Multi-Tissue Stable Isotope Analysis Reveals the Feeding Ecology of Dominant Shark Bycatch Species in the Northern South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Stable Isotope Analysis
2.3. Trophic Position
2.4. Trophic Niche Estimation
2.5. Data Analysis
3. Results
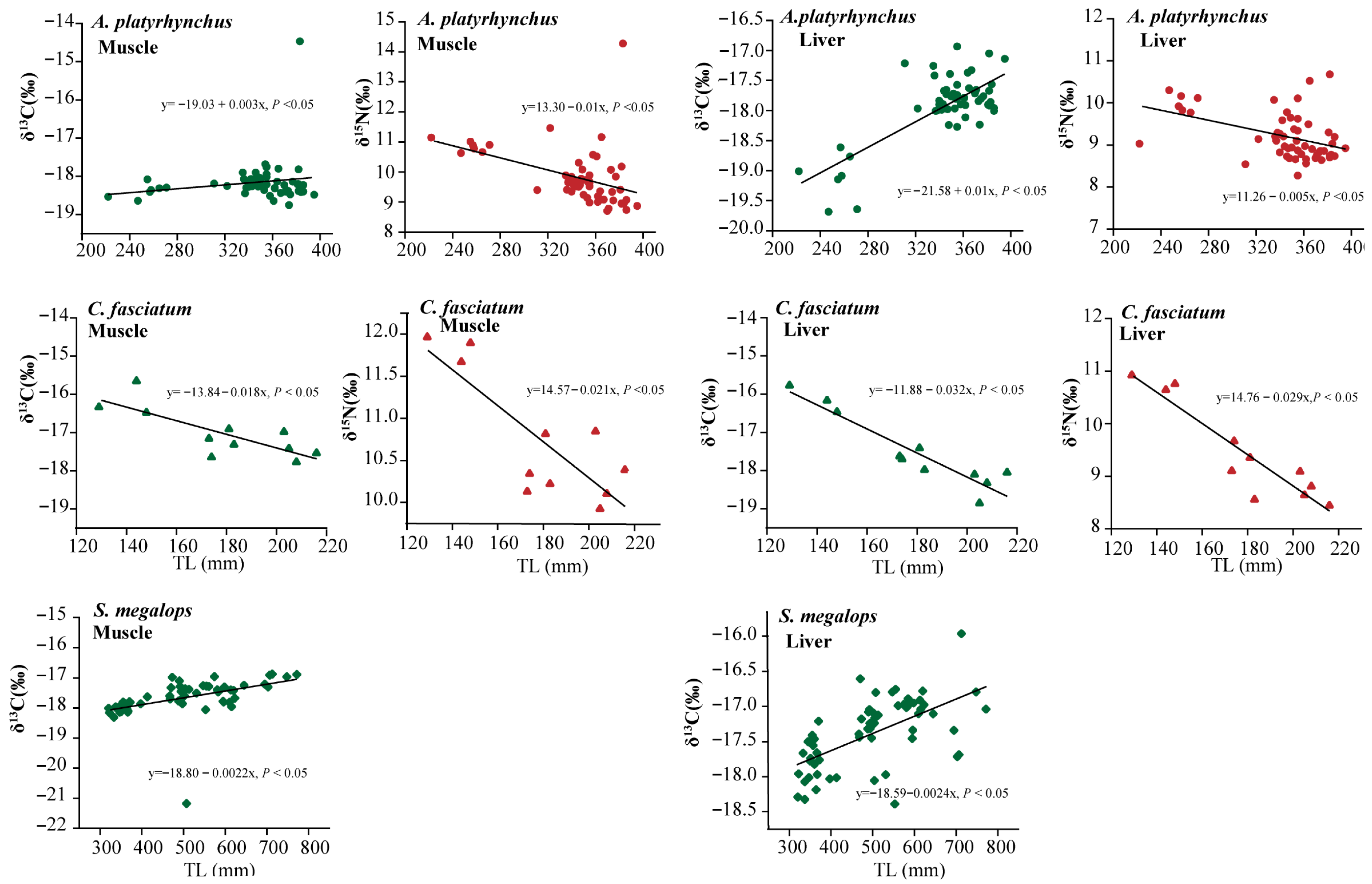
3.1. Multi-Tissue Stable Isotope Analysis
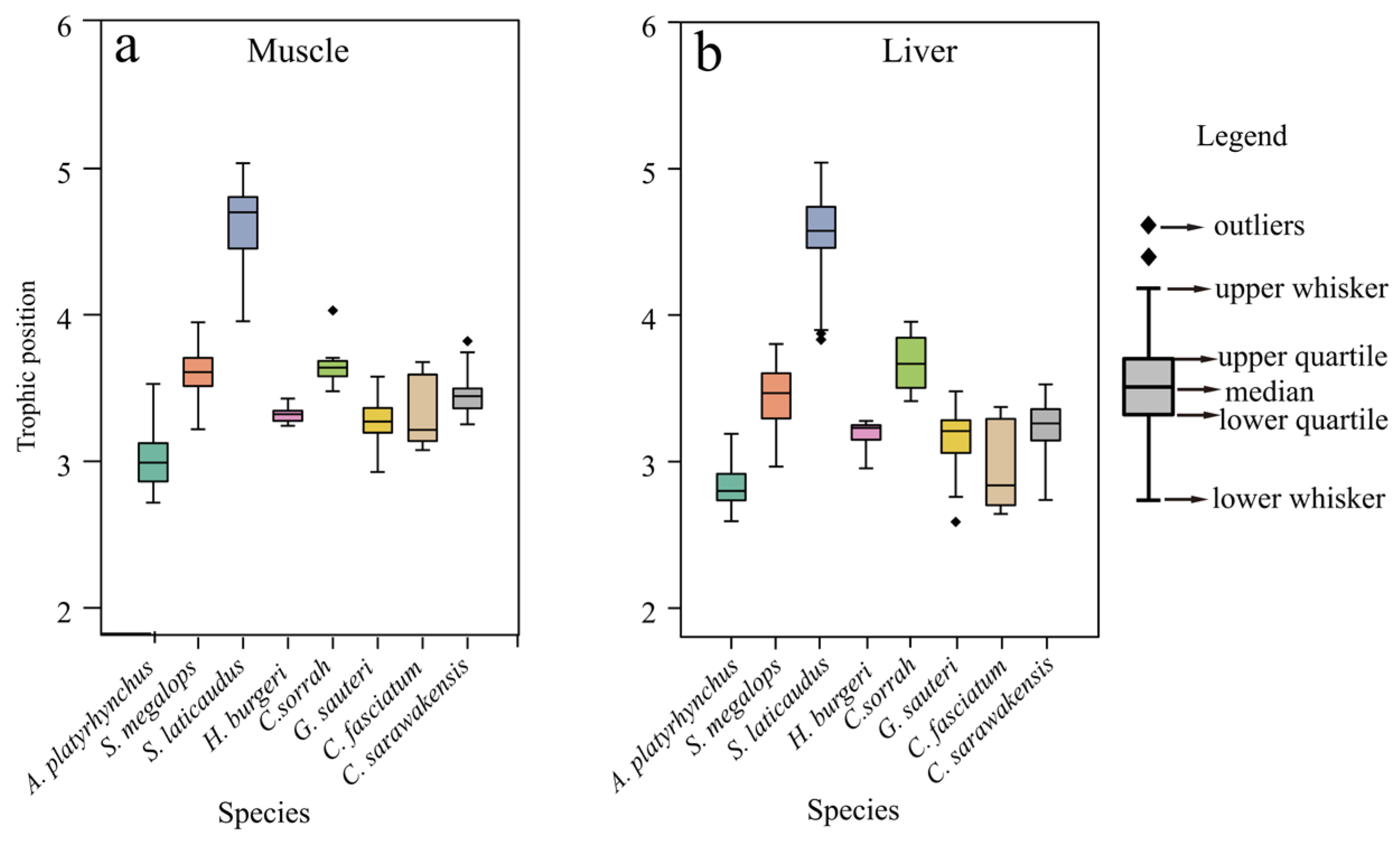
3.2. Trophic Position Comparison
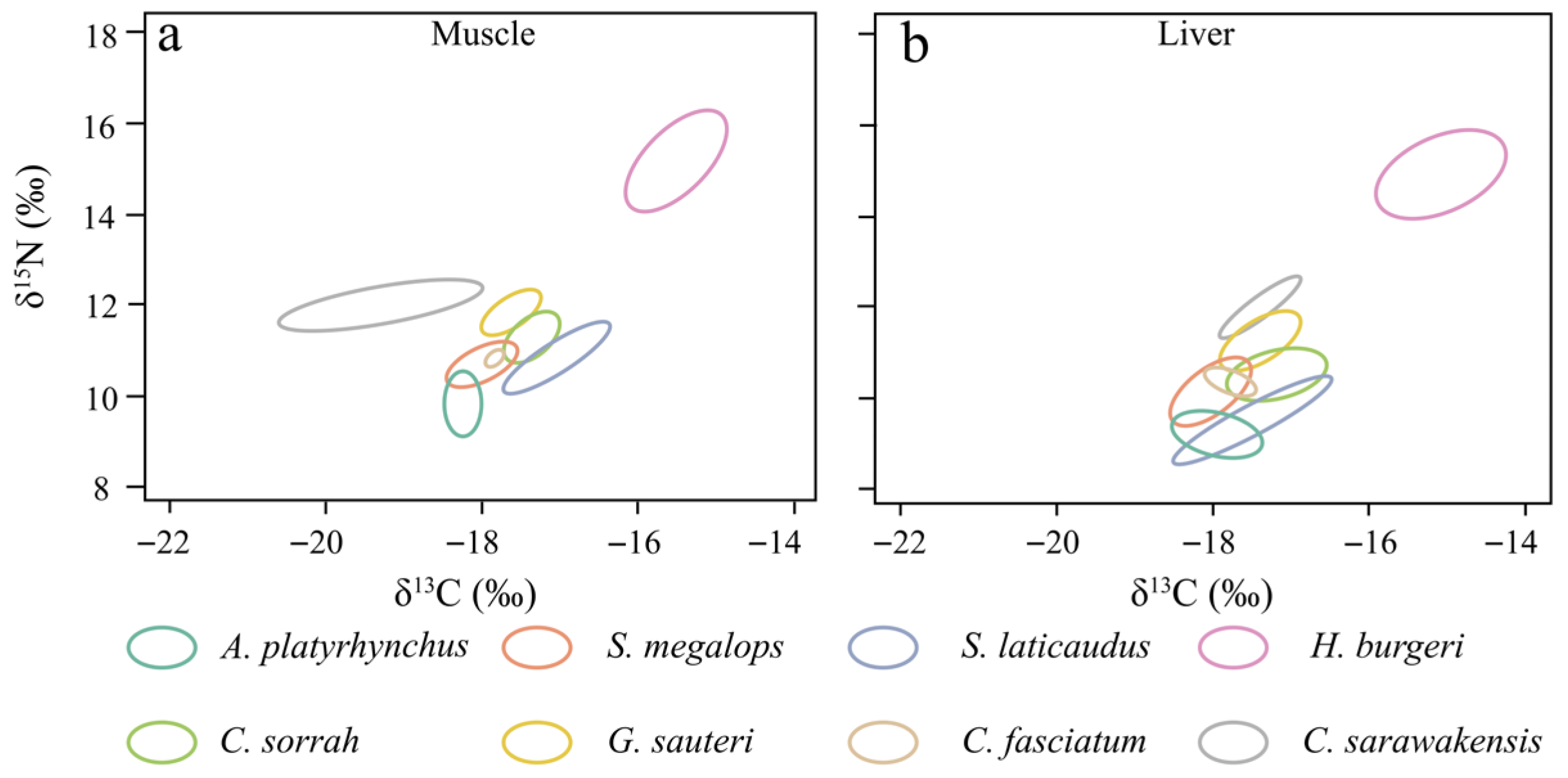
3.3. Trophic Niche Analysis
4. Discussion
4.1. δ13C and δ15N Ratios
4.2. Isotopic Trophic Niche
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCS | Northern South China Sea |
| TP | Trophic Position |
| SEA | Standard Ellipse Area |
| SEAc | the Corrected Standard Ellipse Area |
| TL | Total Length |
References
- Kechang, N.; Yining, L.; Zehao, S.; Fangliang, H.E.; Jingyun, F. Community assembly: The relative importance of neutral theory and niche theory. Biodivers. Sci. 2009, 17, 579–593. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- Shipley, O.N.; Brooks, E.J.; Madigan, D.J.; Sweeting, C.J.; Dean Grubbs, R. Stable isotope analysis in deep-sea chondrichthyans: Recent challenges, ecological insights, and future directions. Rev. Fish Biol. Fish. 2017, 27, 481–497. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Skomal, G.B.; Fisk, A.T. Stable isotopes from multiple tissues reveal diet switching in sharks. Mar. Ecol. Prog. Ser. 2005, 302, 199–206. [Google Scholar] [CrossRef]
- Estupiñán-Montaño, C.; Galván-Magaña, F.; Sánchez-González, A.; Elorriaga-Verplancken, F.R.; Delgado-Huertas, A.; Páez-Rosas, D. Dietary ontogeny of the blue shark, Prionace glauca, based on the analysis of δ13C and δ15N in vertebrae. Mar. Biol. 2019, 166, 101. [Google Scholar] [CrossRef]
- Rounick, J.S.; Winterbourn, M.J. Stable carbon isotopes and carbon flow in ecosystems. BioScience 1986, 36, 171–177. [Google Scholar] [CrossRef]
- Yu, C.J.; Joung, S.J.; Hsu, H.H.; Liu, K.M.; Yamaguchi, A. Stable Isotope Analysis of Two Filter-Feeding Sharks in the Northwestern Pacific Ocean. Fishes 2025, 10, 249. [Google Scholar] [CrossRef]
- Hobson, K.A.; Piatt, J.F.; Pitocchelli, J. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 1994, 63, 786–798. [Google Scholar] [CrossRef]
- Ma, H.; Ma, M. Stable carbon isotopes in seagrasses: Variability in ratios and use in ecological studies. Mar. Ecol. Prog. Ser. 1996, 140, 285–298. [Google Scholar] [CrossRef]
- Vizzini, S.; Mazzola, A. Seasonal variations in the stable carbon and nitrogen isotope ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Mar. Biol. 2003, 142, 1009–1018. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Middelburg, J.J.; Holthuijsen, S.J.; Jouta, J.; Compton, T.J.; van der Heide, T.; Piersma, T.; Sinninghe Damsté, J.S.; van der Veer, H.W.; Schouten, S. Benthic primary producers are key to sustain the Wadden Sea food web: Stable carbon isotope analysis at landscape scale. Ecology 2017, 98, 1498–1512. [Google Scholar] [CrossRef]
