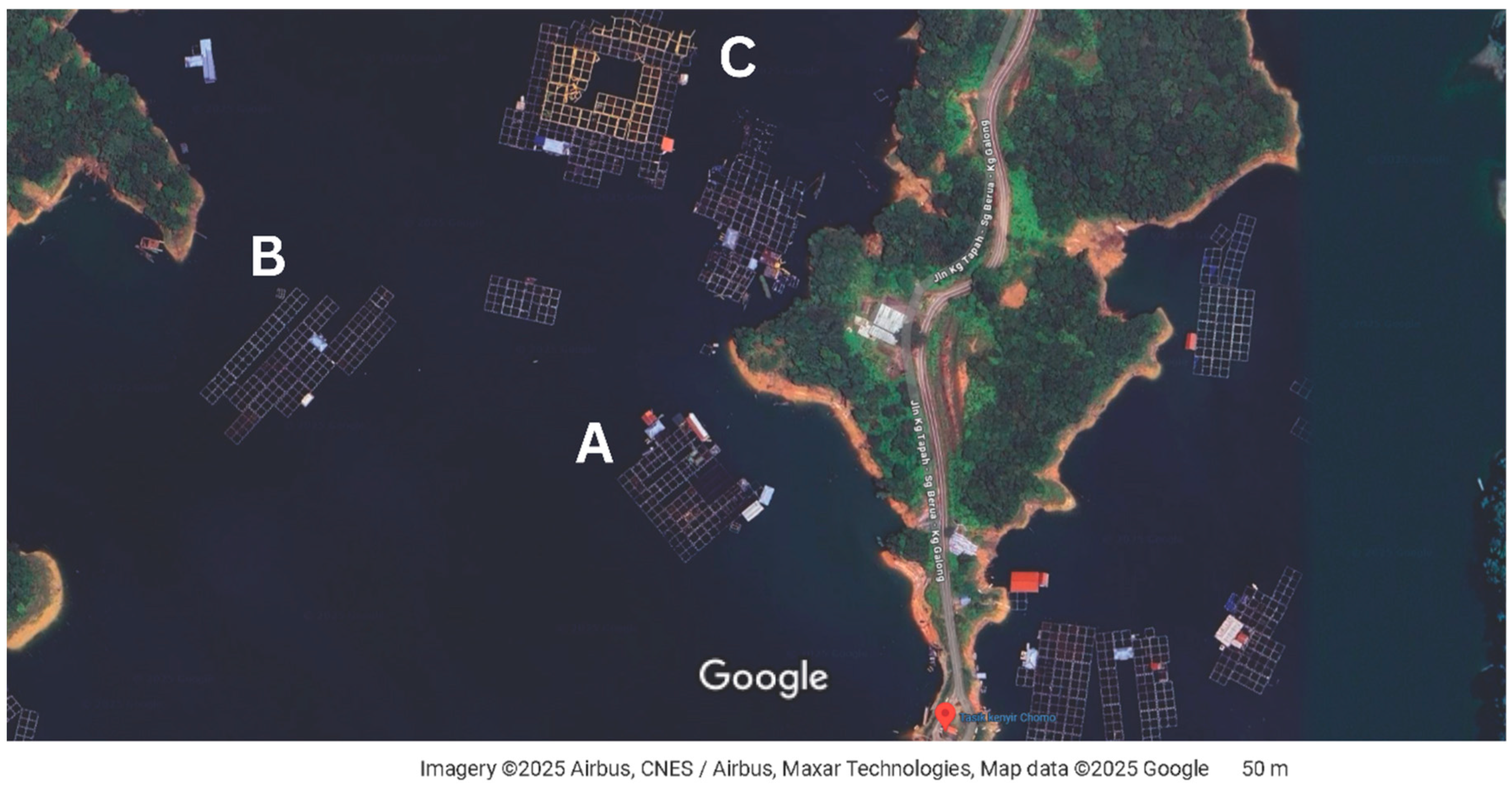
3.1. Nutrient Analysis of Red Kenyir™ and Wild Tilapia
Wild tilapia showed significantly higher caloric value (132.79 ± 4.69 kcal/100 g) than Red Kenyir™ groups (77.38–113.46 kcal/100 g), driven mainly by its higher total fat value. In terms of intra-group variability, caloric values were also higher in Red Kenyir™ sample C (113.46 ± 3.76 kcal/100 g), driven mainly by its higher protein fraction as compared to Red Kenyir™ samples A and B (77.38–85.46 kcal/100 g). Abd-Allah and colleagues reported apparent differences between wild and cultured Nile tilapia in Egypt (Assiut), in energy values, where they found cultured tilapia to contain significantly more calories (100.73 ± 1.44 kcal/100 g) than wild fish (83.29 ± 0.06 kcal/100 g). Notably, the energy range observed in our study (77.38–132.79 kcal/100 g) was broader than that reported by them (73.75–124.77 kcal/100 g), indicating greater variability across strains and culture treatments [
15]. In Bangladesh, Islam and his colleagues reported that cage-cultured tilapia exhibited higher caloric values than wild fish, with elevated energy (126.73 vs. 97.62 kcal/100 g). This opposite pattern emerged in our study, where wild red tilapia recorded substantially higher caloric value than cultured red tilapia [
16]. Similarly, Francis et al. (2024) found that farmed tilapia in Sabah exhibited higher energy (94.50 kcal/100 g) compared with their wild counterparts (82.50 kcal/100 g) [
7]. In contrast, our findings on Red Tilapia showed the opposite trend, where wild fish retained the highest energy density (132.79 kcal/100 g). Nevertheless, Al-Taee et al. (2022) reported that wild Nile tilapia contained a higher energy value (167.82 kcal/100 g) compared to cultured fish (152.13 kcal/100 g) [
