Comparative Physiological Profiling of Abalone (Haliotis iris): Insights from Wild and Aquaculture Broodstock
Abstract
1. Introduction
2. Materials and Methods
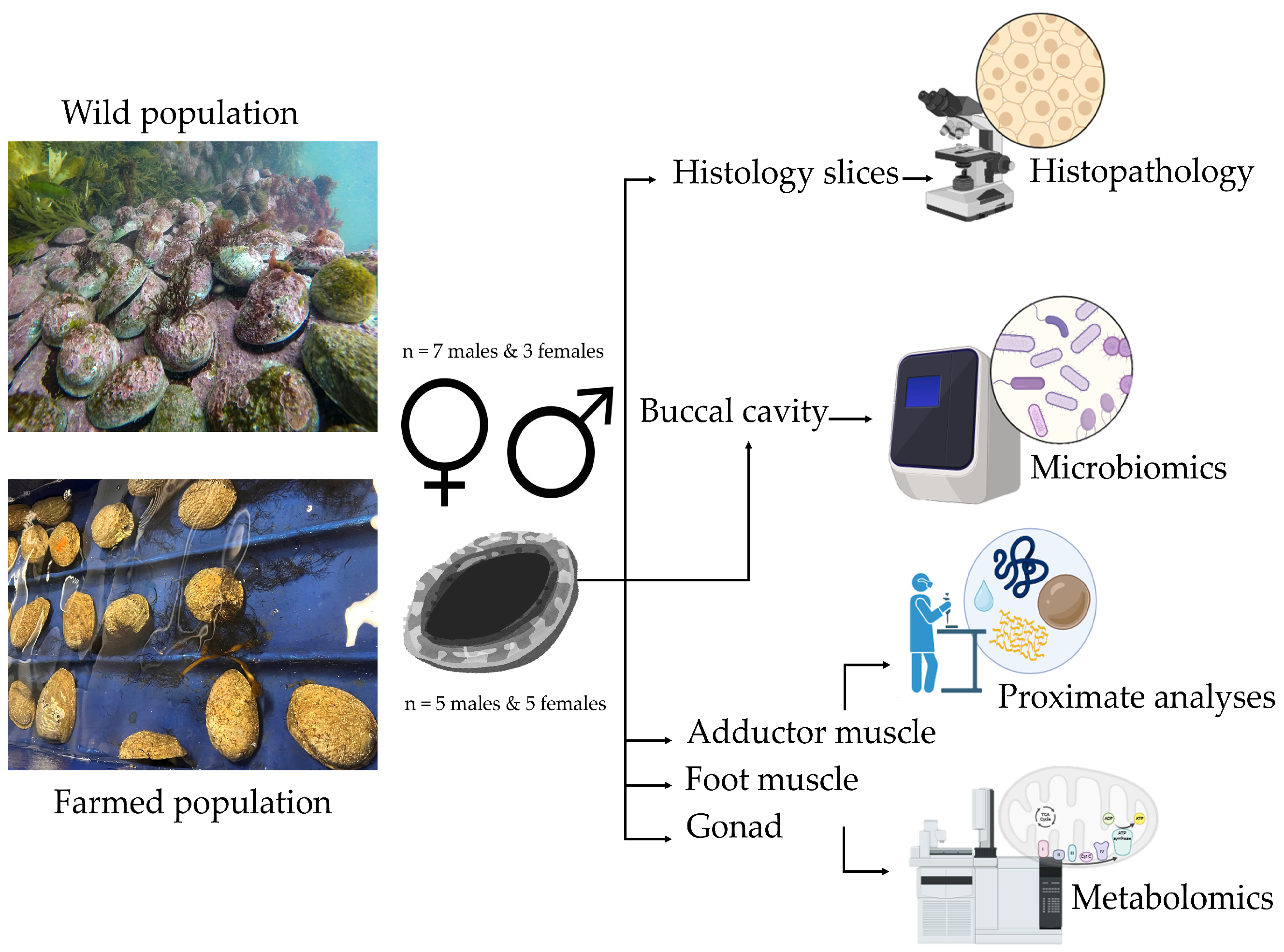
2.1. Experimental Design and Sampling
2.2. Histopathology
2.3. Microbiome Processing
2.4. Proximate Analyses
2.5. Metabolomics
2.6. Statistical Analyses
2.6.1. Morphometrics
2.6.2. Histopathology
2.6.3. Microbiome
2.6.4. Proximate Analyses
2.6.5. Metabolomics
3. Results
3.1. Morphometrics
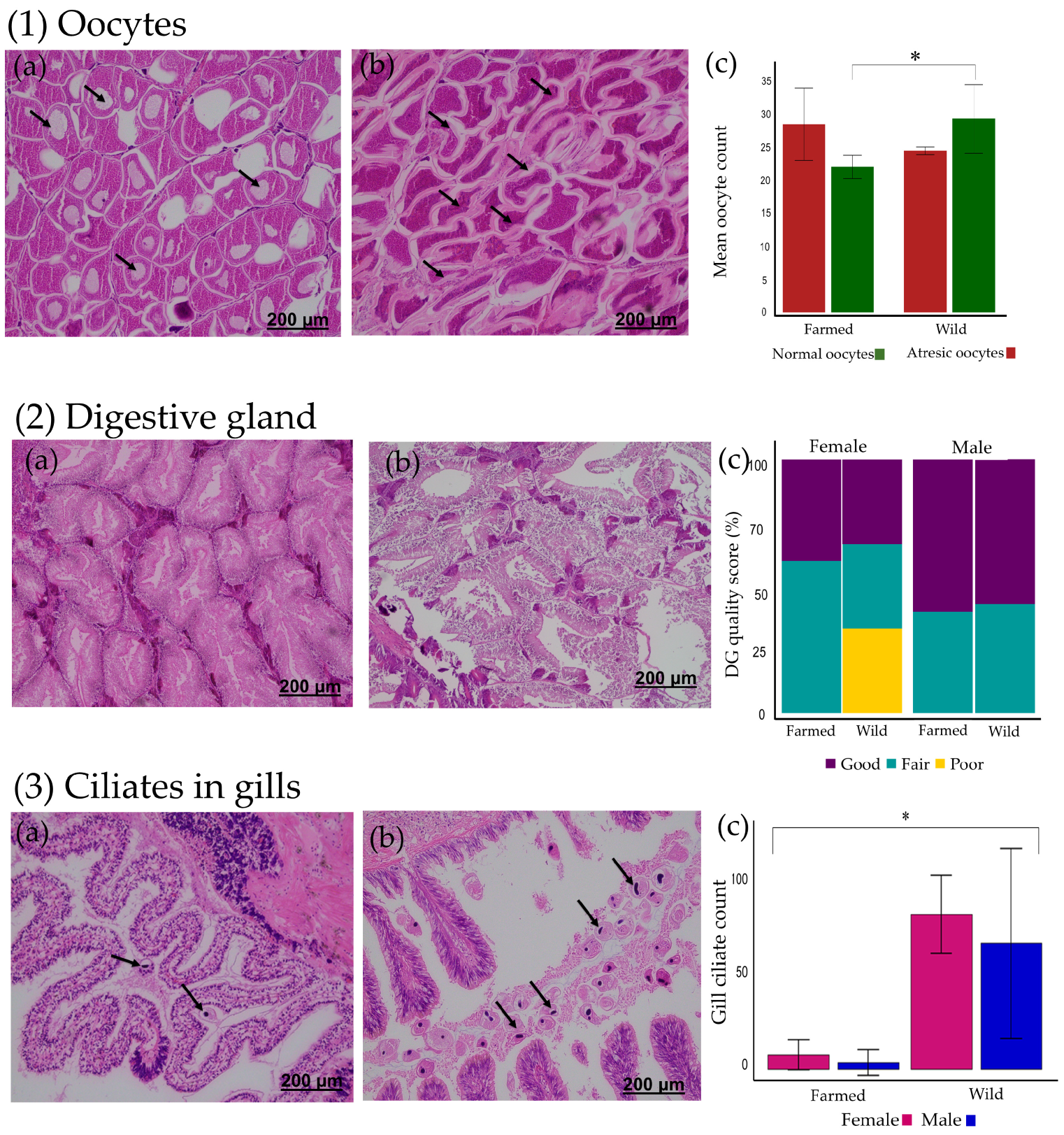
3.2. Histopathology
