Air Nanobubbles Enhance Viable Bacteria Counts, Abundance of Nitrifying Bacteria, and Reduce Nitrite Levels in Marine Recirculation Aquaculture Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Trial and Animal Husbandry Routine
2.2. Flow Cytometry (FCM)
2.3. Total Bacteria Plate Counts
2.4. Collection of Water Samples for 16s rRNA Analysis
2.5. Library Preparation and Illumia Novaseq Sequencing
2.6. Sequence Analysis
2.7. Statistical Analysis
3. Results
3.1. Water Quality
3.2. Fish Growth Performance
3.3. Flow Cytometry and Total Bacterial Plate Counts
3.4. LNA and HNA Bacteria Abundance
3.5. Microbiome Profile of Water Samples from RAS with and Without Nanobubbles
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, F.; Bostock, J.; Fletcher, D. Review of Recirculation Aquaculture System Technologies and Their Commercial Application. 2014. Available online: http://www.hie.co.uk/common/handlers/download-document.ashx?id=236008c4-f52a-48d9-9084-54e89e965573 (accessed on 12 May 2025).
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A Review on the Research Status and Development Trend of Equipment in Water Treatment Processes of Recirculating Aquaculture Systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and Applications of Microbubble and Nanobubble Technology for Water Treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental Characteristics and Applications in Food Processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Lin, X.; Liu, R.; Bartlam, M.; Wang, Y. Characteristics, Biodiversity, and Cultivation Strategy of Low Nucleic Acid Content Bacteria. Front. Microbiol. 2022, 13, 900669. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Wu, S.; Mortimer, R.J.G.; Pan, G. Nanobubble Technology in Environmental Engineering: Revolutionization Potential and Challenges; ACS Publications: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Domingos, J.A.; Huang, Q.; Liu, H.; Dong, H.T.; Khongcharoen, N.; Van, P.T.; Nghia, N.H.; Giang, P.T.; The Viet, P.; St-Hilaire, S. Air-Nanobubbles Ineffective to Reduce Pathogenic Bacteria in Fresh and Brackish Waters. bioRxiv 2021. [Google Scholar] [CrossRef]
- Battino, R.; Clever, H.L. The Solubility of Gases in Liquids. Chem. Rev. 1966, 66, 395–463. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the Existence and the Stability of Nano-Bubbles in Water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Rahmawati, A.I.; Saputra, R.N.; Hidayatullah, A.; Dwiarto, A.; Junaedi, H.; Cahyadi, D.; Saputra, H.K.H.; Prabowo, W.T.; Kartamiharja, U.K.A.; Shafira, H. Enhancement of Penaeus vannamei Shrimp Growth Using Nanobubble in Indoor Raceway Pond. Aquac. Fish 2021, 6, 277–282. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Zhang, Y.; Zhang, R.; Inaba, K.; Osato, T.; Zhao, X.; Han, Y.; Ren, T. Oxygen Nanobubble-Induced Hyperoxia: Effects on Growth, Digestive Enzyme Activity, Intestinal Morphology, and Biochemical Parameters in Kuruma Prawn (Penaeus japonicus). Aquac. Rep. 2025, 43, 102882. [Google Scholar] [CrossRef]
- Heriyati, E.; Rustadi, R.; Isnansetyo, A.; Triyatmo, B.; Istiqomah, I.; Deendarlianto, D.; Budhijanto, W. Microbubble Aeration in A Recirculating Aquaculture System (RAS) Increased Dissolved Oxygen, Fish Culture Performance, and Stress Resistance of Red Tilapia (Oreochromis Sp.). Trends Sci. 2022, 19, 6251. [Google Scholar] [CrossRef]