- Schmidt, K.; McClelland, J.W.; Mente, E.; Montoya, J.P.; Atkinson, A.; Voss, M. Trophic-level interpretation based on δ15N values: Implications of tissue-specific fractionation and amino acid composition. Mar. Ecol. Prog. Ser. 2004, 266, 43–58. [Google Scholar] [CrossRef]
- Stephens, R.B.; Shipley, O.N.; Moll, R.J. Meta-analysis and critical review of trophic discrimination factors (Δ13C and Δ15N): Importance of tissue, trophic level and diet source. Funct. Ecol. 2023, 37, 2535–2548. [Google Scholar] [CrossRef]
- Pethybridge, H.; Choy, C.A.; Logan, J.M.; Allain, V.; Lorrain, A.; Bodin, N.; Somes, C.J.; Young, J.; Ménard, F.; Langlais, C. A global meta-analysis of marine predator nitrogen stable isotopes: Relationships between trophic structure and environmental conditions. Glob. Ecol. Biogeogr. 2018, 27, 1043–1055. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 375–449. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Michener, R.H.; Kaufman, L. Stable isotope ratios as tracers in marine food webs: An update. In Stable Isotopes in Ecology and Environmental Science; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 238–282. [Google Scholar] [CrossRef]
- Deeken, D.; Macdonald, C.; Gainsbury, A.; Green, M.L.; Cassill, D.L. Maternal risk-management elucidates the evolution of reproductive adaptations in sharks by means of natural selection. Sci. Rep. 2024, 14, 20088. [Google Scholar] [CrossRef]
- Niella, Y.; Raoult, V.; Gaston, T.; Peddemors, V.M.; Harcourt, R.; Smoothey, A.F. Overcoming multi-year impacts of maternal isotope signatures using multi-tracers and fast turnover tissues in juvenile sharks. Chemosphere 2021, 269, 129393. [Google Scholar] [CrossRef]
- Pahl, K.B.; Yurkowski, D.J.; Wintner, S.P.; Cliff, G.; Dicken, M.L.; Hussey, N.E. Determining the appropriate pretreatment procedures and the utility of liver tissue for bulk stable isotope (δ13C and δ15N) studies in sharks. J. Fish Biol. 2021, 98, 829–841. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021; Volume 19. [Google Scholar]
- Zhang, Q.; Yang, S. Species, geography distribution and resource of chondrichthian fishes of China. J. Xiamen Univ. Nat. Sci. 2005, 44, 207–211. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Simpfendorfer, C.A.; Davidson, L.N.K.; Fordham, S.V.; Bräutigam, A.; Sant, G.; Welch, D.J. Challenges and Priorities in Shark and Ray Conservation. Curr. Biol. 2017, 27, R565–R572. [Google Scholar] [CrossRef]
- Porcher, I.F.; Darvell, B.W. Shark fishing vs. conservation: Analysis and synthesis. Sustainability 2022, 14, 9548. [Google Scholar] [CrossRef]
