Oral L-Dopa Disrupts Behavioral Self-Control in Male Fighting Fish (Betta splendens)
Abstract
1. Introduction
2. Experiment I: Materials and Method
2.1. Animals and Housing
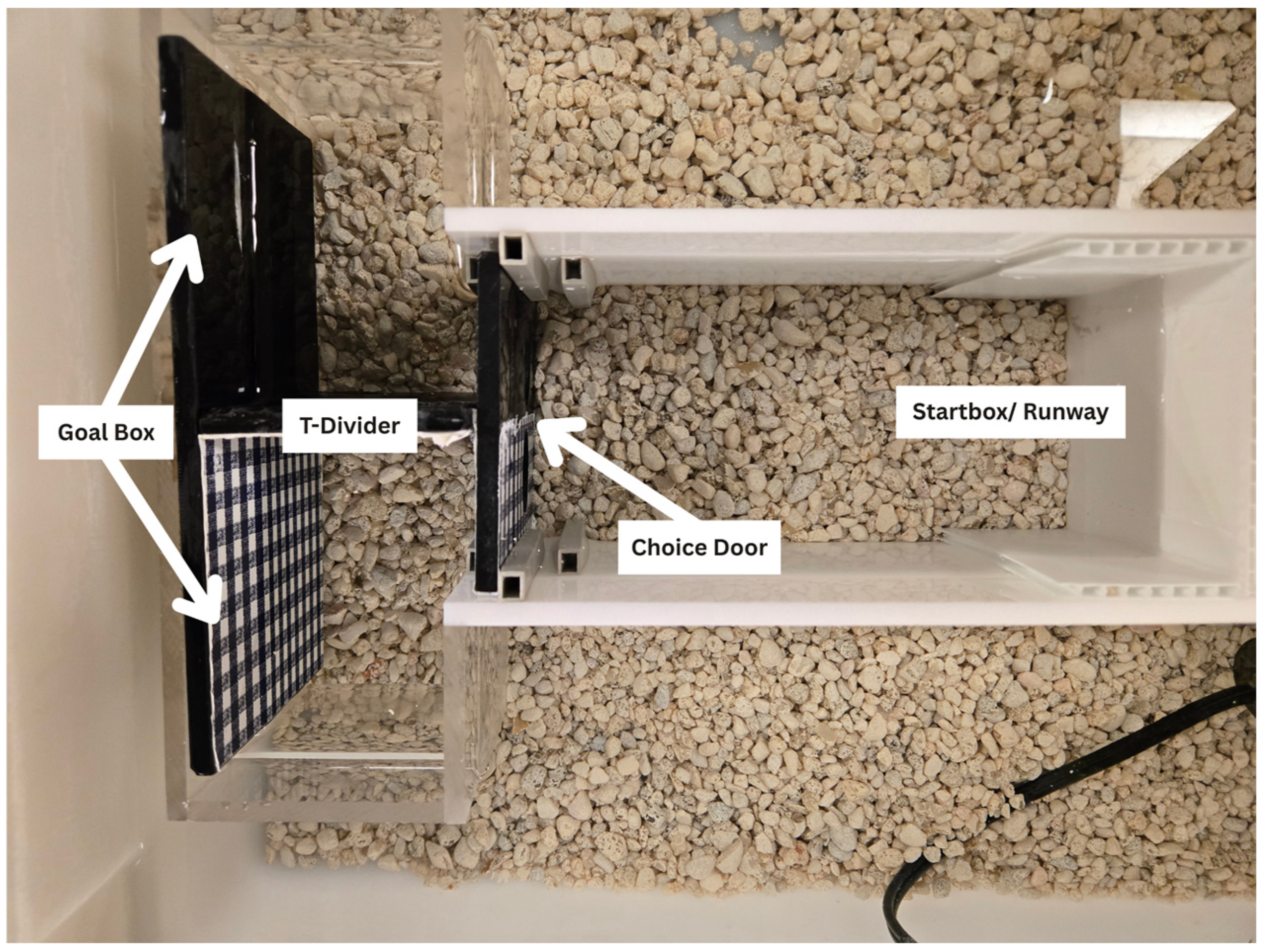
2.2. Materials and Apparatus
2.3. Procedure
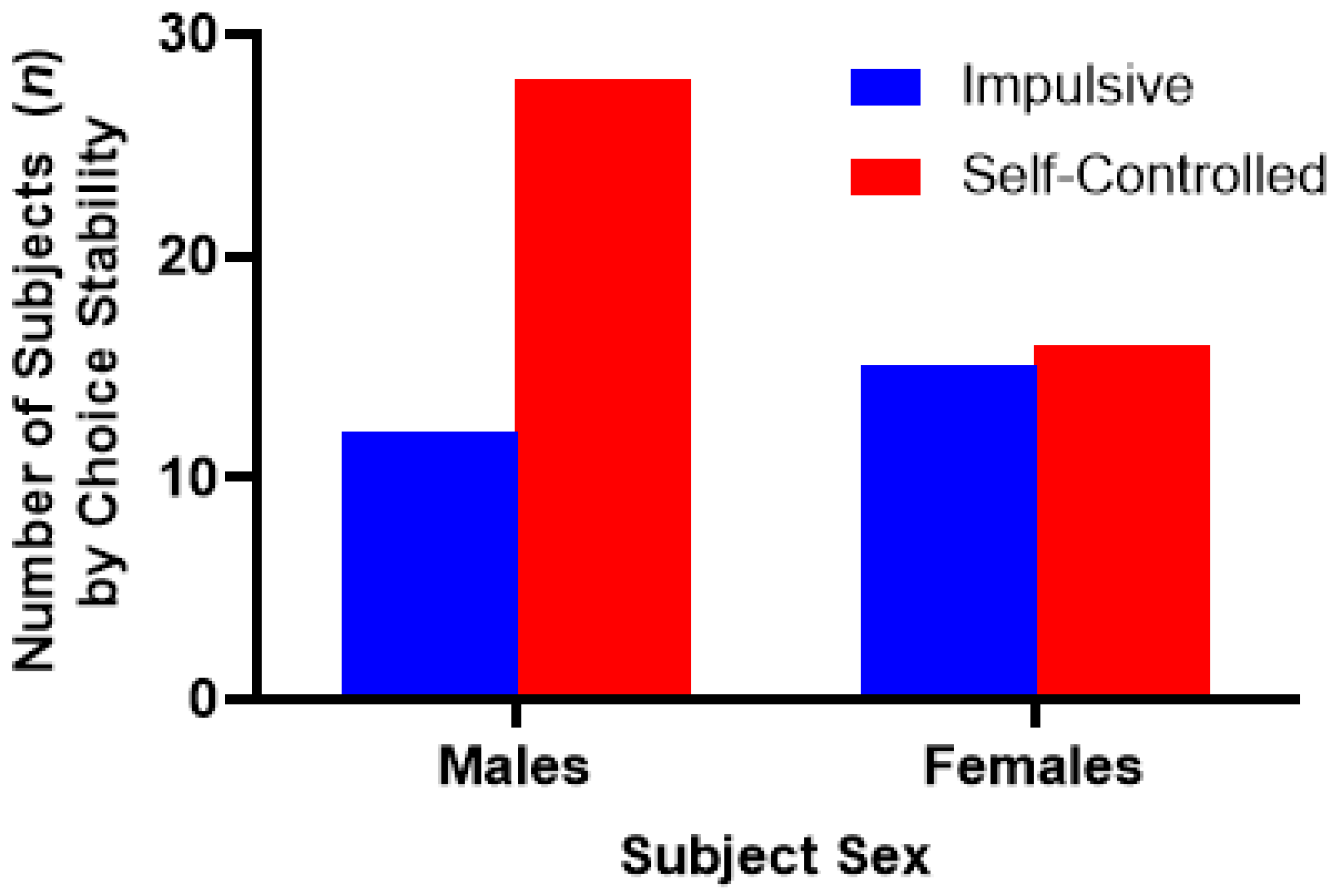
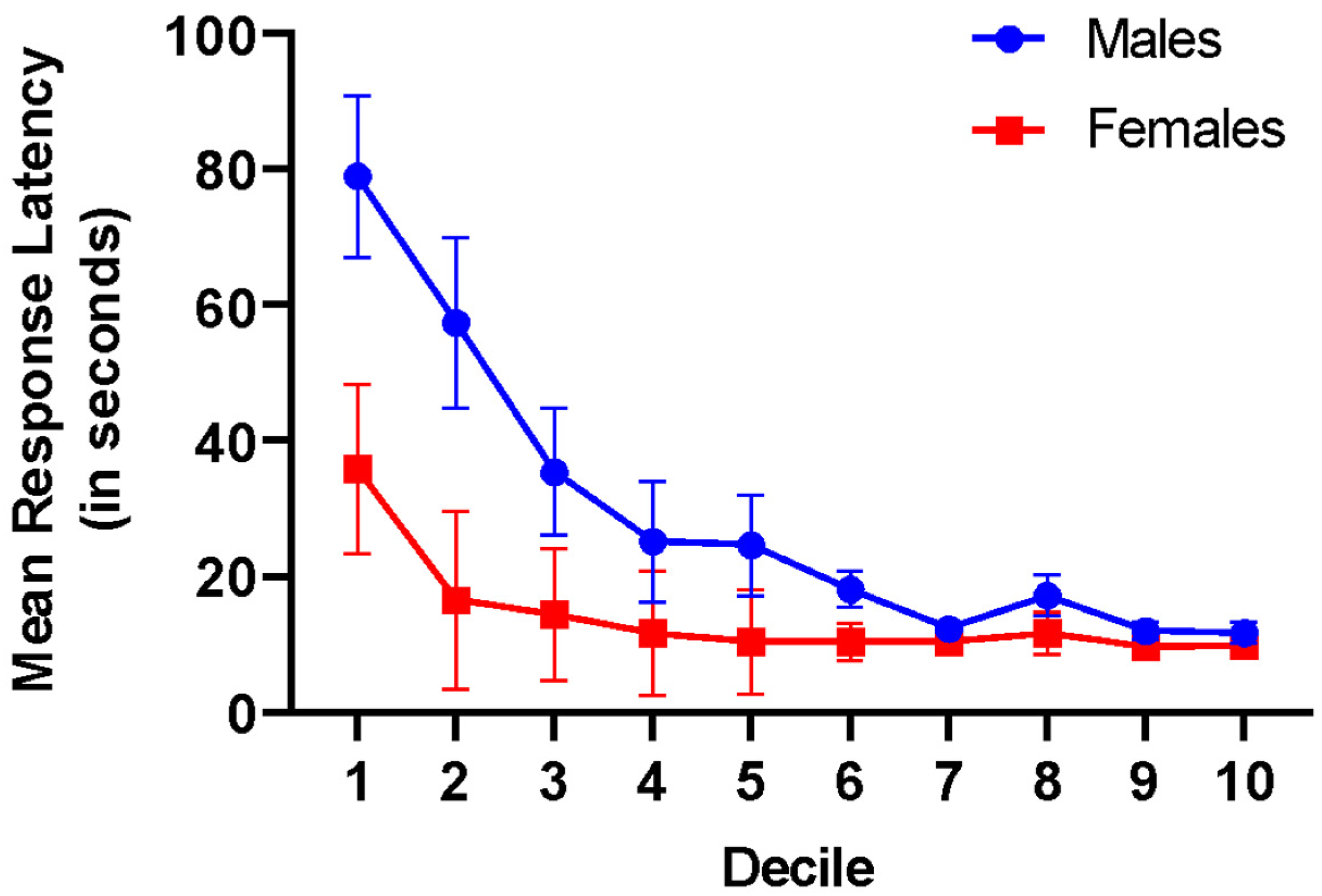
3. Experiment I: Results
4. Experiment I: Discussion
5. Experiment II: Introduction
6. Experiment II: Materials and Method
6.1. Animals and Housing
6.2. Procedure
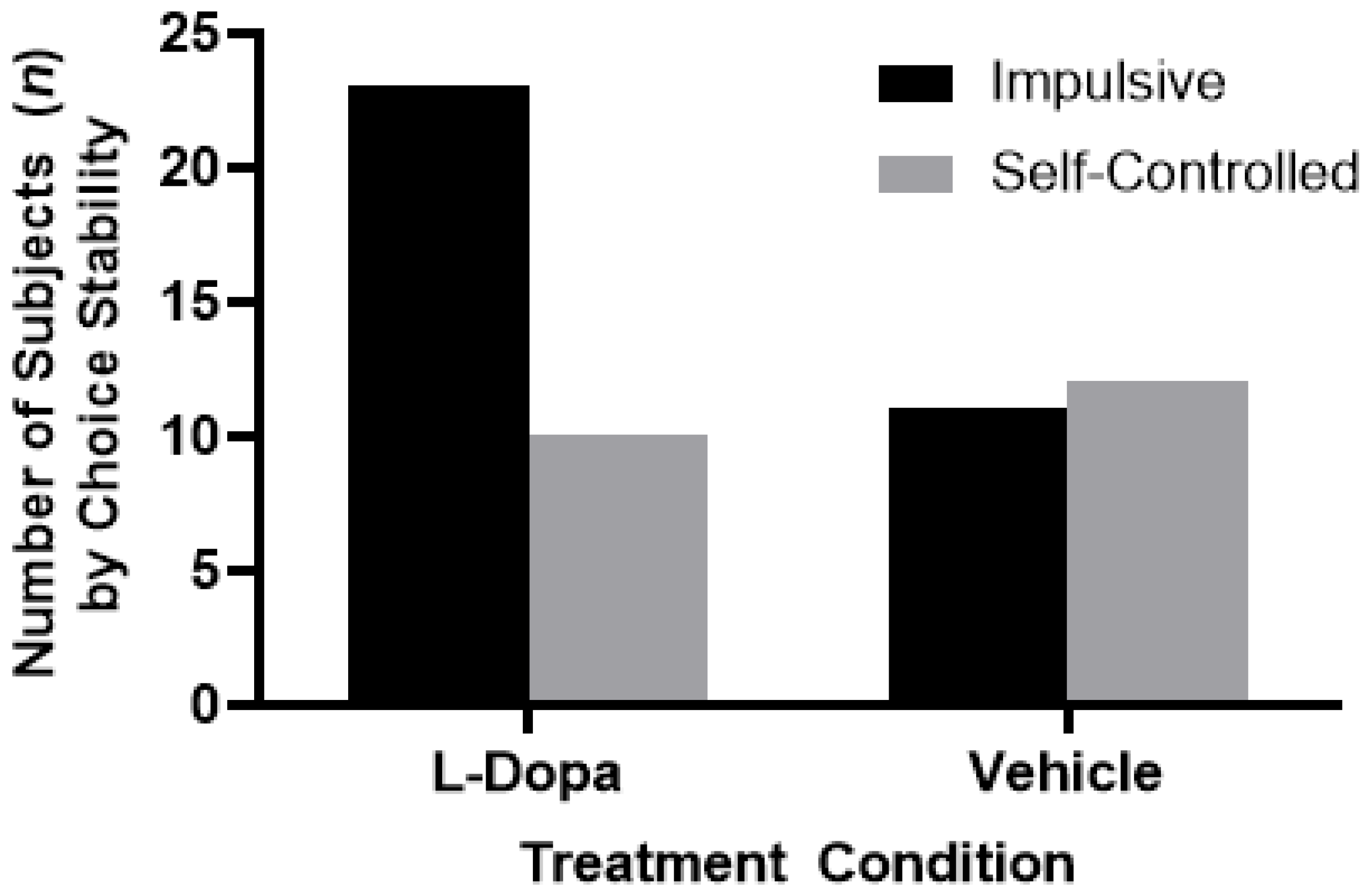
7. Experiment II: Results
8. Experiment II: Discussion
9. General Discussion
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ainslie, G.W. Impulse control in pigeons. J. Exp. Anal. Behav. 1974, 21, 485–489. [Google Scholar] [CrossRef]
- Rachlin, H.; Green, L. Commitment, choice and self-control. J. Exp. Anal. Behav. 1972, 17, 15–22. [Google Scholar] [CrossRef]
- Logue, A.W. Research on self-control: An integrating framework. Behav. Brain Sci. 1988, 11, 665–679. [Google Scholar] [CrossRef]
- van Baal, S.T.; Walasek, L.; Verdejo-García, A.; Hohwy, J. Impulsivity and self-control as timeless concepts: A conceptual analysis of intertemporal choice. Decision 2025, 12, 165–189. [Google Scholar] [CrossRef]