- Linh, N.V.; Khongcharoen, N.; Nguyen, D.-H.; Dien, L.T.; Rungrueng, N.; Jhunkeaw, C.; Sangpo, P.; Senapin, S.; Uttarotai, T.; Panphut, W. Effects of Hyperoxia during Oxygen Nanobubble Treatment on Innate Immunity, Growth Performance, Gill Histology, and Gut Microbiome in Nile Tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2023, 143, 109191. [Google Scholar] [CrossRef]
- Cardona, E.; Gueguen, Y.; Magré, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial Community Characterization of Water and Intestine of the Shrimp Litopenaeus stylirostris in a Biofloc System. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Microorganisms in Biofloc Aquaculture System. Aquac. Rep. 2022, 26, 101300. [Google Scholar] [CrossRef]
- Del’Duca, A.; Cesar, D.E.; Freato, T.A.; Azevedo, R.d.S.; Rodrigues, E.M.; Abreu, P.C. Variability of the Nitrifying Bacteria in the Biofilm and Water Column of a Recirculating Aquaculture System for Tilapia (Oreochromis niloticus) Production. Aquac. Res. 2019, 50, 2537–2544. [Google Scholar] [CrossRef]
- Ward, B.B.; Arp, D.J.; Klotz, M.G. Nitrification; American Society for Microbiology Press: Washington, DC, USA, 2011. [Google Scholar]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in Recirculating Aquaculture Systems and Their Management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Chew, X.Z.; Gibson-Kueh, S.; Jerry, D.R.; Shen, X. Comparison of Intestinal Bacterial Communities in Asymptomatic and Diseased Asian Seabass (Lates calcarifer) with Chronic Enteritis and Mixed Bacterial Infections. Aquaculture 2023, 572, 739516. [Google Scholar] [CrossRef]
- Kelly, C.; Salinas, I. Under Pressure: Interactions between Commensal Microbiota and the Teleost Immune System. Front. Immunol. 2017, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xu, W.; Wei, J.; Sun, J. Impact of Environmental Bacterial Communities on Fish Health in Marine Recirculating Aquaculture Systems. Vet. Microbiol. 2017, 203, 34–39. [Google Scholar] [CrossRef] [PubMed]
- You, J.L.; Cao, L.X.; Liu, G.F.; Zhou, S.N.; Tan, H.M.; Lin, Y.C. Isolation and Characterization of Actinomycetes Antagonistic to Pathogenic Vibrio spp. from Nearshore Marine Sediments. World J. Microbiol. Biotechnol. 2005, 21, 679–682. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and Managing Microbes in Aquaculture—Towards a Sustainable Industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef]
- Ganesh, E.A.; Das, S.; Chandrasekar, K.; Arun, G.; Balamurugan, S. Monitoring of Total Heterotrophic Bacteria and Vibrio spp. in an Aquaculture Pond. Curr. Res. J. Biol. Sci. 2010, 2, 48–52. Available online: https://www.researchgate.net/publication/267606862 (accessed on 12 May 2025).
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Ahmad, W.; Zhu, X.-Y.; Chen, J.; Austin, B. Viable but Nonculturable Bacteria and Their Resuscitation: Implications for Cultivating Uncultured Marine Microorganisms. Mar. Life Sci. Technol. 2021, 3, 189–203. [Google Scholar] [CrossRef]
