Comparative Demography of Five Holocentridae Species from American Samoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
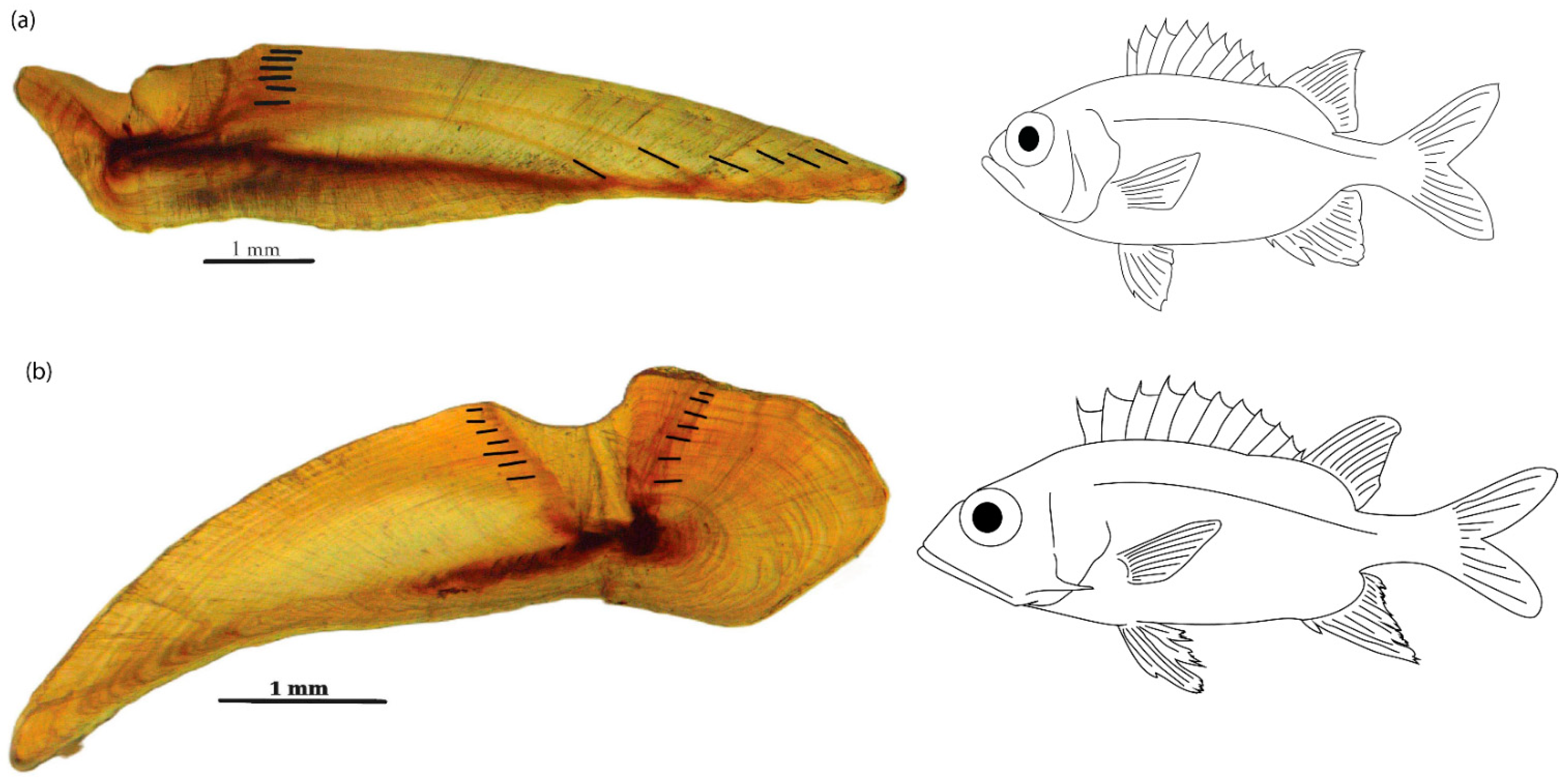