- Shoemaker, L.G.; Barner, A.K.; Bittleston, L.S.; Teufel, A.I. Quantifying the relative importance of variation in predation and the environment for species coexistence. Ecol. Lett. 2020, 23, 939–950. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Shiffman, D.S.; Byrnes, E.E.; Hammerschlag-Peyer, C.M.; Hammerschlag, N. Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat. Ecol. 2017, 51, 435–448. [Google Scholar] [CrossRef]
- Chen, W.K.; Liu, K.M. Reproductive biology of whitespotted bamboo shark Chiloscyllium plagiosum in northern waters off Taiwan. Fish. Sci. 2006, 72, 1215–1224. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Ju, P.; Xiao, J.; Chen, M. Reproductive biology of the Pacific spadenose shark Scoliodon macrorhynchos, a heavily exploited species in the Southern Taiwan Strait. Mar. Coast. Fish. 2022, 14, e210216. [Google Scholar] [CrossRef]
- Ming-ru, C.; Shu-yuan, Q.I.U.; Sheng-yun, Y. The reproductive biology of the Spadenose Shark, Scoliodon laticaudus, from southern Fujian coastal waters. Acta Oceanol. Sin. 2001, 23, 92–98. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.; Su, L.; Chen, Z.Z.; Qiu, Y.S. An extensive assessment of exploitation indicators for multispecies fisheries in the South China Sea to inform more practical and precise management in China. Ecol. Indic. 2025, 173, 113363. [Google Scholar] [CrossRef]
- Arai, T.; Azri, A. Diversity, occurrence and conservation of sharks in the southern South China Sea. PLoS ONE 2019, 14, e0213864. [Google Scholar] [CrossRef]
- Du, J.; Ding, L.; Su, S.; Hu, W.; Wang, Y.; Loh, K.-H.; Yang, S.; Chen, M.; Roeroe, K.A.; Songploy, S. Setting conservation priorities for marine sharks in China and the Association of Southeast Asian Nations (ASEAN) seas: What are the benefits of a 30% conservation target? Front. Mar. Sci. 2022, 9, 933291. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, X.; Huang, Z.; Sun, M.; Chen, Z.; Zhang, K. Stock Assessment of Four Dominant Shark Bycatch Species in Bottom Trawl Fisheries in the Northern South China Sea. Sustainability 2022, 14, 3722. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montana, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Kim, S.L.; Koch, P.L. Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ. Biol. Fishes 2012, 95, 53–63. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Dong, J.Y.; Sun, X.; Zhan, Q.P.; Wang, L.L.; Zhang, X.M. Trophic Structure of Fishery Assemblage in Surrounding Waters of Lingshan Island Based on Stable Isotope Analysis. Period. Ocean Univ. China 2022, 52, 40–51. [Google Scholar] [CrossRef]
- Kim, S.L.; Casper, D.R.; Galván-Magaña, F.; Ochoa-Díaz, R.; Hernández-Aguilar, S.B.; Koch, P.L. Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study. Environ. Biol. Fishes 2012, 95, 37–52. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaa, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Compagno, L.J.V. FAO Species Catalogue Vol. 4. Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date. Part 2. Carcharhiniformes; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984; pp. 251–655. [Google Scholar]