- Śliwa-Dominiak, J.; Czechowska, K.; Blanco, A.; Sielatycka, K.; Radaczyńska, M.; Skonieczna-Żydecka, K.; Marlicz, W.; Łoniewski, I. Flow Cytometry in Microbiology: A Review of the Current State in Microbiome Research, Probiotics, and Industrial Manufacturing. Cytom. Part A 2025, 107, 145–164. [Google Scholar] [CrossRef]
- Khan, M.M.; Pyle, B.H.; Camper, A.K. Specific and Rapid Enumeration of Viable but Nonculturable and Viable-Culturable Gram-Negative Bacteria by Using Flow Cytometry. Appl. Environ. Microbiol. 2010, 76, 5088–5096. [Google Scholar] [CrossRef]
- Lebaron, P.; Servais, P.; Agogué, H.; Courties, C.; Joux, F. Does the High Nucleic Acid Content of Individual Bacterial Cells Allow Us To Discriminate between Active Cells and Inactive Cells in Aquatic Systems? Appl. Environ. Microbiol. 2001, 67, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, P.; Servais, P.; Baudoux, A.-C.; Bourrain, M.; Courties, C.; Parthuisot, N. Variations of Bacterial-Specific Activity with Cell Size and Nucleic Acid Content Assessed by Flow Cytometry. Aquatic. Microbial. Ecol. 2002, 28, 131–140. [Google Scholar] [CrossRef]
- Marwiyah, U.C.; Mahasri, G.; Ratnasari, R.E.; Wiradana, P.A. Total Plate Count and Identification of Vibrio in Pacific White Shrimp (Litophenaeus vannamei) from Ponds and in Those Exposed to Immunogenic Protein Membrane Zoothamnium penaei. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 236, p. 012087. [Google Scholar] [CrossRef]
- Majaneva, M.; Diserud, O.H.; Eagle, S.H.C.; Boström, E.; Hajibabaei, M.; Ekrem, T. Environmental DNA Filtration Techniques Affect Recovered Biodiversity. Sci. Rep. 2018, 8, 4682. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Qu, X.; Peng, W.; Yu, Y.; Zhang, M. Nutrients Drive the Structures of Bacterial Communities in Sediments and Surface Waters in the River-Lake System of Poyang Lake. Water 2019, 11, 930. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.M.; Ape, F.; Manini, E.; Mirto, S.; Luna, G.M. Temporal Changes in Microbial Communities Beneath Fish Farm Sediments Are Related to Organic Enrichment and Fish Biomass Over a Production Cycle. Front. Mar. Sci. 2020, 7, 524. [Google Scholar] [CrossRef]
- Fossmark, R.O.; Vadstein, O.; Rosten, T.W.; Bakke, I.; Košeto, D.; Bugten, A.V.; Helberg, G.A.; Nesje, J.; Jørgensen, N.O.G.; Raspati, G.; et al. Effects of Reduced Organic Matter Loading through Membrane Filtration on the Microbial Community Dynamics in Recirculating Aquaculture Systems (RAS) with Atlantic Salmon Parr (Salmo salar). Aquaculture 2020, 524, 735268. [Google Scholar] [CrossRef]
- Bugten, A.V.; Attramadal, K.J.K.; Fossmark, R.O.; Rosten, T.W.; Vadstein, O.; Bakke, I. Changes in Rearing Water Microbiomes in RAS Induced by Membrane Filtration Alters the Hindgut Microbiomes of Atlantic Salmon (Salmo salar) Parr. Aquaculture 2022, 548, 737661. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLoS One 2013, 8, e65339. [Google Scholar] [CrossRef]
- Van Beijnen, J.; Yan, G. A Breath of Fresh Air: How Nanobubbles Can Make Aquaculture More Sustainable. 2021. Available online: https://thefishsite.com/articles/a-breath-of-fresh-air-how-nanobubbles-can-make-aquaculture-more-sustainable-dissolved-oxygen (accessed on 12 May 2025).