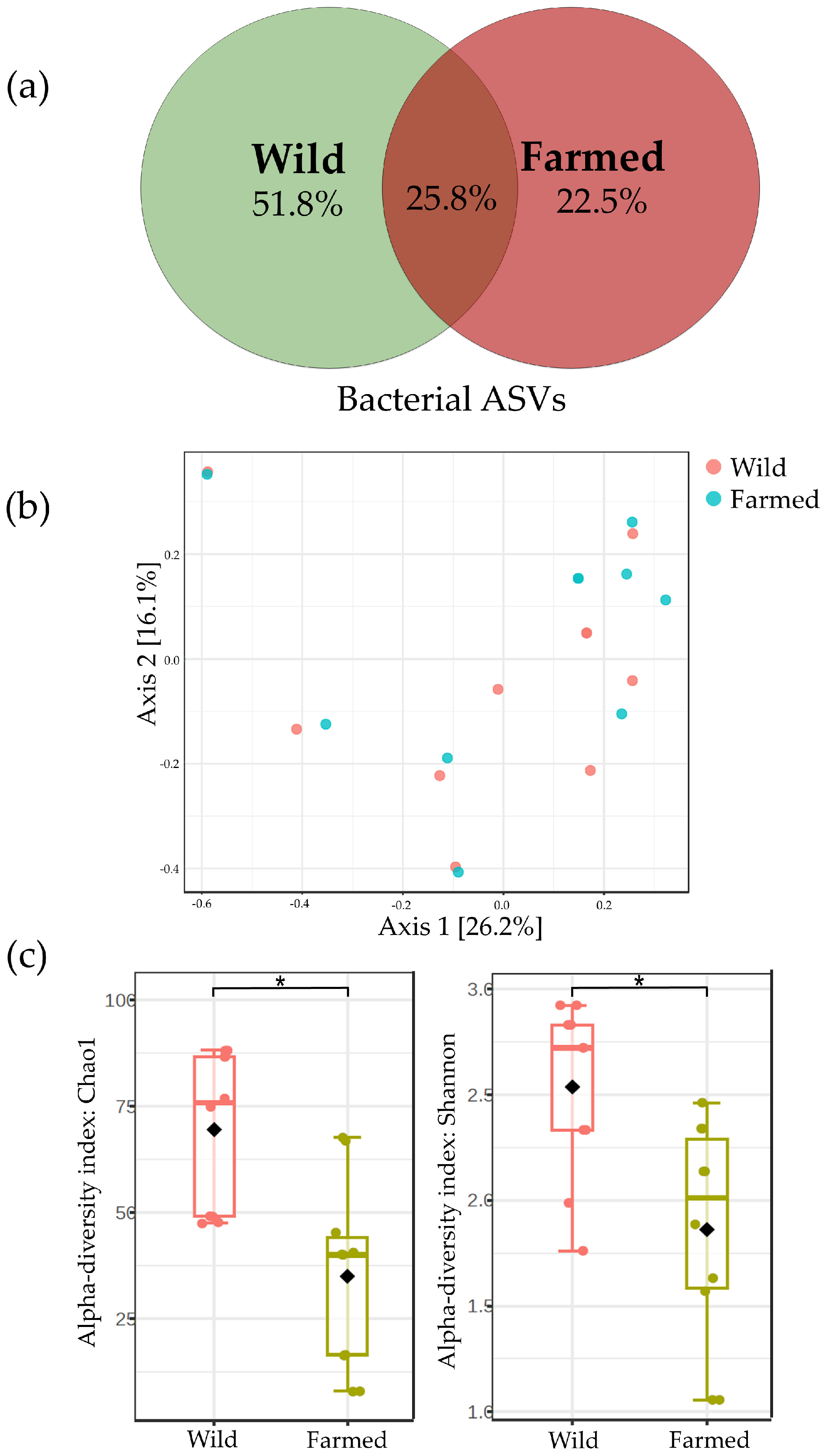
3.3. Microbiome
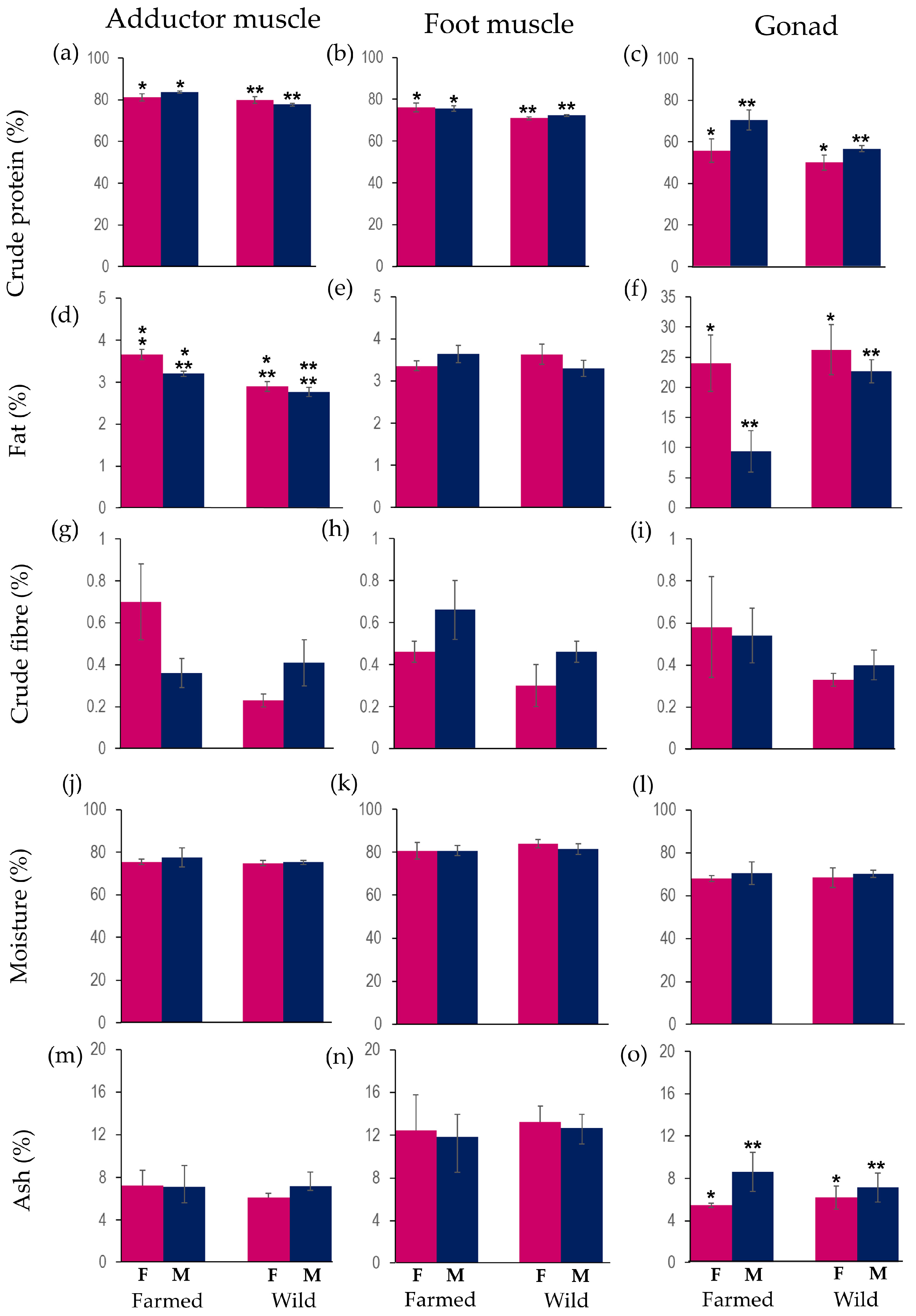
3.4. Proximate Composition of Tissues
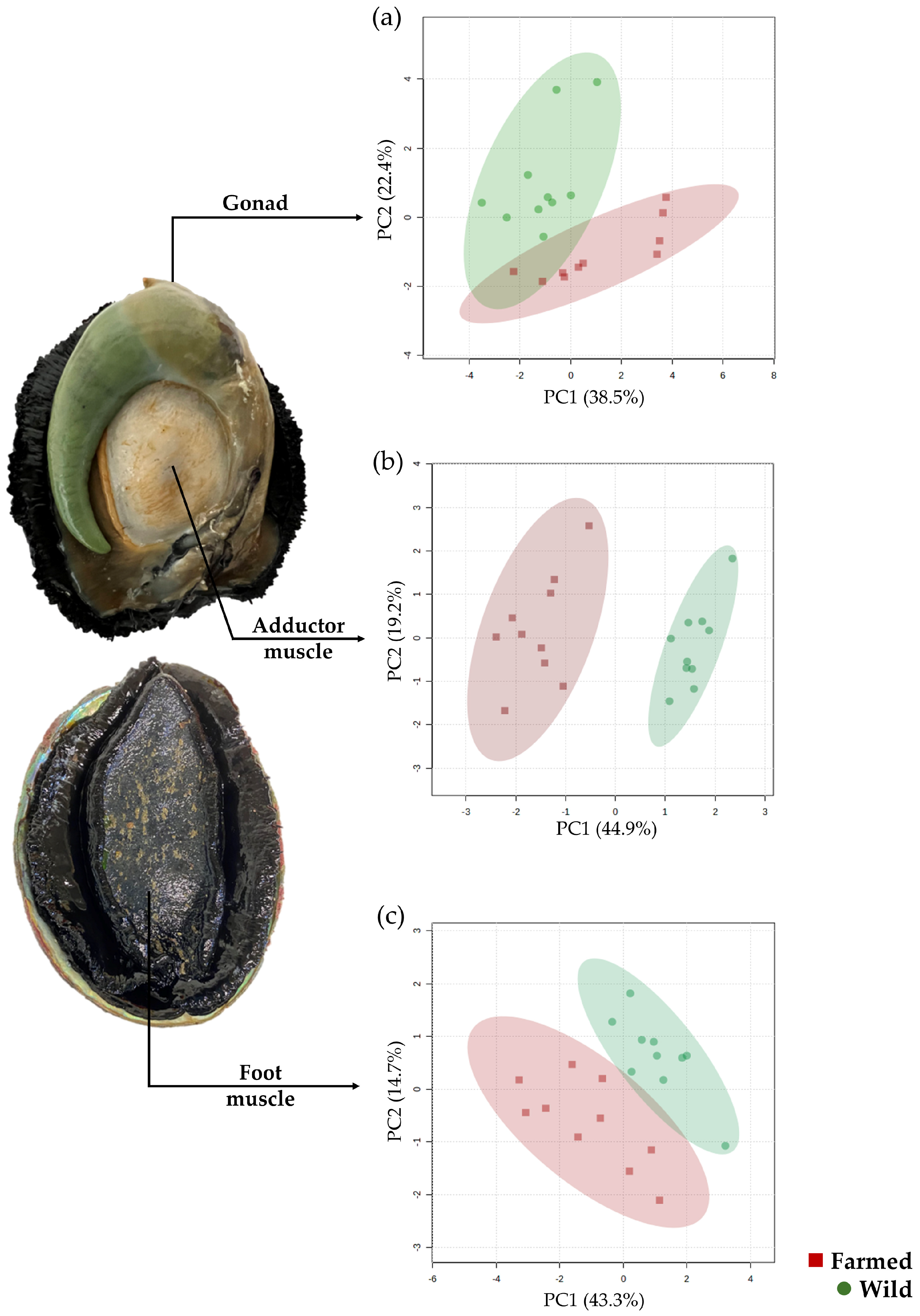
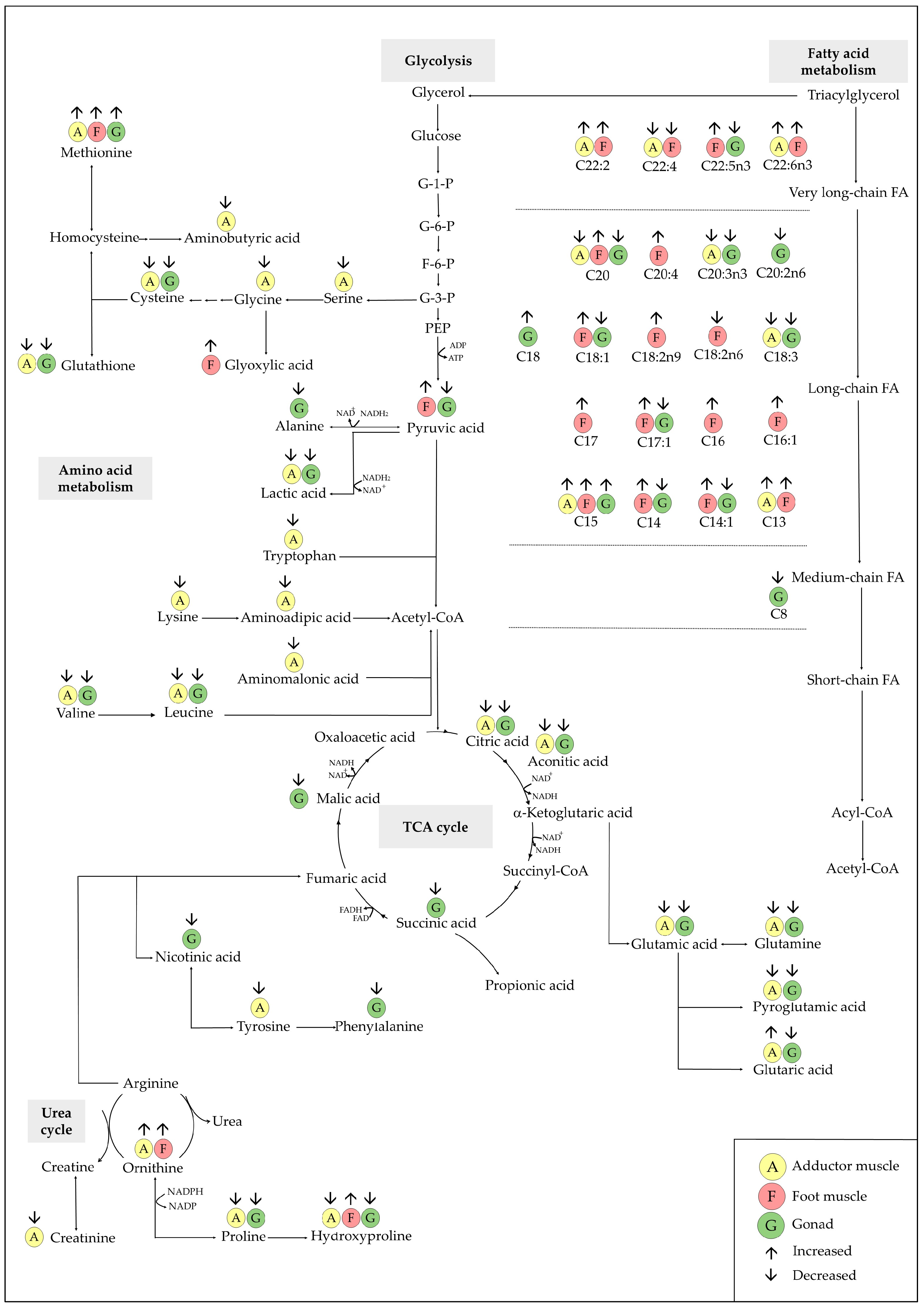
3.5. Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AOAC | Association of Official Agricultural Chemists |
| ASV | Amplicon sequence variants |
| DG | Digestive gland |
| EI | Electron impact |
| GC-MS | Gas chromatography–mass spectrometry |
| gDNA | Genomic deoxyribonucleic acid |
| glog | Generalised log transformed |