- Nakaya, K.; Sato, K. Taxonomic review of Apristurus platyrhynchus and related species from the Pacific Ocean (Chondrichthyes, Carcharhiniformes, Scyliorhinidae). Ichthyol. Res. 2000, 47, 223–230. [Google Scholar] [CrossRef]
- Yano, T.; Ohshimo, S.; Sakai, T.; Yoda, M. Filling gaps in the biology and habitat use of two spurdog sharks (Squalus japonicus and Squalus brevirostris) in the East China Sea. Mar. Freshw. Res. 2020, 71, 1719–1731. [Google Scholar] [CrossRef]
- Braccini, J.M.; Gillanders, B.M.; Walker, T.I. Sources of variation in the feeding ecology of the piked spurdog (Squalus megalops): Implications for inferring predator–prey interactions from overall dietary composition. ICES J. Mar. Sci. 2005, 62, 1076–1094. [Google Scholar] [CrossRef]
- Nakaya, K.; Kawauchi, J. A review of the genus Apristurus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) from Taiwanese waters. Zootaxa 2013, 3752, 130–171. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Y.; Ma, W.; Zhu, W.; Wang, H.; Zhou, Q.; Li, J.; Wang, A.; Li, X.; Xu, Q. δ13C and δ15N stable isotopes demonstrate seasonal changes in the food web of coral reefs at the Wuzhizhou Island of the South China sea. Ecol. Indic. 2023, 146, 109852. [Google Scholar] [CrossRef]
- Wenbo, Z.; Honghui, H.; Chunhou, L.I.; Yong, L.I.U.; Zhanhui, Q.I.; Shannan, X.U.; Huaxue, L.I.U. Study on carbon and nitrogen stable isotopes of main fishery species in typical gulf, southern China. South China Fish. Sci. 2019, 15, 9–14. [Google Scholar] [CrossRef]
- Wanru, Z.; Qingxia, L.; Honghui, H.; Xiaoqing, Q.; Jiajun, L.; Jianhua, C. Study on stable isotopes of carbon and nitrogen of main fishery organisms in the southwestern waters of Daya Bay, South China Sea in winter 2020. J. Trop. Oceanogr. 2022, 41, 147–155. [Google Scholar] [CrossRef]
- Shi, J.; Wang, T.; Li, C.; Zhao, J.; Kang, Z.; Song, X.; Liu, Y. Food web structure and trophic diversity for the fishes of four islands in the Pearl River Estuary, China. Ecol. Indic. 2024, 160, 111916. [Google Scholar] [CrossRef]
- Xingyu, Q.; Qingxia, L.; Zuozhi, C.; Yancong, C.; Shouhui, D.; Honghui, H. The trophic structure of main fishery organisms in the southwestern continental shelf of the Nansha Islands in spring. J. Fish. Sci. China 2024, 31, 1524–1538. (In Chinese) [Google Scholar] [CrossRef]
- Galindo-Rosado, M.A.; Galván-Magaña, F.; Torres-Rojas, Y.E.; Delgado-Huertas, A.; Aguiñiga-García, S. Use of δ15N and δ13C in reconstructing the ontogenetic feeding habits of silky shark (Carcharhinus falciformis): Reassessing their trophic role in the Eastern Tropical Pacific Ocean. Environ. Biol. Fishes 2023, 106, 657–671. [Google Scholar] [CrossRef]