17]. This is consistent with the trend recorded in the present study, which found caloric values in our samples. These divergent patterns highlight how species strain culture environment, and feeding regimes may differentially influence nutrient partitioning, with aquaculture promoting lipid accumulation in some tilapia populations but enhancing protein deposition in others.
Wild tilapia showed significantly higher fat content (4.30 ± 0.36 g/100 g) than Red Kenyir™ groups (0.25–1.37 g/100 g). No significant differences in fat content were observed within the Red Kenyir™ groups. The proximate composition of Stirling Nile tilapia and red hybrid tilapia showed crude fat of 2.07% and 0.33%, respectively [
1]. Al-Taee et al. (2022) reported that wild Nile tilapia contained fat (7.67%) compared to cultured fish (6.97% fat) [
17]. Job et al. (2015) reported that wild Nile tilapia contained 0.57% crude fat while cultured fish contained 0.30% [
18]. These findings align with current findings as wild tilapia recorded higher fat content. However, two studies have also shown opposite trends compared to the current findings. Abd-Allah et al. (2016) reported cultured fish contained higher fat (2.82%) levels compared to wild tilapia, which recorded 1.20% [
15]. Francis et al. (2024) found that farmed tilapia in Sabah exhibited higher fat (3.55%) compared with their wild counterparts (1.35% fat) [
7]. The higher fat content in wild tilapia may be attributed to differences in diet, habitat, and activity levels, where natural feeding patterns and diverse food sources can promote greater lipid accumulation. Meanwhile, aquaculture fishes such as Red Kenyir™, often raised on formulated feeds and under controlled conditions, tend to develop leaner flesh due to standardized diets and possibly higher stocking densities, which influence their proximate composition and caloric profile. However, variations compared to previous findings also elucidated the roles of other environmental factors, such as feed type and time, that could affect fat content.