| HSD | Honest Significant Difference |
| MCF | Methyl chloroformate |
| NIST | National Institute of Standards and Technology |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| PCR | Polymerase chain reaction |
| PERMANOVA | Permutational multivariate analysis of variance |
| rRNA | Ribosomal ribonucleic acid |
| TACC | Total Allowable Commercial Catch |
| TCA | Tricarboxylic acid cycle |
References
- Cook, P.A. Worldwide Abalone Production Statistics. J. Shellfish Res. 2019, 38, 401–404. [Google Scholar] [CrossRef]
- Walton, K.; Marshall, B.A.; Rawlence, N.J.; Spencer, H.G. Haliotis virginea Gmelin, 1791 and a new abalone from Aotearoa New Zealand (Mollusca: Gastropoda: Haliotidae). Molluscan Res. 2024, 44, 305–315. [Google Scholar] [CrossRef]
- Walton, K.; Spencer, G.H.; Rawlence, N. ‘The Pāua That Clings to the Sea’: A New Species of Abalone Found Only in Waters off a Remote NZ Island Chain. 2024. Available online: https://theconversation.com/the-paua-that-clings-to-the-sea-a-new-species-of-abalone-found-only-in-waters-off-a-remote-nz-island-chain-236568 (accessed on 29 September 2025).