2.2. Age and Growth
2.3. Reproduction
2.4. Mortality
3. Results
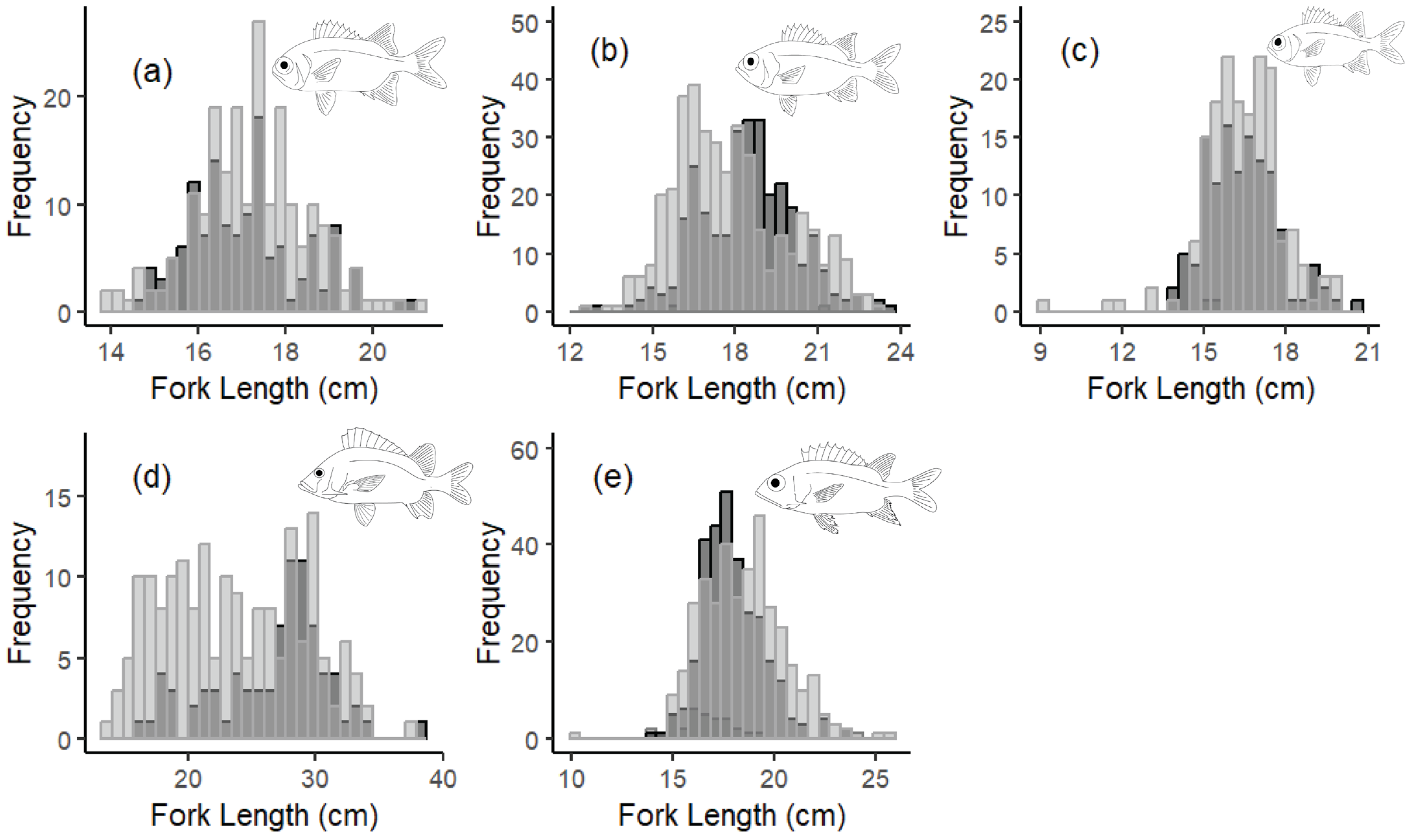
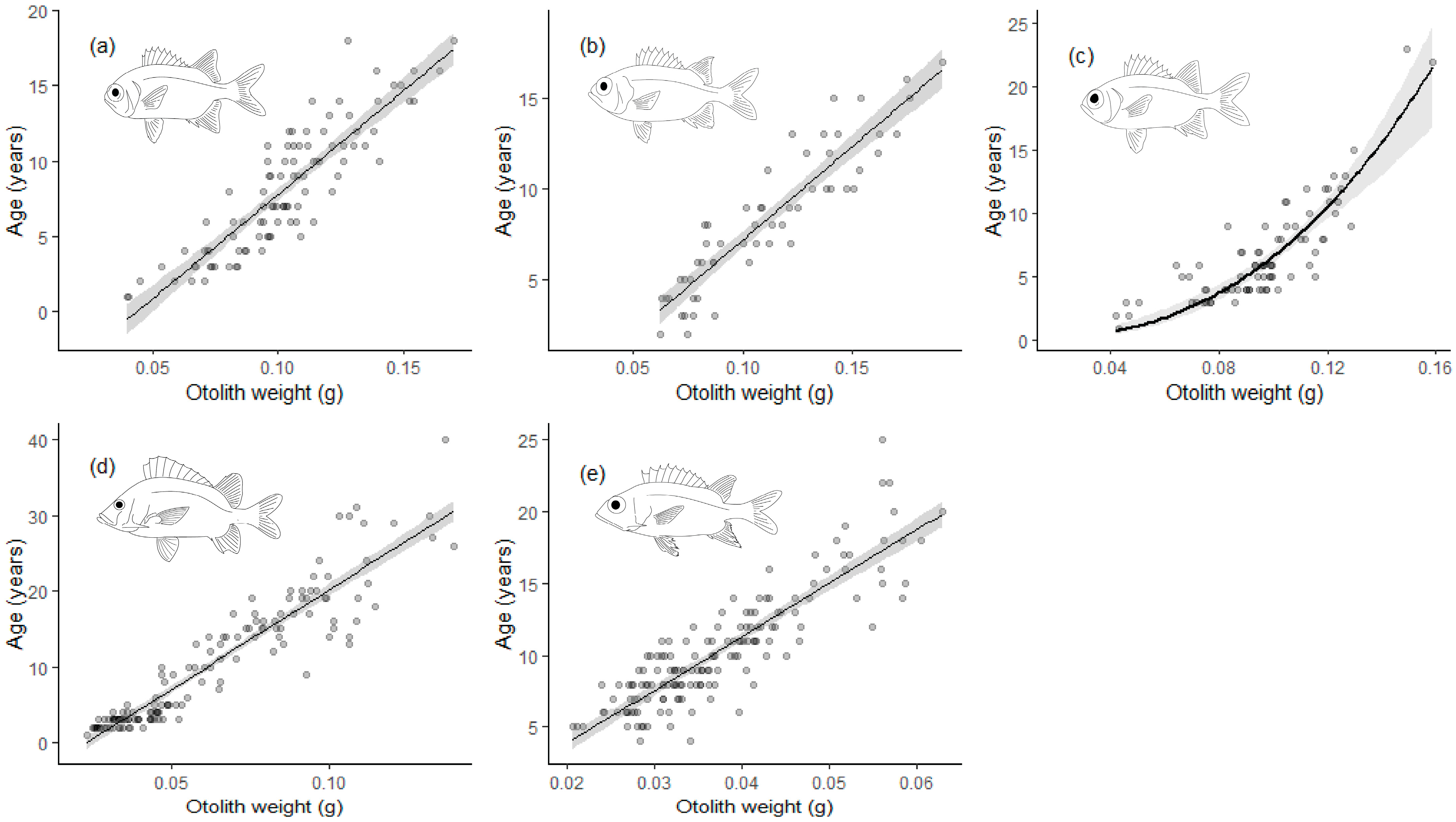