- Yao, G.-J.; Ren, J.-Q.; Zhou, F.; Liu, Y.-D.; Li, W. Micro-Nano Aeration Is a Promising Alternative for Achieving High-Rate Partial Nitrification. Sci. Total Environ. 2021, 795, 148899. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kurata, K. Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth. Horttechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Qu, J.; Yang, H.; Liu, Y.; Qi, H.; Wang, Y.; Zhang, Q. The Study of Natural Biofilm Formation and Microbial Community Structure for Recirculating Aquaculture System. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 742, p. 012018. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis Infection in Marine Fish Caused by Tenacibaculum maritimum: A Review. Dis. Aquat. Organ. 2006, 71, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendaño-Herrera, R.; Rodkhum, C. Tenacibaculosis Caused by Tenacibaculum maritimum: Updated Knowledge of This Marine Bacterial Fish Pathogen. Front. Cell. Infect. Microbiol. 2023, 12, 1068000. [Google Scholar] [CrossRef]
- Jefferson, K.K. What Drives Bacteria to Produce a Biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Widigdo, B.; Yuhana, M.; Iswantari, A.; Madonsa, C.; Sapitri, I.D.; Wardiatno, Y.; Hakim, A.A.; Nazar, F. The Impact of Nitrifying Probiotic to Population Growth of Pathogenic Bacteria, Vibrio sp., and Toxic Nitrogen Gasses in Marine Shrimp Culture Media under Laboratory Condition. J. Pengelolaan Sumberd. Alam Dan Lingkung. (J. Nat. Resour. Environ. Manag.) 2021, 11, 130–140. [Google Scholar] [CrossRef]
- Hassenrück, C.; Reinwald, H.; Kunzmann, A.; Tiedemann, I.; Gärdes, A. Effects of Thermal Stress on the Gut Microbiome of Juvenile Milkfish (Chanos chanos). Microorganisms 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, L.; Xia, Z. Impact of Groundwater Salinity on Bioremediation Enhanced by Micro-Nano Bubbles. Materials 2013, 6, 3676–3687. [Google Scholar] [CrossRef]
- Davey, H.; Guyot, S. Estimation of Microbial Viability Using Flow Cytometry. Curr. Protoc. Cytom. 2020, 93, e72. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L. Growth Measurement. In Methods for General and Molecular Microbiology; Wiley Online Library: New York, NY, USA, 2007; pp. 172–199. [Google Scholar] [CrossRef]
- Malmberg, C. Evaluation of Flow Cytometry as Replacement for Plating in In Vitro Measurements of Competitive Growth under Antibiotic Stress. 2013, p. 13. Available online: https://uu.diva-portal.org/smash/record.jsf?pid=diva2%3A639728&dswid=4462 (accessed on 12 May 2025).
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6. [Google Scholar]
- Endo, H.; Nakayama, J.; Hayashi, T. Application of Flow Cytometry to Environmental Control in Marine Aquaculture. Mater. Sci. Eng. C 2000, 12, 83–88. [Google Scholar] [CrossRef]
- Bernard, L.; Courties, C.; Servais, P.; Troussellier, M.; Petit, M.; Lebaron, P. Relationships among Bacterial Cell Size, Productivity, and Genetic Diversity in Aquatic Environments Using Cell Sorting and Flow Cytometry. Microb. Ecol. 2000, 40, 148–158. [Google Scholar] [CrossRef]
- Fiedler, C.J.; Schönher, C.; Proksch, P.; Kerschbaumer, D.J.; Mayr, E.; Zunabovic-Pichler, M.; Domig, K.J.; Perfler, R. Assessment of Microbial Community Dynamics in River Bank Filtrate Using High-Throughput Sequencing and Flow Cytometry. Front. Microbiol. 2018, 9, 2887. [Google Scholar] [CrossRef]
- Props, R.; Monsieurs, P.; Mysara, M.; Clement, L.; Boon, N. Measuring the Biodiversity of Microbial Communities by Flow Cytometry. Methods Ecol. Evol. 2016, 7, 1376–1385. [Google Scholar] [CrossRef]