- Gnanalingam, G.; Pritchard, D.W.; Richards, D.K.; Subritzky, P.; Flack, B.; Hepburn, C.D. Local management to support local fisheries: Rāhui (temporary closure) and bag limits for blackfoot abalone (Haliotis iris) in southern New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2320–2333. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Alfaro, A.C.; Venter, L.; Ericson, J.A.; Ragg, N.L.C.; McCowan, T.; Mundy, C. Metabolomics approach reveals size-specific variations of blackfoot abalone (Haliotis iris) in Chatham Islands, New Zealand. Fish. Res. 2023, 262, 106645. [Google Scholar] [CrossRef]
- Ryder, F.J.; Sainsbury, K.J.; Hepburn, C.D.; Pritchard, D.W.; Gnanalingam, G. Re-assessment of a blackfoot abalone (Haliotis iris) population in Peraki Bay, New Zealand, after 45 years. N. Z. J. Mar. Freshw. Res. 2025, 59, 164–182. [Google Scholar] [CrossRef]
- Luo, Q.; Hamid, N.; Oey, I.; Leong, S.Y.; Kantono, K.; Alfaro, A.; Lu, J. Physicochemical changes in New Zealand abalone (Haliotis iris) with pulsed electric field (PEF) processing and heat treatments. LWT 2019, 115, 108438. [Google Scholar] [CrossRef]
- Bagarinao, N.C.; Kaur, L.; Boland, M. Effects of ultrasound treatments on tenderness and in vitro protein digestibility of New Zealand abalone, Haliotis iris. Foods 2020, 9, 1122. [Google Scholar] [CrossRef]
- Cook, P.A. Worldwide abalone production: An update. N. Z. J. Mar. Freshw. Res. 2025, 59, 4–10. [Google Scholar] [CrossRef]
- Li, X.; Huang, D.; Pan, M.; Sahandi, J.; Wu, Z.; Mai, K.; Zhang, W. Nutrition and feeds for abalone: Current knowledge and future directions. Rev. Aquac. 2024, 16, 1555–1579. [Google Scholar] [CrossRef]
- Hernández-Casas, S.; Seijo, J.C.; Beltrán-Morales, L.F.; Hernández-Flores, Á.; Arreguín-Sánchez, F.; Ponce-Díaz, G. Analysis of supply and demand in the international market of major abalone fisheries and aquaculture production. Mar. Policy 2023, 148, 105405. [Google Scholar] [CrossRef]
- Virgin, S.D.; Gerrity, S.; Schiel, D.R. Recreational fishing effects on New Zealand abalone (pāua, Haliotis iris) after five years of fishery closure: A matrix-based approach. Mar. Environ. Res. 2025, 210, 107276. [Google Scholar] [CrossRef]
- Wilkinson, V.; Morris, T. New Zealand Situation and Capabilities—Emerging and Future Platforms in New Zealand’s Bioeconomy. Coriolis (Research—Consulting—Strategy) for the Ministry of Business, Innovation and Employment, v1.00a. 2003. Available online: https://www.mbie.govt.nz/assets/new-zealand-situation-and-capabilities.pdf (accessed on 20 September 2025).
- Evans, B.; Bartlett, J.; Sweijd, N.; Cook, P.; Elliott, N. Loss of genetic variation at microsatellite loci in hatchery produced abalone in Australia (Haliotis rubra) and South Africa (Haliotis midae). Aquaculture 2004, 233, 109–127. [Google Scholar] [CrossRef]
- Li, Q.; Park, C.; Endo, T.; Kijima, A. Loss of genetic variation at microsatellite loci in hatchery strains of the Pacific abalone (Haliotis discus hannai). Aquaculture 2004, 235, 207–222. [Google Scholar] [CrossRef]
- Biandolino, F.; Parlapiano, I.; Grattagliano, A.; Fanelli, G.; Prato, E. Comparative characteristics of percentage edibility, condition index, biochemical constituents and lipids nutritional quality indices of wild and farmed scallops (Flexopecten glaber). Water 2020, 12, 1777. [Google Scholar] [CrossRef]
- Hall, S.A.; Méthé, D.; Stewart-Clark, S.E.; Clark, K.F.; Tremblay, R. Comparison of absorption efficiency and metabolic rate between wild and aquaculture oysters (Crassostrea virginica). Aquac. Rep. 2020, 16, 100263. [Google Scholar] [CrossRef]
- Benito, D.; Ahvo, A.; Nuutinen, J.; Bilbao, D.; Saenz, J.; Etxebarria, N.; Lekube, X.; Izagirre, U.; Lehtonen, K.K.; Marigómez, I. Influence of season-depending ecological variables on biomarker baseline levels in mussels (Mytilus trossulus) from two Baltic Sea subregions. Sci. Total Environ. 2019, 689, 1087–1103. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking biomarkers for the health and welfare of aquaculture fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- Sabetian, A.; Hoang, L.H.; Zhang, J.; Lilkendey, J. Tracing transgenerational plasticity through ova fatty acid biomarkers in Giant Kōkopu (Galaxias argenteus). N. Z. J. Mar. Freshw. Res. 2025, 59, 356–367. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef]
- Sabetian, A.; Hoang, L.H.; Zhang, J.; White, W.L.; Chen, T.; Wang, H.; Lilkendey, J. Fatty acid biomarkers reveal maternal energy allocation strategies in spawning Snapper (Chrysophrys auratus). N. Z. J. Mar. Freshw. Res. 2025, 59, 1117–1130. [Google Scholar] [CrossRef]
- Brosset, P.; Cooke, S.J.; Schull, Q.; Trenkel, V.M.; Soudant, P.; Lebigre, C. Physiological biomarkers and fisheries management. Rev. Fish Biol. Fish. 2021, 31, 797–819. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Alfaro, A.C.; Mundy, C.; Petersen, J.; Ragg, N.L. Omics research on abalone (Haliotis spp.): Current state and perspectives. Aquaculture 2022, 547, 737438. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Young, T. Showcasing metabolomic applications in aquaculture: A review. Rev. Aquac. 2018, 10, 135–152. [Google Scholar] [CrossRef]
- Delorme, N.J.; Venter, L.; Rolton, A.; Ericson, J.A. Integrating animal health and stress assessment tools using the green-lipped mussel Perna canaliculus as a case study. J. Shellfish Res. 2021, 40, 93–112. [Google Scholar] [CrossRef]