- Cherel, Y.; Hobson, K.A. Geographical variation in carbon stable isotope signatures of marine predators: A tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 2007, 329, 281–287. [Google Scholar] [CrossRef]
- Avsar, D. Age, growth, reproduction and feeding of the spurdog (Squalus acanthias Linnaeus, 1758) in the South-eastern Black Sea. Estuar. Coast. Shelf Sci. 2001, 52, 269–278. [Google Scholar] [CrossRef]
- Navia, A.F.; Mejía-Falla, P.A.; Giraldo, A. Feeding ecology of elasmobranch fishes in coastal waters of the Colombian Eastern Tropical Pacific. BMC Ecol. 2007, 7, 8. [Google Scholar] [CrossRef]
- Lim, K.C.; Then, A.Y.-H.; Loh, K.-H. Feeding ecology and reproductive biology of small coastal sharks in Malaysian waters. PeerJ 2023, 11, e15849. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, Y.; Chen, X.; Dai, X.; Zhu, J. Trophic ecology of sharks in the mid-east Pacific ocean inferred from stable isotopes. J. Ocean Univ. China 2014, 13, 278–282. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, D.S.; Frazier, B.S.; Kucklick, J.R.; Abel, D.; Brandes, J.; Sancho, G. Feeding Ecology of the Sandbar Shark in South Carolina Estuaries Revealed through δ13C and δ15N Stable Isotope Analysis. Mar. Coast. Fish. 2014, 6, 156–169. [Google Scholar] [CrossRef]
- Thomas, S.; Purushottama, G.B.; Nataraja, G.D.; Kizhakudan, S.J. Fishery and biological characteristics of the spadenose shark Scoliodon laticaudus Müller & Henle, 1838 from the Eastern Arabian Sea. Reg. Stud. Mar. Sci. 2020, 34, 101085. [Google Scholar] [CrossRef]
- Bora, G.; Dsouza, S.; Shanker, K. Diet composition and variation in four commonly landed and threatened shark species in Maharashtra, India. Reg. Stud. Mar. Sci. 2024, 74, 103531. [Google Scholar] [CrossRef]
- Jew, M. Evaluating the Trophic Habits and Dietary Overlap of Two Deep-Sea Catsharks (Apristurus brunneus and Parmaturus xaniurus) in Central California, USA. 2021. Available online: https://digitalcommons.csumb.edu/caps_thes_all/1116 (accessed on 20 August 2025).
- Laptikhovsky, V.V.; Arkhipkin, A.I.; Henderson, A.C. Feeding habits and dietary overlap in spiny dogfish Squalus acanthias (Squalidae) and narrowmouth catshark Schroederichthys bivius (Scyliorhinidae). J. Mar. Biol. Assoc. U. K. 2001, 81, 1015–1018. [Google Scholar] [CrossRef]
- Lopez, S.; Zapata-Hernández, G.; Bustamante, C.; Sellanes, J.; Meléndez, R. Trophic ecology of the dusky catshark Bythaelurus canescens (Günther, 1878)(Chondrychthyes: Scyliorhinidae) in the southeast Pacific Ocean. J. Appl. Ichthyol. 2013, 29, 751–756. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Hemigaleidae. Halaelurus burgeri. In Species Identification Guide for Fishery Purposes; The Living Marine Resources of the Western Central Pacific. Volume 2. Cephalopods, Crustaceans, Holothurians and Sharks; Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1998; pp. 1305–1311. [Google Scholar]