Protein was also relatively higher in wild tilapia (22.95 ± 1.17 g/100 g) than Red Kenyir™ samples A and B, although protein content for sample C (26.54 ± 0.20 g/100 g) is significantly higher than both wild and other aquaculture groups. Despite receiving the highest-quality feed (Fish Meal 1; 28% crude protein) (as detailed in
Table 1), Red Kenyir™ sample A exhibited the lowest protein content. This indicates that protein levels may depend on fish meal quality and factors like feeding frequency, feed storage, and fish metabolism. The protein values observed in the present study (22.95–26.54 g/100 g) were higher than those reported in many earlier studies, though the relative trends between wild and cultured tilapia showed both consistencies and contradictions. Garduño-Lugo et al. (2007) recorded crude protein levels of 17.0% in Stirling Nile tilapia and 17.8% in red hybrid tilapia [
1], while Al-Taee et al. (2022) similarly found higher protein content in wild Nile tilapia (16.88%) compared with cultured fish (15.26%) [
17]. Job et al. (2015) reported minimal differences between wild (17.40%) and cultured (17.10%) Nile tilapia [
18], whereas Abd-Allah et al. (2016) noted the reverse pattern, with cultured fish containing more protein (17.91%) than wild fish (17.50%) [
15]. In Bangladesh, Islam et al. (2021) also reported slightly elevated protein levels in cage-cultured tilapia (16.03%) compared with wild fish (15.87%) [
16]. Conversely, Francis et al. (2024) found higher protein in wild tilapia (16.90%) than in farmed counterparts (15.65%) [
7], which aligns with the findings of Raymond et al. (2020), who observed protein levels of 20.2–21.9% in wild fish compared to 16.6–18.9% in farmed fish [
19]. Collectively, these studies highlight that protein content in tilapia is highly variable across strains, culture systems, and environments, and the elevated values found in the Red Kenyir™ sample C suggest that aquaculture practices can, under certain conditions, yield protein levels even higher than those typically reported for wild populations.
In terms of moisture, wild tilapia (70.96 ± 0.76 g/100 g) had lower values than Red Kenyir™ sample A and sample B (>77 g/100 g), consistent with its higher protein and fat. Red Kenyir™ Sample C recorded reduced moisture (70.27 ± 0.76 g/100 g), consistent with an inverse relationship between protein and water retention in fish muscle. Our findings are consistent with several previous reports. The proximate composition of Stirling Nile tilapia and red hybrid tilapia showed moisture levels of 79.1% and 80.0% [
1]. Al-Taee et al. (2022) reported that cultured tilapia exhibited slightly higher moisture (75.22%) compared to wild Nile tilapia [
17]. According to Raymond et al. (2020), farmed tilapia exhibited higher moisture content (75.2–78.1%) than wild tilapia (71.2–73.1%) [
19]. These reports align with higher moisture content in cultured tilapia than in wild tilapia. However, several reports show the opposite trend. Abd-Allah et al. (2016) reported wild fish retained more moisture compared to cultured Nile tilapia (79.63% vs. 77.17%) [
15]. In Bangladesh, Islam et al. (2021) reported wild tilapia retained more moisture (80.97% vs. 79.12%) [
16]. Two reports noted minor differences between farmed and wild tilapia regarding moisture content. Francis et al. (2024) found that farmed tilapia in Sabah exhibited only minor differences between farmed and wild tilapia (80.25% vs. 78.13% moisture) [
7]. Job et al. (2015) reported that wild Nile tilapia contained 80.90% moisture while cultured fish contained 80.80% [
18]. The moisture-protein-fat relationship can explain these variations in moisture reports. The moisture–protein trend aligns with previous findings that reported higher protein levels generally coincide with lower moisture in fish, including tilapia, as protein matrices have a limited water-binding capacity [
20]. Here, Red Kenyir
™ sample C had the highest protein and the lowest moisture, demonstrating this pattern. Similarly, the moisture–fat inverse relationships were evident in W, which had the highest fat content but reduced moisture (70.96 ± 0.76 g/100 g). This aligns with previous findings that reported fat deposition displaces water in muscle tissue, lowering water-holding capacity [
21,
22]. Overall, the moisture content of tilapia muscle is closely linked to its protein and fat composition, and may reflect physiological and ecological influences on flesh quality.