- Abeyesinghe, S.M.; Nicol, C.J.; Hartnell, S.J.; Wathes, C.M. Can domestic fowl, Gallus gallus domesticus, show self-control? Anim. Behav. 2005, 70, 1–11. [Google Scholar] [CrossRef]
- Tobin, H.; Logue, A.W. Self-control across species (Columba livia, Homo sapiens, and Rattus norvegicus). J. Comp. Psychol. 1994, 108, 126–133. [Google Scholar] [CrossRef]
- Van Haaren, F.; Van Hest, A.; Van De Poll, N.E. Self-control in male and female rats. J. Exp. Anal. Behav. 1988, 49, 201–211. [Google Scholar] [CrossRef]
- MacLean, E.L.; Hare, B.; Nunn, C.L.; Addessi, E.; Amici, F.; Anderson, R.C.; Filippo, A.; Baker, J.M.; Bania, A.E.; Barnard, A.M.; et al. The evolution of self-control. Proc. Natl. Acad. Sci. USA 2014, 111, E2140–E2148. [Google Scholar] [CrossRef]
- Miller, R.; Boeckle, M.; Jelbert, S.A.; Frohnwieser, A.; Wascher, C.A.; Clayton, N.S. Self-control in crows, parrots and nonhuman primates. Wiley Interdiscip. Rev. Cogn. Sci. 2019, 10, e1504. [Google Scholar] [CrossRef]
- Gobbo, E.; Zupan Šemrov, M. Dogs exhibiting high levels of aggressive reactivity show impaired self-control abilities. Front. Vet. Sci. 2022, 9, 869068. [Google Scholar] [CrossRef]