- Belleggia, M.; Colonello, J.; Cortés, F.; Figueroa, D.E. Eating catch of the day: The diet of porbeagle shark Lamna nasus (Bonnaterre 1788) based on stomach content analysis, and the interaction with trawl fisheries in the south-western Atlantic (52° S–56° S). J. Fish Biol. 2021, 99, 1591–1601. [Google Scholar] [CrossRef]
- Joyce, W.N.; Campana, S.E.; Natanson, L.J.; Kohler, N.E.; Pratt, H.L., Jr.; Jensen, C.F. Analysis of stomach contents of the porbeagle shark (Lamna nasus Bonnaterre) in the northwest Atlantic. ICES J. Mar. Sci. 2002, 59, 1263–1269. [Google Scholar] [CrossRef]
- Edje, B.O.; Ishaque, A.B.; Chigbu, P. Spatial and Temporal Patterns of δ13C and δ15N of Suspended Particulate Organic Matter in Maryland Coastal Bays, USA. Water 2020, 12, 2345. [Google Scholar] [CrossRef]
- Pastore, A.I.; Barabás, G.; Bimler, M.D.; Mayfield, M.M.; Miller, T.E. The evolution of niche overlap and competitive differences. Nat. Ecol. Evol. 2021, 5, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Pelage, L.; Lucena-Frédou, F.; Eduardo, L.N.; Le Loc’h, F.; Bertrand, A.; Lira, A.S.; Frédou, T. Competing with each other: Fish isotopic niche in two resource availability contexts. Front. Mar. Sci. 2022, 9, 975091. [Google Scholar] [CrossRef]
- Leurs, G.; Nieuwenhuis, B.O.; Zuidewind, T.J.; Hijner, N.; Olff, H.; Govers, L.L. Where land meets sea: Intertidal areas as key-habitats for sharks and rays. Fish Fish. 2023, 24, 407–426. [Google Scholar] [CrossRef]
- Weideli, O.C.; Daly, R.; Peel, L.R.; Heithaus, M.R.; Shivji, M.S.; Planes, S.; Papastamatiou, Y.P. Elucidating the role of competition in driving spatial and trophic niche patterns in sympatric juvenile sharks. Oecologia 2023, 201, 673–688. [Google Scholar] [CrossRef]
- Priester, C.R.; Dierking, J.; Hansen, T.; Abecasis, D.; Fontes, J.M.; Afonso, P. Trophic ecology and coastal niche partitioning of two sympatric shark species in the Azores (mid-Atlantic). Mar. Ecol. Prog. Ser. 2024, 726, 113–130. [Google Scholar] [CrossRef]
- Pantoja-Echevarría, L.M.; Tamburin, E.; Elorriaga-Verplancken, F.R.; Marmolejo-Rodríguez, A.J.; Galván-Magaña, F.; Tripp-Valdez, A.; Lara, A.; Jonathan, M.P.; Sujitha, S.B.; Delgado-Huertas, A.; et al. How to stay together? Habitat use by three sympatric sharks in the western coast of Baja California Sur, Mexico. Environ. Sci. Pollut. Res. 2022, 29, 61685–61697. [Google Scholar] [CrossRef]
- Cook, N.D.; Jenkins, A.; Perry, S.L.; Perkins, S.E.; Cable, J. Temporal niche partitioning as a potential mechanism for coexistence in two sympatric mesopredator sharks. Front. Mar. Sci. 2024, 11, 1443357. [Google Scholar] [CrossRef]