- Foladori, P.; Bruni, L.; Tamburini, S.; Ziglio, G. Direct Quantification of Bacterial Biomass in Influent, Effluent and Activated Sludge of Wastewater Treatment Plants by Using Flow Cytometry. Water Res. 2010, 44, 3807–3818. [Google Scholar] [CrossRef]
- Ma, L.; Mao, G.; Liu, J.; Yu, H.; Gao, G.; Wang, Y. Rapid Quantification of Bacteria and Viruses in Influent, Settled Water, Activated Sludge and Effluent from a Wastewater Treatment Plant Using Flow Cytometry. Water Sci. Technol. 2013, 68, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Manti, A.; Boi, P.; Falcioni, T.; Canonico, B.; Ventura, A.; Sisti, D.; Pianetti, A.; Balsamo, M.; Papa, S. Bacterial Cell Monitoring in Wastewater Treatment Plants by Flow Cytometry. Water Environ. Res. 2008, 80, 346–354. [Google Scholar] [CrossRef] [PubMed]
- De Roy, K.; Clement, L.; Thas, O.; Wang, Y.; Boon, N. Flow Cytometry for Fast Microbial Community Fingerprinting. Water Res. 2012, 46, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Van Nevel, S.; Koetzsch, S.; Proctor, C.R.; Besmer, M.D.; Prest, E.I.; Vrouwenvelder, J.S.; Knezev, A.; Boon, N.; Hammes, F. Flow Cytometric Bacterial Cell Counts Challenge Conventional Heterotrophic Plate Counts for Routine Microbiological Drinking Water Monitoring. Water Res. 2017, 113, 191–206. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Mize, S.V.; Johnson, D.; Brown, B.L. Flow Cytometric Detection of Waterborne Bacteria Metabolic Response to Anthropogenic Chemical Inputs to Aquatic Ecosystems. Cells 2025, 14, 352. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Z.; Ma, L.; Ji, Y.; Bartlam, M.; Wang, Y. Spatio-Temporal Variations of High and Low Nucleic Acid Content Bacteria in an Exorheic River. PLoS One 2016, 11, e0153678. [Google Scholar] [CrossRef]
- Santos, M.; Oliveira, H.; Pereira, J.L.; Pereira, M.J.; Gonçalves, F.J.M.; Vidal, T. Flow Cytometry Analysis of Low/High DNA Content (LNA/HNA) Bacteria as Bioindicator of Water Quality Evaluation. Ecol. Indic. 2019, 103, 774–781. [Google Scholar] [CrossRef]
- Wang, Y.; Hammes, F.; Boon, N.; Chami, M.; Egli, T. Isolation and Characterization of Low Nucleic Acid (LNA)-Content Bacteria. ISME J. 2009, 3, 889–902. [Google Scholar] [CrossRef]
- Le Boucher, R.; Chung, W.; Ng, J.K.L.; Tan, L.S.E.; Lee, C.S. Balancing Stocking Density and Feed Intake for Barramundi (Lates calcarifer) Raised in Recirculating Aquaculture Systems. Aquac. Res. 2024, 2024, 2264274. [Google Scholar] [CrossRef]
- Galang, D.P.; Ashari, A.K.; Sulmatiwi, L.; Mahasri, G.; Prayogo; Sari, L.A. The Oxygen Content and Dissolved Oxygen Consumption Level of White Shrimp Litopenaeus vannamei in the Nanobubble Cultivation System. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012014. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of Dissolved Oxygen and Fish Size on Nile Tilapia, Oreochromis niloticus (L.): Growth Performance, Whole-Body Composition, and Innate Immunity. Aquac. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Gao, Y.; Huang, B. Hypoxia Tolerance, Hematological, and Biochemical Response in Juvenile Turbot (Scophthalmus maximus. L). Aquaculture 2021, 535, 736380. [Google Scholar] [CrossRef]
- Mallya, Y.J. The Effects of Dissolved Oxygen on Fish Growth in Aquaculture. The United Nations University Fisheries Training Programme, Final Project 2007. Available online: https://www.grocentre.is/static/gro/publication/58/document/yovita07prf.pdf (accessed on 12 May 2025).