No significant differences were observed in ash (1.31–1.55 g/100 g) and total carbohydrate content (0.15–1.50 g/100 g) between wild and Red Kenyir™ tilapia, nor among the Red Kenyir™ groups (sample A–C). Dietary fiber was not detected in either aquaculture or wild samples. Garduño-Lugo et al. (2007) observed ash levels of 0.65% in Stirling Nile tilapia and 0.12% in red hybrid tilapia, lower than the current study’s values [
1]. Similarly, Olopade et al. (2016) reported ash of 1.36% in wild tilapia, while Al-Taee et al. (2022) found slightly higher values in cultured fish (1.86%) [
17,
23]. Job et al. (2015) noted comparable ash contents between wild (1.20%) and cultured Nile tilapia (1.31%), along with carbohydrate levels of 0.22% and 0.20%, respectively, consistent with the minimal differences observed here [
18]. Abd-Allah et al. (2016) also demonstrated only modest variation between cultured (1.17%) and wild (1.04%) Nile tilapia in Egypt [
15]. In Bangladesh, Islam et al. (2021) reported lower ash contents in both cage-cultured (0.53%) and wild (0.34%) tilapia compared with the present values [
16]. A similar trend was reported by Francis et al. (2024), who observed comparable ash levels between cultured and wild groups (9.22% vs. 8.51%), and by Raymond et al. (2020), who recorded higher ash in wild (1.8–1.9%) than farmed tilapia (1.2–1.4%) [
7,
19]. Collectively, these findings indicate that while ash and carbohydrate contents vary across species, culture systems, and geographical regions, differences between wild and farmed tilapia are generally small and often not statistically significant, supporting the patterns observed in the current study.
Overall, the proximate analysis revealed distinct nutritional differences between Red Kenyir™ and wild tilapia. Wild tilapia exhibited significantly higher caloric value and fat content, while protein was highest in Red Kenyir™ sample C. Moisture content was generally greater in aquaculture groups A and B, but lower in wild fish and Red Kenyir™ sample C, consistent with their higher protein and fat fractions. In contrast, ash and carbohydrate contents showed no significant variation across groups, and dietary fiber was not detected. These findings highlight that while wild tilapia retained greater energy density through fat deposition, Red Kenyir™ tilapia demonstrated comparable nutritional quality, with certain groups (sample C) achieving superior protein levels under controlled aquaculture conditions, such as perhaps better feed timing, quality and aquaculture practice.
Table 2 summarizes the proximate and nutrient analysis of aquaculture Red Kenyir™ (A, B and C) and wild (W) red tilapia (
Oreochromis sp.).
For mineral content, no significant differences (
p > 0.05) were observed between wild and Red Kenyir™ tilapia nor among the Red Kenyir™ groups (sample A–C) across all measured minerals. However, numerically, wild tilapia showed slightly higher calcium (79.43 ± 0.82 mg/100 g vs. 27.26–57.86 mg/100 g in Red Kenyir groups) and zinc (0.43 ± 0.01 mg/100 g vs. 0.28–0.38 mg/100 g), while Red Kenyir groups generally had higher magnesium and phosphorus. Numerical variation was also observed among the Red Kenyir groups; sample C showed the highest phosphorus (229.99 ± 6.94 mg/100 g) and potassium (473.57 ± 9.64 mg/100 g). Sample A had slightly higher sodium (127.05 ± 5.50 mg/100 g) than samples B and C. Previous studies have reported similar variability in tilapia mineral profiles across regions and production systems. Garduño-Lugo et al. (2007) found lower calcium in Stirling Nile and red hybrid tilapia (13.6–14.2 mg/100 g) [
1], while Olopade et al. (2016) reported calcium levels of 32.4 mg/100 g in wild tilapia [
23]. Al-Taee et al. (2022) observed higher calcium in cultured Nile tilapia (86.2 mg/100 g) compared to wild fish (71.5 mg/100 g) [
17], and Job et al. (2015) also noted minor differences between wild and cultured tilapia for phosphorus and sodium [
18]. Similarly, Abd-Allah et al. (2016) in Egypt and Islam et al. (2021) in Bangladesh reported minor but inconsistent variations in minerals between farmed and wild fish [
15,
16], while Francis et al. (2024) and Raymond et al. (2020) highlighted that differences in mineral content are often modest and influenced by environmental and dietary factors [
7,
19]. This observation aligns with the previous findings that noted the diet and environment’s significant impact on tilapia’s mineral levels [
10]. In short, these findings are consistent with the present results, suggesting that mineral composition in tilapia is relatively stable, with only minor variations attributable to diet, water quality, and culture conditions.
Table 3 summarizes the minerals analysis of aquaculture Red Kenyir™ (A, B and C) and wild (W) red tilapia (
Oreochromis sp.).