- Klimley, A.P.; Ketchum, J.T.; Lara-Lizardi, F.; Papastamatiou, Y.P.; Hoyos-Padilla, E.M. Evidence for spatial and temporal resource partitioning of sharks at Roca Partida, an isolated pinnacle in the eastern Pacific. Environ. Biol. Fishes 2022, 105, 1963–1974. [Google Scholar] [CrossRef]
- Fontaine, P.; Jensen, C.C.; Matich, P.; Rooker, J.R.; Wells, R.J.D. Predicting habitat suitability for the co-occurrence of an estuarine mesopredator and two top predatory fishes. Front. Fish Sci. 2024, 2, 1443923. [Google Scholar] [CrossRef]
- Speed, C.W.; Field, I.C.; Meekan, M.G.; Bradshaw, C.J.A. Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 2010, 408, 275–293. [Google Scholar] [CrossRef]
- Kajiura, S.M.; Tellman, S.L. Quantification of massive seasonal aggregations of blacktip sharks (Carcharhinus limbatus) in Southeast Florida. PLoS ONE 2016, 11, e0150911. [Google Scholar] [CrossRef] [PubMed]
- Curnick, D.J.; Carlisle, A.B.; Gollock, M.J.; Schallert, R.J.; Hussey, N.E. Evidence for dynamic resource partitioning between two sympatric reef shark species within the British Indian Ocean Territory. J. Fish Biol. 2019, 94, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, J.J.; Heithaus, M.R. Dietary niche overlap in a nearshore elasmobranch mesopredator community. Mar. Ecol. Prog. Ser. 2011, 425, 247–260. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- May, R.M.; Arthur, R.H.M. Niche Overlap as a Function of Environmental Variability. Proc. Natl. Acad. Sci. USA 1972, 69, 1109–1113. [Google Scholar] [CrossRef]
- Mengal, K.; Zhang, X. Intraspecific variations in climatic niche of sharks and their relatives: Patterns, drivers, and molecular mechanisms. Rev. Fish Biol. Fish. 2025. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Campbell, H.A.; Cramp, R.L.; Burke, C.L.; Micheli-Campbell, M.A.; Pillans, R.D.; Lyon, B.J.; Franklin, C.E. Niche partitioning between river shark species is driven by seasonal fluctuations in environmental salinity. Funct. Ecol. 2020, 34, 2170–2185. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]

| Tissues | Species | n | TL (mm) | δ13C (‰) | δ15N (‰) | ||
|---|---|---|---|---|---|---|---|
| Rang | Mean | Rang | Mean | ||||
| Muscle | A. platyrhynchus | 55 | 222~395 | −18.76~−14.47 | −18.16 | 8.70~14.28 | 9.81 |
| S. megalops | 63 | 320~772 | −21.18~−16.87 | −17.66 | 10.40~12.89 | 11.75 | |
| S. laticaudus | 135 | 207~690 | −18.10~−14.62 | −15.50 | 9.80~16.58 | 15.10 | |
| H. burgeri | 11 | 435~582 | −17.95~−17.62 | −17.82 | 10.48~11.12 | 10.74 | |
| C. sorrah | 13 | 267~1000 | −21.46~−16.71 | −19.29 | 11.28~13.16 | 11.91 | |
| G. sauteri | 34 | 201~412 | −18.96~−17.13 | −17.98 | 9.41~11.62 | 10.61 | |
| C. fasciatum | 11 | 129~216 | −17.77~−15.65 | −17.02 | 9.92~11.96 | 10.75 | |
| C. sarawakensis | 127 | 182~470 | −17.93~−15.30 | −17.34 | 10.52~15.21 | 11.21 | |
| Liver | A. platyrhynchus | 55 | 222~395 | −19.68~−16.93 | −17.92 | 8.27~10.67 | 9.20 |