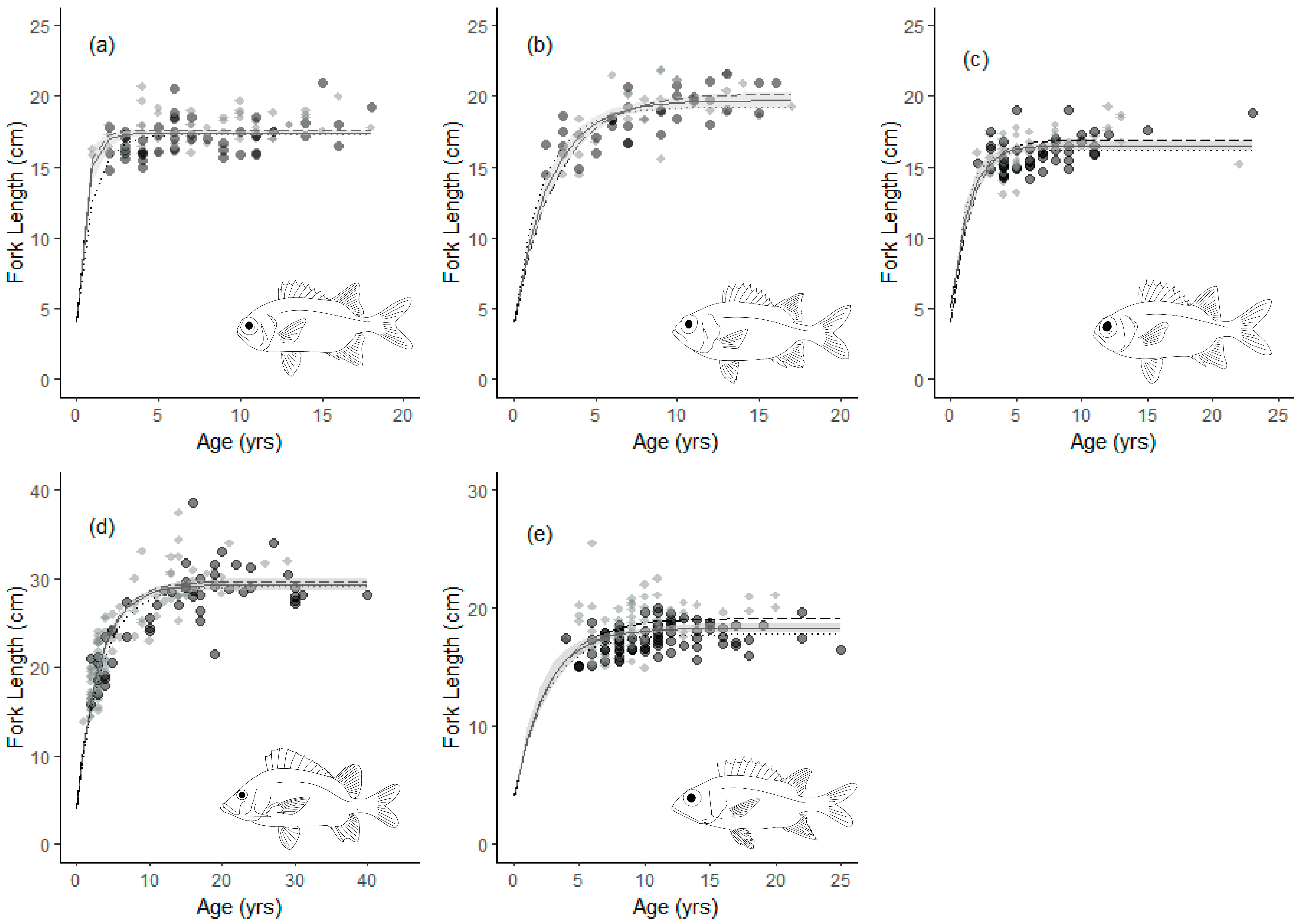
3.1. Age and Growth
3.2. Reproduction
3.3. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 27 October 2024).