- Bardon-Albaret, A.; Saillant, E.A. Effects of Hypoxia and Elevated Ammonia Concentration on the Viability of Red Snapper Embryos and Early Larvae. Aquaculture 2016, 459, 148–155. [Google Scholar] [CrossRef]
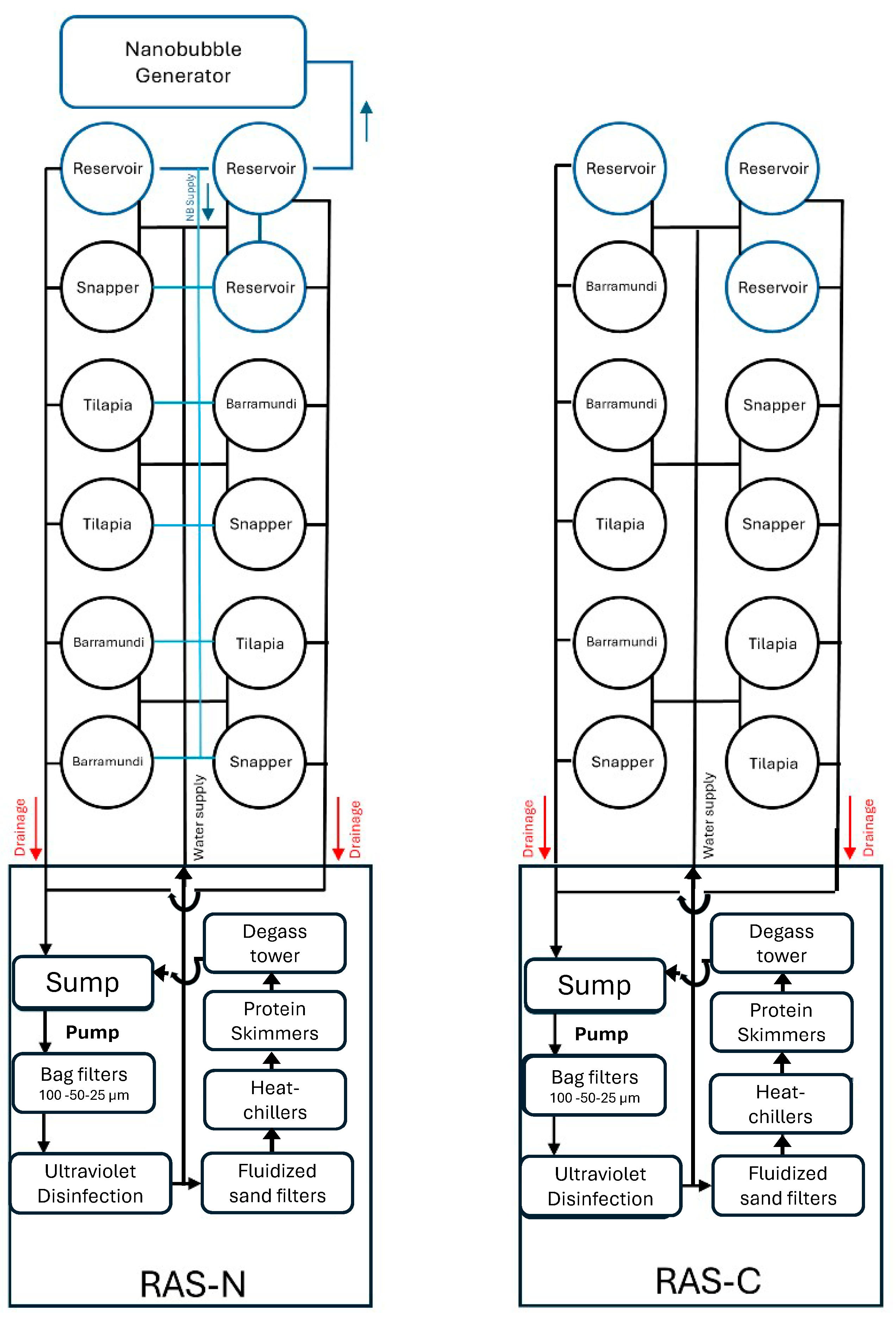
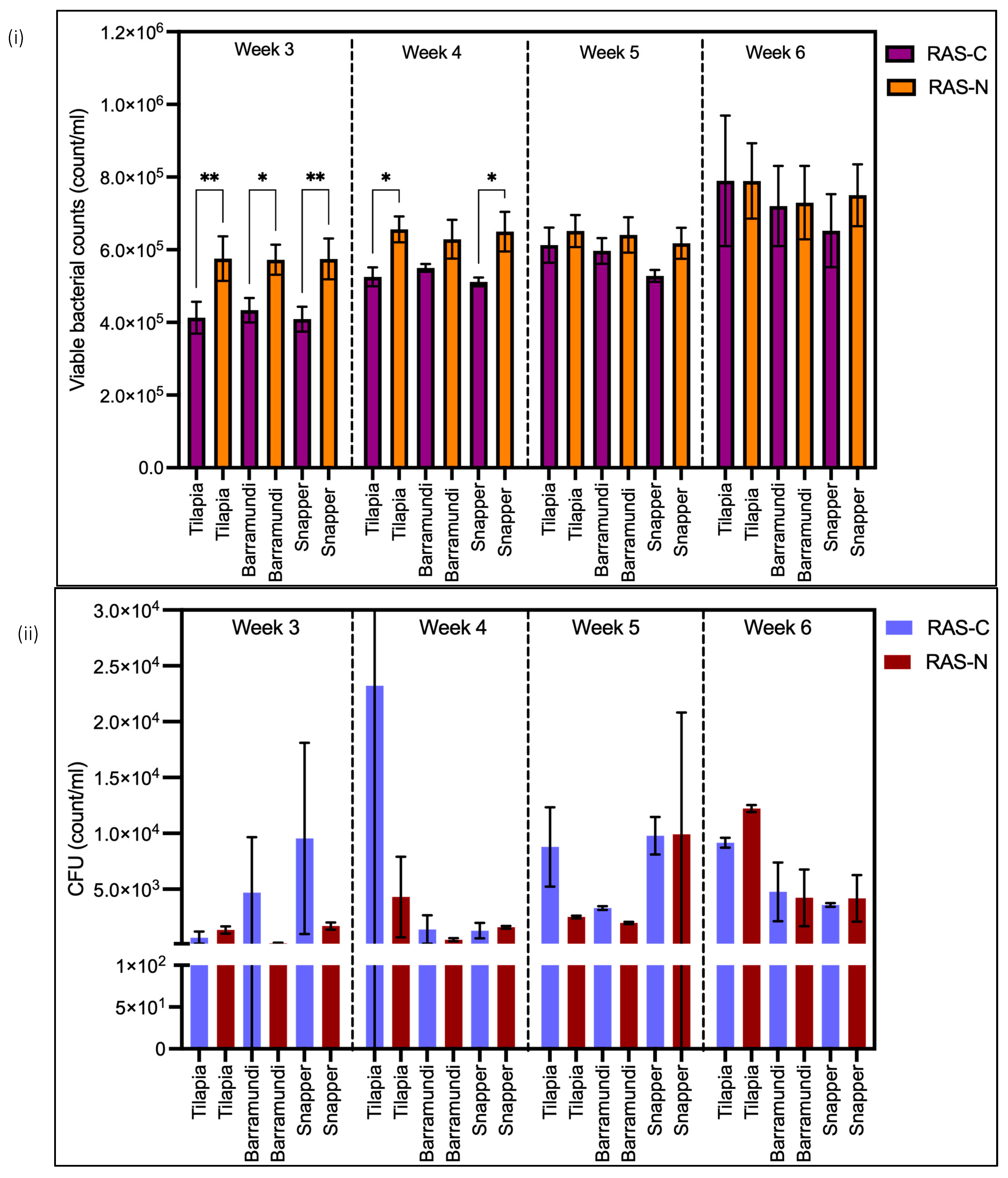


| Water Parameter | RAS-C | RAS-N |
|---|---|---|
| pH | 8.00 ± 0.09 | 8.05 ± 0.07 |
| TAN (mg/L) | 0.12 ± 0.06 | 0.12 ± 0.06 |
| Nitrite (mg/L) | 1.40 ± 0.25 a | 0.99 ± 0.16 b |
| Nitrate (mg/L) | 165.6 ± 27.7 | 182.2 ± 29.8 |
| Temperature (°C) | 28.6 ± 0.5 | 28.6 ± 0.4 |
| Salinity (ppt) | 24.8 ± 0.6 | 25.2 ± 0.5 |
| DO (mg/L) | 6.61 ± 0.34 | 6.44 ± 0.24 |
| DO (saturation, %) | 89.4 ± 5.7 | 87.7 ± 5.9 |
| Treatment | Species | Initial Body Weight (g) | Final Body Weight (g) | Total Feed Intake (g) | SGR | FCR | Survival (%) | Final Total Weight (g) | Final Condition Factor |
|---|---|---|---|---|---|---|---|---|---|