- Hooper, C.; Day, R.; Slocombe, R.; Benkendorff, K.; Handlinger, J.; Goulias, J. Effects of severe heat stress on immune function, biochemistry and histopathology in farmed Australian abalone (hybrid Haliotis laevigata × Haliotis rubra). Aquaculture 2014, 432, 26–37. [Google Scholar] [CrossRef]
- Villasante, A.; Catalán, N.; Rojas, R.; Lohrmann, K.B.; Romero, J. Microbiota of the digestive gland of red abalone (Haliotis rufescens) is affected by withering syndrome. Microorganisms 2020, 8, 1411. [Google Scholar] [CrossRef]
- Ganogpichayagrai, A.; Suksaard, C. Proximate composition, vitamin and mineral composition, antioxidant capacity, and anticancer activity of Acanthopanax trifoliatus. J. Adv. Pharm. Technol. Res. 2020, 11, 179–183. [Google Scholar] [CrossRef]
- García-López, Á.; Pascual, E.; Sarasquete, C.; Martínez-Rodríguez, G. Disruption of gonadal maturation in cultured Senegalese sole Solea senegalensis Kaup by continuous light and/or constant temperature regimes. Aquaculture 2006, 261, 789–798. [Google Scholar] [CrossRef]
- Stentiford, G.; Massoud, M.; Al-Mudhhi, S.; Al-Sarawi, M.; Al-Enezi, M.; Lyons, B. Histopathological survey of potential biomarkers for the assessment of contaminant related biological effects in species of fish and shellfish collected from Kuwait Bay, Arabian Gulf. Mar. Environ. Res. 2014, 98, 60–67. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An important tool to assess the welfare of aquatic animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Copedo, J.S.; Webb, S.C.; Delisle, L.; Knight, B.; Ragg, N.L.; Laroche, O.; Venter, L.; Alfaro, A.C. Elucidating divergent growth and climate vulnerability in abalone (Haliotis iris): A multi-year snapshot. Mar. Environ. Res. 2025, 207, 107090. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.W. Histological Techniques for Marine Bivalve Mollusks and Crustaceans; National Centers for Coastal Ocean Service, National Ocean Service, NOAA: Silver Spring, MD, USA, 2004; Volume 5. [Google Scholar]
- Copedo, J.S.; Webb, S.C.; Ragg, N.L.; Venter, L.; Alfaro, A.C. Histopathological investigation of four populations of abalone (Haliotis iris) exhibiting divergent growth performance. J. Invertebr. Pathol. 2024, 202, 108042. [Google Scholar] [CrossRef] [PubMed]
- Beninger, P.G. Caveat observator: The many faces of pre-spawning atresia in marine bivalve reproductive cycles. Mar. Biol. 2017, 164, 163. [Google Scholar] [CrossRef]
- Bullon, N.; Alfaro, A.C.; Guo, J.; Copedo, J.; Nguyen, T.V.; Seyfoddin, A. Expanding the menu for New Zealand farmed abalone: Dietary inclusion of insect meal and grape marc (effects on gastrointestinal microbiome, digestive morphology, and muscle metabolome). N. Z. J. Mar. Freshw. Res. 2025, 59, 31–60. [Google Scholar] [CrossRef]
- Bullon, N.; Seyfoddin, A.; Hamid, N.; Manivannan, M.; Alfaro, A.C. Effects of insect meal and grape marc in the nutritional profile, growth, and digestibility of juvenile New Zealand farmed abalone. Aquac. Int. 2024, 32, 1507–1536. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Nguyen, T.V.; Venter, L.; Ericson, J.A.; Sharma, S.; Ragg, N.L.; Mundy, C. The effects of live transport on metabolism and stress responses of abalone (Haliotis iris). Metabolites 2021, 11, 748. [Google Scholar] [CrossRef]
- Smart, K.F.; Aggio, R.B.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 1709–1729. [Google Scholar] [CrossRef]
- Young, T.; Walker, S.P.; Alfaro, A.C.; Fletcher, L.M.; Murray, J.S.; Lulijwa, R.; Symonds, J. Impact of acute handling stress, anaesthesia, and euthanasia on fish plasma biochemistry: Implications for veterinary screening and metabolomic sampling. Fish Physiol. Biochem. 2019, 45, 1485–1494. [Google Scholar] [CrossRef]
- Chen, T. Libracef Version 0.2: A Tool for Building Agilent Library Files from .cef Files of Mass Hunter Unknown Analysis. Available online: https://github.com/mzzzhunter/libracef/releases/tag/v0.2 (accessed on 1 January 2025).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Archer, S.D.; Lee, K.C.; Caruso, T.; King-Miaow, K.; Harvey, M.; Huang, D.; Wainwright, B.J.; Pointing, S.B. Air mass source determines airborne microbial diversity at the ocean–atmosphere interface of the Great Barrier Reef marine ecosystem. ISME J. 2020, 14, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Schimmel, B.C.; Glauser, G.; Klinkhamer, P.G.; Erb, M. Leafminer attack accelerates the development of soil-dwelling conspecific pupae via plant-mediated changes in belowground volatiles. New Phytol. 2022, 234, 280–294. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Wang, S.; Chen, H.; Cai, S.; Feng, J. NMR-based metabolomic analysis of Haliotis diversicolor exposed to thermal and hypoxic stresses. Sci. Total Environ. 2016, 545, 280–288. [Google Scholar] [CrossRef]
- Koyama, M.; Furukawa, F.; Koga, Y.; Funayama, S.; Furukawa, S.; Baba, O.; Lin, C.-C.; Hwang, P.-P.; Moriyama, S.; Okumura, S.-I. Gluconeogenesis and glycogen metabolism during development of Pacific abalone, Haliotis discus hannai. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R619–R633. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Z.; Yu, W.; Luo, X.; Ke, C.; You, W. The taste characteristics and metabolite variations of two Pacific abalone strains with different glycogen contents. LWT 2024, 195, 115820. [Google Scholar] [CrossRef]