- Goldin, M.R. Field Guide to the Samoan Archipelago: Fish, Wildlife, and Protected Areas; Bess Press: Honolulu, HI, USA, 2002; ISBN 1573061115. [Google Scholar]
- Greenfield, D.W. Holocentridae: Squirrelfishes (Soldierfishes). In The Living Marine Resources of the Western Central Atlantic 2; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; pp. 1192–1196. [Google Scholar]
- Randall, J.E. Shore Fishes of Hawaii, 20th ed.; University of Hawaii Press: Honolulu, HI, USA, 2010; ISBN 978-0-8248-3427-2. [Google Scholar]
- Hobson, E.S. Feeding Relationships of Teleostean Fishes on Coral Reefs in Kona. Fish. Bull. 1974, 72, 915–1031. [Google Scholar]
- Rocha, L.A.; Rosa, I.L. Baseline Assessment of Reef Fish Assemblages of Parcel Manuel Luiz Marine State Park, Maranhão, North-East Brazil. J. Fish Biol. 2001, 58, 985–998. [Google Scholar] [CrossRef]
- Dee, A.J.; Parrish, J.D. Reproductive and Trophic Ecology of the Soldierfish Myripristis amaena in Tropical Fisheries. Fish. Bull. 1994, 92, 516–530. [Google Scholar]
- Levine, A.; Allen, S.D. American Samoa as a Fishing Community; NOAA Tech. Memo; NOAA: Silver Spring, MD, USA, 2009; Volume ATM-NMFS-PIFSC-19. [Google Scholar]
- WPRFMC. Annual Stock Assessment and Fishery Evaluation (SAFE) Report for the American Samoa Archipelago Fishery Ecosystem Plan 2024; Remington, T., Pardee, C., DeMello, J., Ishizaki, A., Eds.; Western Pacific Regional Fishery Management Council: Honolulu, HI, USA, 2025. [Google Scholar]
- Fogg, L.G.; Cortesi, F.; Lecchini, D.; Gache, C.; Marshall, N.J.; de Busserolles, F. Development of Dim-Light Vision in the Nocturnal Reef Fish Family Holocentridae. I: Retinal Gene Expression. J. Exp. Biol. 2022, 225, jeb244513. [Google Scholar] [CrossRef]
- De Busserolles, F.; Cortesi, F.; Fogg, L.; Stieb, S.M.; Luehrmann, M.; Marshall, N.J. The Visual Ecology of Holocentridae, a Nocturnal Coral Reef Fish Family with a Deep-Sea-like Multibank Retina. J. Exp. Biol. 2021, 224, jeb233098. [Google Scholar] [CrossRef]