- Cheng, K.; Peña, J.; Porter, M.A.; Irwin, J.D. Self-control in honeybees. Psychon. Bull. Rev. 2002, 9, 259–263. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Starving honeybees lose self-control. Biol. Lett. 2015, 11, 20140820. [Google Scholar] [CrossRef]
- Schnell, A.K.; Boeckle, M.; Rivera, M.; Clayton, N.S.; Hanlon, R.T. Cuttlefish exert self-control in a delay of gratification task. Proc. R. Soc. B 2021, 288, 20203161. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Ng, S.; Gray, Q.; Cowie, S. Pigeons’ (Columba livia) intertemporal choice in binary-choice and patch-leaving contexts. J. Comp. Psychol. 2025, 139, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Tobin, H.; Chelonis, J.J.; Logue, A.W. Choice in self-control paradigms using rats. Psychol. Rec. 1993, 43, 441–453. [Google Scholar]
- Pepperberg, I.M.; Rosenberger, V.A. Delayed gratification: A grey parrot (Psittacus erithacus) will wait for more tokens. J. Comp. Psychol. 2022, 136, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, T.D. Token reinforcement: A review and analysis. J. Exp. Anal. Behav. 2009, 91, 257–286. [Google Scholar] [CrossRef]
- Aellen, M.; Dufour, V.; Bshary, R. Cleaner fish and other wrasse match primates in their ability to delay gratification. Anim. Behav. 2021, 176, 125–143. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution Of Life Histories; Oxford Academic: Oxford, UK, 1998. [Google Scholar] [CrossRef]