- Livingstone, D.R.; De Zwaan, A. Carbohydrate metabolism of gastropods. In Metabolic Biochemistry and Molecular Biomechanics; Elsevier: Amsterdam, The Netherlands, 1983; pp. 177–242. [Google Scholar]
- Martinez-Cruz, O.; Sanchez-Paz, A.; Garcia-Carreño, F.; Jimenez-Gutierrez, L.; Muhlia-Almaza, A. Invertebrates Mitochondrial Function and Energetic Challenges; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Wang, X.; Li, E.; Chen, L. A review of carbohydrate nutrition and metabolism in crustaceans. N. Am. J. Aquac. 2016, 78, 178–187. [Google Scholar] [CrossRef]
- Li, B.; Song, K.; Meng, J.; Li, L.; Zhang, G. Integrated application of transcriptomics and metabolomics provides insights into glycogen content regulation in the Pacific oyster Crassostrea gigas. BMC Genom. 2017, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.; Andrew, N.; Kim, S. Demographic variation in the New Zealand abalone Haliotis iris. Mar. Freshw. Res. 2006, 57, 215–224. [Google Scholar] [CrossRef]
- Poore, G.C. Ecology of New Zealand abalones, Haliotis species (Mollusca: Gastropoda) 3. Growth. N. Z. J. Mar. Freshw. Res. 1972, 6, 534–559. [Google Scholar] [CrossRef]
- Morash, A.J.; Alter, K. Effects of environmental and farm stress on abalone physiology: Perspectives for abalone aquaculture in the face of global climate change. Rev. Aquac. 2016, 8, 342–368. [Google Scholar] [CrossRef]
- Moss, G.A. Effect of temperature on the breeding cycle and spawning success of the New Zealand abalone, Haliotis australis. N. Z. J. Mar. Freshw. Res. 1998, 32, 139–146. [Google Scholar] [CrossRef]
- Wood, A.; Buxton, C. Aspects of the biology of the abalone Haliotis midae (Linne, 1758) on the east coast of South Africa. 2. Reproduction. S. Afr. J. Mar. Sci. 1996, 17, 69–78. [Google Scholar] [CrossRef]
- Corriero, A.; Zupa, R.; Mylonas, C.C.; Passantino, L. Atresia of ovarian follicles in fishes, and implications and uses in aquaculture and fisheries. J. Fish Dis. 2021, 44, 1271–1291. [Google Scholar] [CrossRef]
- Osterheld, K.; Davidson, J.; Comeau, L.A.; Audet, C.; Hori, T.; Tremblay, R. Reproductive investment and gonad development in triploid mussels, Mytilus Edulis. Aquaculture 2024, 593, 741315. [Google Scholar] [CrossRef]
- Venter, L.; Loots, D.T.; Vosloo, A.; Jansen van Rensburg, P.; Lindeque, J.Z. Abalone growth and associated aspects: Now from a metabolic perspective. Rev. Aquac. 2018, 10, 451–473. [Google Scholar] [CrossRef]
- Ayres, D.W.P. Effect of Diet and Sex-Sorting on Growth and Gonad Development in Farmed South African Abalone, Haliotis midae. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 2013. [Google Scholar]
- Kim, M.A.; Kim, T.H.; Lee, S.; Nam, B.H.; Lee, J.S.; Jang, W.; Sohn, Y.C. Ovarian transcriptome profiles associated with sexual maturation in Pacific abalone (Haliotis discus hannai). Genes Genom. 2020, 42, 1179–1188. [Google Scholar] [CrossRef]
- Straub, D.; Blackwell, N.; Langarica-Fuentes, A.; Peltzer, A.; Nahnsen, S.; Kleindienst, S. Interpretations of environmental microbial community studies are biased by the selected 16S rRNA (gene) amplicon sequencing pipeline. Front. Microbiol. 2020, 11, 550420. [Google Scholar] [CrossRef] [PubMed]
- Soh, M.; Tay, Y.C.; Lee, C.S.; Low, A.; Orban, L.; Jaafar, Z.; Seedorf, H. The intestinal digesta microbiota of tropical marine fish is largely uncultured and distinct from surrounding water microbiota. NPJ Biofilms Microbiomes 2024, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Bullon, N.; Seyfoddin, A.; Alfaro, A.C. The role of aquafeeds in abalone nutrition and health: A comprehensive review. J. World Aquac. Soc. 2023, 54, 7–31. [Google Scholar] [CrossRef]
- Jiang, J.-Z.; Zhao, W.; Liu, G.-F.; Wang, J.-Y. Relationships between and formation dynamics of the microbiota of consumers, producers, and the environment in an abalone aquatic system. PLoS ONE 2017, 12, e0182590. [Google Scholar] [CrossRef]
- Muznebin, F.; Alfaro, A.C.; Webb, S.C. Occurrence of Perkinsus olseni and other parasites in New Zealand black-footed abalone (Haliotis iris). N. Z. J. Mar. Freshw. Res. 2023, 57, 261–281. [Google Scholar] [CrossRef]
- Choi, M.-J.; Oh, Y.D.; Kim, Y.R.; Lim, H.K.; Kim, J.-M. Intestinal microbial diversity is higher in Pacific abalone (Haliotis discus hannai) with slower growth rates. Aquaculture 2021, 537, 736500. [Google Scholar] [CrossRef]
- Guo, Z.; Hou, X.; Chang, L.; Du, Z.; Shi, K.; Song, A.; Liang, Z.; Gu, J. Effects of different feeding patterns on growth, enzyme activity, and intestinal microbiome of the juvenile Pacific abalone Haliotis discus hannai. Aquac. Rep. 2024, 39, 102427. [Google Scholar] [CrossRef]
- Parker-Graham, C.A.; Eetemadi, A.; Yazdi, Z.; Marshman, B.C.; Loeher, M.; Richey, C.A.; Barnum, S.; Moore, J.D.; Soto, E. Effect of oxytetracycline treatment on the gastrointestinal microbiome of critically endangered white abalone (Haliotis sorenseni) treated for withering syndrome. Aquaculture 2020, 526, 735411. [Google Scholar] [CrossRef]