- Fogg, L.G.; Chung, W.S.; Justin Marshall, N.; Cortesi, F.; De Busserolles, F. Multiple Rod Layers Increase the Speed and Sensitivity of Vision in Nocturnal Reef Fishes. Proc. R. Soc. B Biol. Sci. 2023, 290, 20231749. [Google Scholar] [CrossRef]
- Anbalagan, T.; Murugan, A.; Jawahar, P.; Vijayanand, P.; Saravanan, R.; Veerappan, N. Age and Growth of Squirrel Fish, Sargocentron Rubrum, (Forsskal, 1775) from Cuddalore Waters, Southeast Coast of India. Indian J. Geomar. Sci. 2016, 45, 1742–1748. [Google Scholar]
- Mohammad, A.S.; Mehanna, S.F.; Osman, Y.A.A.; El-Mahdy, S.M. Age, Growth and Population Parameters of the Spiny Squirrelfish, Sargocentron spiniferum (Forsskål, 1775) from Shalateen Fishing Area, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 469–480. [Google Scholar] [CrossRef]
- Dee, A.J.; Radtke, R.L. Age and Growth of the Brick Soldierfish, Myripristis amaena. Coral Reefs 1989, 8, 79–85. [Google Scholar] [CrossRef]
- Pacific Islands Fisheries Science Center. American Samoa Commercial Fisheries BioSampling (CFBS) from 2010-06-15 to 2010-08-15; Pacific Islands Fisheries Science Center: Honolulu, HI, USA, 2025. [Google Scholar]
- Sundberg, M.; Humphreys, R.; Lowe, M.K.; Cruz, E.; Gourley, J.; Ochavillo, D. Status of Life History Sampling Conducted through the Commercial Fisheries Bio-Sampling Programs in the Western Pacific Territories of American Samoa and Guam and in the Commonwealth of the Northern Mariana Islands. In Pacific Islands Fisheries Science Center Administrative Report; NOAA: Honolulu, HI, USA, 2015; pp. 1–56. [Google Scholar] [CrossRef]
- Taylor, B.M.; Oyafuso, Z.S.; Trianni, M.S. Life History of the Orange-Striped Emperor Lethrinus obsoletus from the Mariana Islands. Ichthyol. Res. 2017, 64, 423–432. [Google Scholar] [CrossRef]
- Tyler, J.C.; Johnson, D.G.; Brothers, E.B.; Tyler, D.M.; Smith, L.C. Comparative Early Life Histories of Western Atlantic Squirrelfishes (Holocentridae): Age and Settlement of Rhynchichthys, Meeki, and Juvenile Stages. Bull. Mar. Sci. 1993, 53, 11261150. [Google Scholar]