Tryptophan level was also relatively higher in wild tilapia (0.19 ± 0.00 g/100 g) than Red Kenyir™ sample A and B (0.13 ± 0.00 and 0.16 ± 0.03 g/100 g, respectively), although tryptophan content for sample (0.23 ± 0.03 g/100 g) is significantly higher than both wild and other aquaculture groups. This trend is consistent with the findings of total protein contents in the proximate analysis. Tryptophan is an essential amino acid that plays important roles in fish protein synthesis and metabolic processes [
24]. A balanced level of tryptophan promotes healthy fish growth and reduces the risk of damage by oxidative stress and diseases [
25]. Higher tryptophan content correlates with improved total protein levels, reflecting better nutritional quality in wild tilapia [
26]. Studies also show that dietary tryptophan supplementation in aquaculture can enhance growth performance and muscle protein deposition, which may explain the significantly higher level observed in sample C, potentially due to better feed formulations [
27]. Thus, improved tryptophan results support its essential role in protein synthesis and relevance to fish growth and human nutrition. This finding also suggests that cultured tilapia, particularly Red Kenyir
™ sample C, may offer better amino acid availability, which could influence feed formulations or growth conditions [
27]. In conclusion, the significantly higher tryptophan content observed, particularly in Red Kenyir™ sample C, highlights its potential nutritional advantage that possibly comes from better feed formulation, aquaculture practice and other environmental factors. However, a more detailed feed composition and amino acids analysis are warranted for more cautious interpretation and further validation.
Table 4 summarizes the tryptophan analysis of aquaculture Red Kenyir™ (A, B and C) and wild (W) red tilapia (
Oreochromis sp.).
For fatty acid composition, no significant differences (
p > 0.05) were observed between wild and Red Kenyir™ tilapia nor among the Red Kenyir™ groups (sample A–C) across all measured minerals. However, numerically, the fatty acid profile revealed markedly higher levels of saturated (1.687 g/100 g), monounsaturated (1.765 g/100 g), and polyunsaturated fatty acids (0.921 g/100 g) in wild tilapia compared with Red Kenyir groups. Among the Red Kenyir groups (samples A, B and C), fatty acid concentrations were generally low, with no consistent differences. Trace amounts of palmitic (C16:0), oleic (C18:1), and lauric acids (C12:0) were detected, but overall, Red Kenyir fish showed a narrower fatty acid profile compared to wild. On the other hand, wild tilapia accumulated substantially more diverse fatty acids in their filets. Farmed tilapia displayed notably lower levels of key SFAs, such as palmitic acid (C16:0; 0.045–0.046 g/100 g) and stearic acid (C18:0; 0.016–0.023 g/100 g), compared to wild tilapia (1.141 g/100 g and 0.266 g/100 g, respectively). Similarly, oleic acid (C18:1n9c), the principal MUFA, was lower in farmed samples (0.026–0.050 g/100 g) than in wild fish (1.403 g/100 g). PUFAs, including linoleic acid (C18:2n6c), linolenic acid (C18:3n3), and docosahexaenoic acid (DHA, C22:6n3), were present only in trace amounts or undetectable in farmed tilapia, whereas wild tilapia contained substantially higher concentrations (0.627, 0.091, and 0.096 g/100 g, respectively). These findings align with previous studies, such as Karapanagiotidis et al. (2017), who reported higher n-3 PUFA levels and favorable n-3/n-6 ratios in wild compared to farmed tilapia, and Suloma et al. (2008), who emphasized that Nile tilapia reared under intensive conditions often exhibit intermediate nutritional quality and require dietary adjustments to enhance n-3 highly unsaturated fatty acids [
22,
28]. These data confirm that farmed tilapia generally contain lower levels of essential fatty acids than their wild counterparts. The observed differences in fatty acid profiles can be attributed to several factors. Dietary composition plays a significant role, as formulated feeds for farmed tilapia often lack sufficient n-3 PUFAs, whereas wild tilapia consumes a more diverse natural diet, which provides essential fatty acids [
29]. Environmental conditions, including water quality, temperature, and genetic strain, can further modulate lipid metabolism and fatty acid composition [
22]. Understanding these factors is critical for optimizing aquaculture practices to enhance the nutritional quality of farmed tilapia and produce filets with fatty acid profiles closer to those of wild fish.
Table 5 summarizes the fatty acid composition analysis of aquaculture Red Kenyir™ (A, B and C) and wild (W) red tilapia (
Oreochromis sp.).