- Yu, W.; Lu, Y.; Shen, Y.; Liu, J.; Gong, S.; Yu, F.; Huang, Z.; Zou, W.; Zhou, M.; Luo, X. Exploring the intestinal microbiota and metabolome profiles associated with feed efficiency in Pacific abalone (Haliotis discus hannai). Front. Microbiol. 2022, 13, 852460. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Tang, B.; Huang, M.; Luo, X.; Ke, C. Effects of Different Diets on Intestinal Microbiota Between Common and Orange-Muscle Mutant of Haliotis gigantea; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Britz, P.J.; Hecht, T. Effect of dietary protein and energy level on growth and body composition of South African abalone, Haliotis midae. Aquaculture 1997, 156, 195–210. [Google Scholar] [CrossRef]
- Bautista-Teruel, M.N.; Millamena, O.M. Diet development and evaluation for juvenile abalone, Haliotis asinina: Protein/energy levels. Aquaculture 1999, 178, 117–126. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Viljoen, C.; Hutchinson, P.; Reinicke, J.; Horgen, F.D.; Howard, L.; Lee, C.S. Effects of diets on the growth performance and shell pigmentation of Pacific abalone. Aquac. Res. 2016, 47, 4004–4014. [Google Scholar] [CrossRef]
- Tung, C.H.; Alfaro, A.C. Effect of dietary protein and temperature on the growth and health of juvenile New Zealand black-footed abalone (Haliotis iris). Aquac. Res. 2011, 42, 366–385. [Google Scholar] [CrossRef]
- Bilbao, A.; Uriarte, I.; del Pino Viera, M.; Sosa, B.; Fernández-Palacios, H.; Hernández-Cruz, C.M. Effect of macroalgae protein levels on some reproductive aspects and physiological parameters for the abalone, Haliotis tuberculata coccinea (Reeve 1846). J. World Aquac. Soc. 2012, 43, 764–777. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Baillie, H.J.; Barrett, S.M.; Volkman, J.K. Effect of diet on the lipid composition of wild and cultured abalone. Aquaculture 1996, 140, 115–127. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Qin, J.G.; Harris, J.O.; Howarth, G.S.; Stone, D.A. Nutritional requirements and use of macroalgae as ingredients in abalone feed. Rev. Aquac. 2014, 5, 121–135. [Google Scholar] [CrossRef]
- Nelson, M.M.; Leighton, D.L.; Phleger, C.F.; Nichols, P.D. Comparison of growth and lipid composition in the green abalone, Haliotis fulgens, provided specific macroalgal diets. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 695–712. [Google Scholar] [CrossRef]
- Mateos, H.T.; Lewandowski, P.; Su, X. Seasonal variations of total lipid and fatty acid contents in muscle, gonad and digestive glands of farmed Jade Tiger hybrid abalone in Australia. Food Chem. 2010, 123, 436–441. [Google Scholar] [CrossRef]
- Mau, A.; Jha, R. Aquaculture of two commercially important molluscs (abalone and limpet): Existing knowledge and future prospects. Rev. Aquac. 2018, 10, 611–625. [Google Scholar] [CrossRef]
- Venter, L.; Alfaro, A.C.; Van Nguyen, T.; Lindeque, J.Z. Metabolite profiling of abalone (Haliotis iris) energy metabolism: A Chatham Islands case study. Metabolomics 2022, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Boulais, M.; Demoy-Schneider, M.; Alavi, S.M.H.; Cosson, J. Spermatozoa motility in bivalves: Signaling, flagellar beating behavior, and energetics. Theriogenology 2019, 136, 15–27. [Google Scholar] [CrossRef]
- Najmudeen, T. Variation in biochemical composition during gonad maturation of the tropical abalone Haliotis varia Linnaeus 1758 (Vetigastropoda: Haliotidae). Mar. Biol. Res. 2007, 3, 454–461. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]

| Two-Away ANOVA | ||||
|---|---|---|---|---|
| Parameter | Tissue | Group | Sex | Group × Sex |
| Crude protein (%) | Adductor muscle | 0.002 | 0.748 | 0.061 |
| Foot muscle | 0.007 | 0.867 | 0.471 | |
| Gonad | 0.051 | 0.017 | 0.333 | |
| Fat (%) | Adductor muscle | 4.83 × 10−5 | 0.018 | 0.204 |
| Foot muscle | 0.616 | 1 | 0.153 | |
| Gonad | 0.056 | 0.016 | 0.141 | |
| Crude fibre (%) | Adductor muscle | 0.511 | 0.704 | 0.34 |
| Foot muscle | 0.098 | 0.059 | 0.813 | |
| Gonad | 0.225 | 0.954 | 0.725 | |
| Moisture (%) | Adductor muscle | 0.307 | 0.223 | 0.439 |
| Foot muscle | 0.391 | 0.137 | 0.303 | |
| Gonad | 0.192 | 0.947 | 0.766 | |
| Ash (%) | Adductor muscle | 0.522 | 0.550 | 0.462 |
| Foot muscle | 0.440 | 0.577 | 0.982 | |
| Gonad | 0.606 | 0.011 | 0.137 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, R.S.; Venter, L.; Azizan, A.; Guo, J.; Carter, J.; Bullon, N.; Chen, T.; Copedo, J.S.; Ragg, N.L.C.; Sabetian, A.; et al. Comparative Physiological Profiling of Abalone (Haliotis iris): Insights from Wild and Aquaculture Broodstock. Fishes 2025, 10, 566. https://doi.org/10.3390/fishes10110566
Sawant RS, Venter L, Azizan A, Guo J, Carter J, Bullon N, Chen T, Copedo JS, Ragg NLC, Sabetian A, et al. Comparative Physiological Profiling of Abalone (Haliotis iris): Insights from Wild and Aquaculture Broodstock. Fishes. 2025; 10(11):566. https://doi.org/10.3390/fishes10110566
Chicago/Turabian StyleSawant, Ruchira S., Leonie Venter, Awanis Azizan, Jinchen Guo, Jack Carter, Natalia Bullon, Tony Chen, Joanna S. Copedo, Norman L. C. Ragg, Armagan Sabetian, and et al. 2025. "Comparative Physiological Profiling of Abalone (Haliotis iris): Insights from Wild and Aquaculture Broodstock" Fishes 10, no. 11: 566. https://doi.org/10.3390/fishes10110566
APA StyleSawant, R. S., Venter, L., Azizan, A., Guo, J., Carter, J., Bullon, N., Chen, T., Copedo, J. S., Ragg, N. L. C., Sabetian, A., & Alfaro, A. C. (2025). Comparative Physiological Profiling of Abalone (Haliotis iris): Insights from Wild and Aquaculture Broodstock. Fishes, 10(11), 566. https://doi.org/10.3390/fishes10110566