- Sullivan-Brown, J.; Bisher, M.E.; Burdine, R.D. Embedding, Serial Sectioning and Staining of Zebrafish Embryos Using JB-4 Resin. Nat. Protoc. 2011, 6, 46–55. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized Terminology for Describing Reproductive Development in Fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Choat, J.H.; Kritzer, J.P.; Ackerman, J.L. Aging in Coral Reef Fishes: Do We Need to Validate the Periodicity of Increment Formation for Every Species of Fish We Collect Age-Based Demographic Data? In Tropical Fish Otoliths: Information for Assessment, Management, and Ecology; Green, B.S., Mapstone, B.D., Carlos, G., Begg, G.A., Eds.; Springer: New York, NY, USA, 2009; pp. 23–48. [Google Scholar]
- Munch, S.B.; Salinas, S. Latitudinal Variation in Lifespan within Species Is Explained by the Metabolic Theory of Ecology. Proc. Natl. Acad. Sci. USA 2009, 106, 13860–13864. [Google Scholar] [CrossRef]
- Taylor, B.M.; Choat, J.H.; DeMartini, E.E.; Hoey, A.S.; Marshell, A.; Priest, M.A.; Rhodes, K.L.; Meekan, M.G. Demographic Plasticity Facilitates Ecological and Economic Resilience in a Commercially Important Reef Fish. J. Anim. Ecol. 2019, 88, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Trip, E.D.L.; Clements, K.D.; Raubenheimer, D.; Choat, J.H. Temperature-Related Variation in Growth Rate, Size, Maturation and Life Span in a Marine Herbivorous Fish over a Latitudinal Gradient. J. Anim. Ecol. 2014, 83, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.M.; Oyafuso, Z.S.; Pardee, C.B.; Ochavillo, D.; Newman, S.J. Comparative Demography of Commercially-Harvested Snappers and an Emperor from American Samoa. PeerJ 2018, 6, e5069. [Google Scholar] [CrossRef] [PubMed]
- Pardee, C.; Taylor, B.M.; Felise, S.; Ochavillo, D.; Cuetos-Bueno, J. Growth and Maturation of Three Commercially Important Coral Reef Species from American Samoa. Fish. Sci. 2020, 86, 985–993. [Google Scholar] [CrossRef]