- Veit, W.; Gascoigne, S.J.; Salguero-Gómez, R. Evolution, complexity, and life history theory. Biol. Theory 2025, 20, 212–221. [Google Scholar] [CrossRef]
- Jaroensutasinee, M.; Jaroensutasinee, K. Sexual size dimorphism and male contest in wild Siamese fighting fish. J. Fish Biol. 2001, 59, 1614–1621. [Google Scholar] [CrossRef]
- Forsatkar, M.N.; Nematollahi, M.A.; Brown, C. Social context modulates aggression and courtship in male Siamese fighting fish, Betta splendens. Behav. Process. 2016, 133, 79–85. [Google Scholar] [CrossRef]
- Simpson, M.J.A. The display of the Siamese fighting fish, Betta splendens. Anim. Behav. Monogr. 1968, 1, 1–73. [Google Scholar] [CrossRef]
- Lichak, M.R.; Barber, J.R.; Kwon, Y.M.; Francis, K.X.; Bendesky, A. Care and use of Siamese fighting fish (Betta splendens) for research. Comp. Med. 2022, 72, 169–180. [Google Scholar] [CrossRef]
- Wooster, E.; Whiting, M.; Nimmo, D.; Sayol, F.; Carthey, A.; Stanton, L.; Ashton, B. Predator-prey interactions as drivers of cognitive evolution (preprint). EcoEvoRxiv 2025. [Google Scholar] [CrossRef]
- Bateman, A.W.; Vos, M.; Anholt, B.R. When to defend: Antipredator defenses and the predation sequence. Am. Nat. 2014, 183, 847–855. [Google Scholar] [CrossRef]