| S. megalops | 63 | 320~772 | −18.45~−15.96 | −17.38 | 9.54~12.38 | 11.18 | |
| S. laticaudus | 135 | 207~690 | −17.78~−13.55 | −15.08 | 11.71~16.59 | 14.85 | |
| H. burgeri | 11 | 435~582 | −18.25~−17.20 | −17.79 | 9.50~10.60 | 10.29 | |
| C. sorrah | 13 | 267~1000 | −17.97~−16.47 | −17.40 | 11.06~12.90 | 11.93 | |
| G. sauteri | 34 | 201~412 | −19.02~−17.26 | −18.03 | 8.25~11.28 | 10.07 | |
| C. fasciatum | 11 | 129~216 | −18.85~−15.77 | −17.50 | 8.44~10.92 | 9.45 | |
| C. sarawakensis | 127 | 182~470 | −19.39~−15.33 | −17.19 | 8.76~11.45 | 10.45 | |
| Tissue | Shark Species | A. platyrhynchus | G. sauteri | H. burgeri | C. fasciatum | C. sarawakensis | S. megalops | C. sorrah | S. laticaudus | SEAc (‰2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | A. platyrhynchus | 1.00 | 0.54 | |||||||
| G. sauteri | 0.00 | 1.00 | 0.45 | |||||||
| H. burgeri | 0.00 | 0.00 | 1.00 | 1.76 | ||||||
| C. fasciatum | 0.00 | 0.00 | 0.00 | 1.00 | 0.06 | |||||
| C. sarawakensis | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.77 | ||||
| S. megalops | 0.16 | 0.00 | 0.00 | 0.95 | 0.00 | 1.00 | 0.57 | |||
| C. sorrah | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.83 | ||
| S. laticaudus | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.10 | 0.02 | 1.00 | 0.56 | |
| Liver | A. platyrhynchus | 1.00 | 0.82 | |||||||
| G. sauteri | 0.00 | 1.00 | 0.84 | |||||||
| H. burgeri | 0.00 | 0.00 | 1.00 | 2.32 | ||||||
| C. fasciatum | 0.00 | 0.00 | 0.00 | 1.00 | 0.26 | |||||
| C. sarawakensis | 0.00 | 0.02 | 0.00 | 0.00 | 1.00 | 0.44 | ||||
| S. megalops | 0.14 | 0.11 | 0.00 | 0.87 | 0.00 | 1.00 | 0.98 | |||
| C. sorrah | 0.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.11 | ||
| S. laticaudus | 0.00 | 0.13 | 0.00 | 0.56 | 0.00 | 0.17 | 0.10 | 1.00 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Xiong, P.; Chen, Z.; Xu, Y. Multi-Tissue Stable Isotope Analysis Reveals the Feeding Ecology of Dominant Shark Bycatch Species in the Northern South China Sea. Fishes 2025, 10, 583. https://doi.org/10.3390/fishes10110583
Zhang K, Xiong P, Chen Z, Xu Y. Multi-Tissue Stable Isotope Analysis Reveals the Feeding Ecology of Dominant Shark Bycatch Species in the Northern South China Sea. Fishes. 2025; 10(11):583. https://doi.org/10.3390/fishes10110583
Chicago/Turabian StyleZhang, Kui, Pengli Xiong, Zuozhi Chen, and Youwei Xu. 2025. "Multi-Tissue Stable Isotope Analysis Reveals the Feeding Ecology of Dominant Shark Bycatch Species in the Northern South China Sea" Fishes 10, no. 11: 583. https://doi.org/10.3390/fishes10110583
APA StyleZhang, K., Xiong, P., Chen, Z., & Xu, Y. (2025). Multi-Tissue Stable Isotope Analysis Reveals the Feeding Ecology of Dominant Shark Bycatch Species in the Northern South China Sea. Fishes, 10(11), 583. https://doi.org/10.3390/fishes10110583