- Craig, P.; Choat, J.H.; Axe, L.M. Population Biology and Harvest of the Coral Reef Surgeonfish Acanthurus Lineatus in American Samoa. Fish. Bull. 1997, 95, 680–693. [Google Scholar]
- Zarco-Perello, S.; Taylor, B.M.; Thepot, V.; Bennett, S.; Hoey, A.S. The Biology, Ecology, Fisheries and Aquaculture of Rabbitfishes (Siganidae). In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2025; pp. 163–193. [Google Scholar]
- McCauley, D.J.; Hoffmann, E.; Young, H.S.; Micheli, F. Night Shift: Expansion of Temporal Niche Use Following Reductions in Predator Density. PLoS ONE 2012, 7, e38871. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Heithaus, M.R.; Serafy, J.E. Influence of Predation Risk and Food Supply on Nocturnal Fish Foraging Distributions along a Mangrove-Seagrass Ecotone. Mar. Ecol. Prog. Ser. 2010, 414, 223–235. [Google Scholar] [CrossRef]
- Berkeley, S.A.; Hixon, M.A.; Larson, R.J.; Love, M.S. Fisheries Sustainability via Protection of Age Structure and Spatial Distribution of Fish Populations. Fisheries 2004, 29, 23–32. [Google Scholar] [CrossRef]
- Barnett, L.A.K.; Branch, T.A.; Ranasinghe, R.A.; Essington, T.E. Old-Growth Fishes Become Scarce under Fishing. Curr. Biol. 2017, 27, 2843–2848.e2. [Google Scholar] [CrossRef] [PubMed]
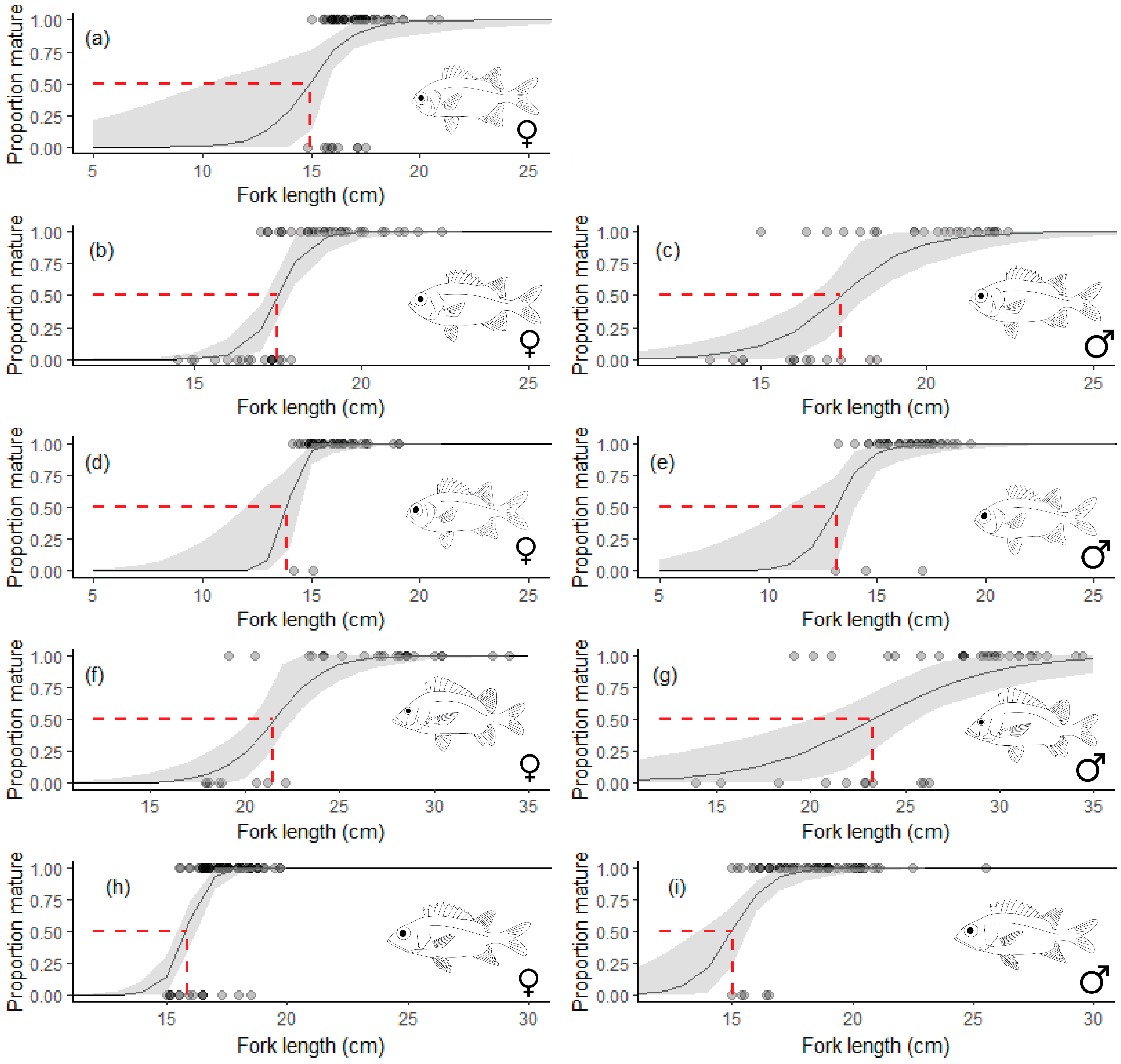

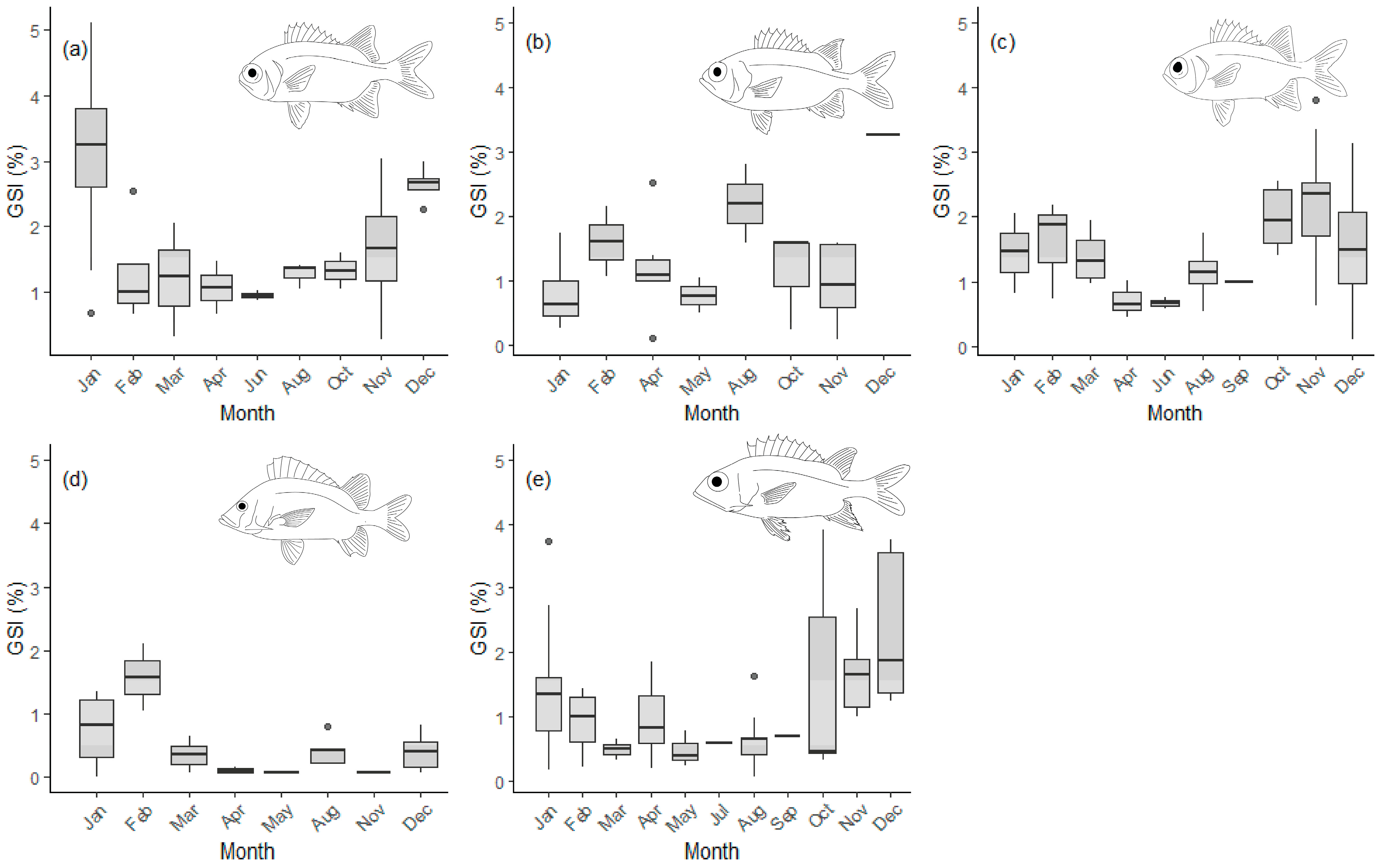
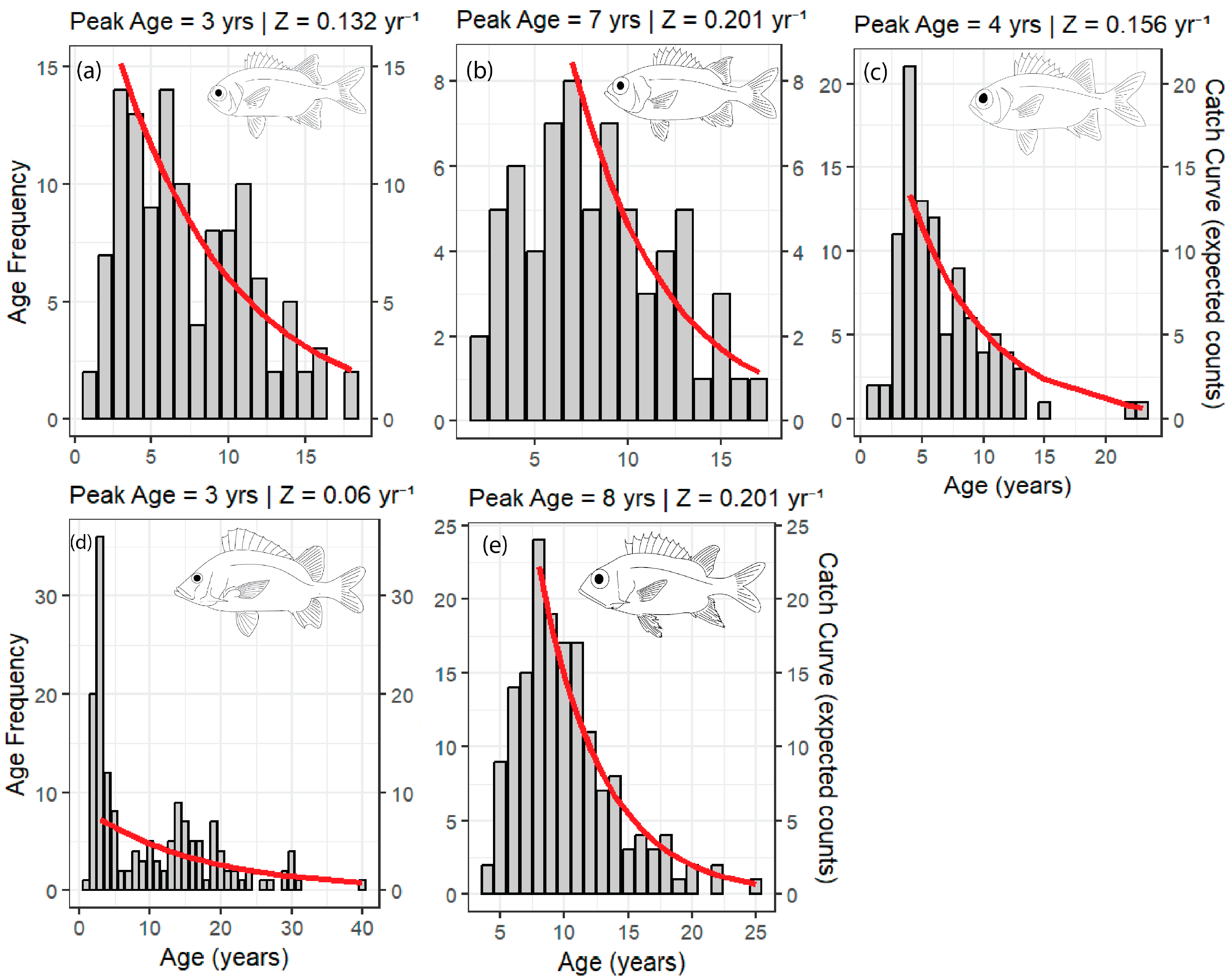
| Species | Sex | LWa | LWb | Naged | tmax | L∞ | K (Year−1) | L50 | A50 | Z (Year−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Myripristis amaena | Female | 49 | 18 | 17.3 (16.9–17.7) | 1.01 (0.78–1.65) | 14.9 (11.2–15.7) | 3.3 (2.9–3.7) | |||
| Male | 70 | 18 | 17.6 (17.4–17.9) | 1.95 (1.46–3.05) | ||||||
| Combined | 1.5 × 10−1 | 2.40 | 119 | 18 | 17.4 (17.2–17.7) | 1.75 (1.32–2.96) | 0.13 (0.08–0.18) | |||
| Myripristis berndti | Female | 36 | 16 | 19.2 (18.7–19.9) | 0.56 (0.45–0.73) | 17.5 (17.2–17.8) | ||||