| RAS-C | Malabar snapper | 178.8 ± 25.6 | 308.2 ± 45.2 | 2250 ± 137 | 1.72 ± 0.12 | 0.93 ± 0.02 | 95.6 ± 0.0 | 4417 ± 178 | 3.13 ± 0.67 |
| RAS-N | 177.0 ± 23.6 | 300.8 ± 46.2 | 2234 ± 91 | 1.74 ± 0.10 | 0.95 ± 0.05 | 97.8 ± 0.0 | 4411 ± 168 | 3.22 ± 0.28 | |
| RAS-C | Barramundi | 132.9 ± 26.9 | 303.8 ± 66.6 | 3390 ± 74 | 3.19 ± 0.03 | 0.76 ± 0.02 | 100.0 | 6076 ± 102 | 2.02 ± 0.14 |
| RAS-N | 132.1 ± 29.9 | 296.8 ± 76.6 | 3332 ± 399 | 3.14 ± 0.19 | 0.77 ± 0.02 | 100.0 | 5937 ± 552 | 2.26 ± 0.28 | |
| RAS-C | Hybrid tilapia | 9.5 ± 3.0 | 85.4 ± 29.7 | 2529 ± 153 | 7.98 ± 0.31 | 0.80 ± 0.03 | 95.8 ± 0.0 | 3272 ± 300 | 3.83 ± 0.54 |
| RAS-N | 9.2 ± 3.0 | 80.7 ± 29.4 | 2405 ± 68 | 7.92 ± 0.10 | 0.81 ± 0.02 | 95.0 ± 0.0 | 3968 ± 160 | 3.51 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sean, A.; Lim, T.S.; Domingos, J.A.; Uichanco, J.A.; Shen, X.; Gibson-Kueh, S. Air Nanobubbles Enhance Viable Bacteria Counts, Abundance of Nitrifying Bacteria, and Reduce Nitrite Levels in Marine Recirculation Aquaculture Systems. Fishes 2025, 10, 550. https://doi.org/10.3390/fishes10110550
Sean A, Lim TS, Domingos JA, Uichanco JA, Shen X, Gibson-Kueh S. Air Nanobubbles Enhance Viable Bacteria Counts, Abundance of Nitrifying Bacteria, and Reduce Nitrite Levels in Marine Recirculation Aquaculture Systems. Fishes. 2025; 10(11):550. https://doi.org/10.3390/fishes10110550
Chicago/Turabian StyleSean, Afifah, Tzer Shyun Lim, Jose A. Domingos, Joseph A. Uichanco, Xueyan Shen, and Susan Gibson-Kueh. 2025. "Air Nanobubbles Enhance Viable Bacteria Counts, Abundance of Nitrifying Bacteria, and Reduce Nitrite Levels in Marine Recirculation Aquaculture Systems" Fishes 10, no. 11: 550. https://doi.org/10.3390/fishes10110550
APA StyleSean, A., Lim, T. S., Domingos, J. A., Uichanco, J. A., Shen, X., & Gibson-Kueh, S. (2025). Air Nanobubbles Enhance Viable Bacteria Counts, Abundance of Nitrifying Bacteria, and Reduce Nitrite Levels in Marine Recirculation Aquaculture Systems. Fishes, 10(11), 550. https://doi.org/10.3390/fishes10110550






