 54,594.43 | 0.2864 | |
| Gamma | 51,316.75 | 51,329.91 | 0.1122 |
| mg/mL | Median | Physiological Ranges (95% CI) |
|---|---|---|
| Total Protein | 21.71 | (5.42–65.42) |
| Cholesterol | 39.66 | (22.08–91.73) |
| Triglycerides | 51.67 | (18.78–184.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelakopoulos, R.; Tsipourlianos, A.; Fytsili, A.E.; Moutou, K.A. Establishing the Physiological Values of Minimally Invasive Biomarkers in Gilthead Sea Bream (Sparus aurata). Fishes 2025, 10, 52. https://doi.org/10.3390/fishes10020052
Angelakopoulos R, Tsipourlianos A, Fytsili AE, Moutou KA. Establishing the Physiological Values of Minimally Invasive Biomarkers in Gilthead Sea Bream (Sparus aurata). Fishes. 2025; 10(2):52. https://doi.org/10.3390/fishes10020052
Chicago/Turabian StyleAngelakopoulos, Rafael, Andreas Tsipourlianos, Alexia E. Fytsili, and Katerina A. Moutou. 2025. "Establishing the Physiological Values of Minimally Invasive Biomarkers in Gilthead Sea Bream (Sparus aurata)" Fishes 10, no. 2: 52. https://doi.org/10.3390/fishes10020052
APA StyleAngelakopoulos, R., Tsipourlianos, A., Fytsili, A. E., & Moutou, K. A. (2025). Establishing the Physiological Values of Minimally Invasive Biomarkers in Gilthead Sea Bream (Sparus aurata). Fishes, 10(2), 52. https://doi.org/10.3390/fishes10020052