| Male | 29 | 17 | 20.1 (19.4–21.4) | 0.38 (0.28–0.51) | 17.4 (16.7–18.3) | |||||
| Combined | 1.3 × 10−1 | 2.44 | 67 | 17 | 19.7 (19.2–20.2) | 0.46 (0.38–0.55) | 0.20 (0.11–0.30) | |||
| Myripristis murdjan | Female | 48 | 23 | 16.1 (15.8–16.5) | 1.03 (0.74–2.30) | 13.9 (12.1–14.3) | ||||
| Male | 49 | 22 | 16.9 (16.4–17.5) | 0.67 (0.53–0.94) | 13.2 (11.2–14.0) | 2.4 (1.5–3.0) | ||||
| Combined | 2.1 × 10−1 | 2.27 | 98 | 23 | 16.5 (16.2–16.8) | 0.76 (0.62–1.06) | 0.16 (0.12–0.20) | |||
| Sargocentron spiniferum | Female | 51 | 40 | 29.1 (28.2–30.2) | 0.28 (0.24–0.35) | 21.5 (20.0–22.6) | ||||
| Male | 107 | 29 | 29.6 (28.8–30.5) | 0.32 (0.30–0.35) | 23.3 (20.3–25.4) | 4.4 (1.9–6.8) | ||||
| Combined | 1.1 × 10−1 | 2.53 | 158 | 40 | 29.3 (28.6–29.9) | 0.32 (0.30–0.35) | 0.06 (0.03–0.09) | |||
| Sargocentron tiere | Female | 81 | 25 | 17.8 (17.4–18.2) | 0.41 (0.33–0.53) | 15.8 (15.5–16.2) | 6.5 (4.9–7.4) | |||
| Male | 78 | 20 | 19.1 (18.5–20.0) | 0.37 (0.28–0.57) | 15.0 (14.0–15.6) | 5.0 (2.1–5.7) | ||||
| Combined | 7.6 × 10−2 | 2.59 | 163 | 25 | 18.3 (18.0–18.7) | 0.42 (0.34–0.54) | 0.20 (0.16–0.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardee, C.; Ochavillo, D.; Taylor, B.M. Comparative Demography of Five Holocentridae Species from American Samoa. Fishes 2025, 10, 596. https://doi.org/10.3390/fishes10110596
Pardee C, Ochavillo D, Taylor BM. Comparative Demography of Five Holocentridae Species from American Samoa. Fishes. 2025; 10(11):596. https://doi.org/10.3390/fishes10110596
Chicago/Turabian StylePardee, Cassandra, Domingo Ochavillo, and Brett M. Taylor. 2025. "Comparative Demography of Five Holocentridae Species from American Samoa" Fishes 10, no. 11: 596. https://doi.org/10.3390/fishes10110596
APA StylePardee, C., Ochavillo, D., & Taylor, B. M. (2025). Comparative Demography of Five Holocentridae Species from American Samoa. Fishes, 10(11), 596. https://doi.org/10.3390/fishes10110596