- Endler, J.A. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vis. Res. 1991, 31, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Baenninger, R.; Kraus, S. Some determinants of aggressive and predatory responses in Betta splendens. J. Comp. Physiol. Psychol. 1981, 95, 220–227. [Google Scholar] [CrossRef]
- Day, S.W.; Higham, T.E.; Holzman, R.; Van Wassenbergh, S. Morphology, kinematics, and dynamics: The mechanics of suction feeding in fishes. Integr. Comp. Biol. 2015, 55, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ferry-Graham, L.A.; Lauder, G.V. Aquatic prey capture in ray-finned fishes: A century of progress and new directions. J. Morphol. 2001, 248, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.; Carroll, A.M.; Collar, D.C.; Day, S.W.; Higham, T.E.; Holzman, R.A. Suction feeding mechanics, performance, and diversity in fishes. Integr. Comp. Biol. 2007, 47, 96–106. [Google Scholar] [CrossRef]
- Gromova, E.S.; Makhotin, V.V. Diversity of feeding methods in Teleosts (Teleostei) in the context of the morphology of their jaw apparatus. Inland Water Biol. 2023, 16, 700–721. [Google Scholar] [CrossRef]
- Cagle, M.D. Sexual Dimorphism and Potential Hormonal Modulation of Feeding Mechanics in Siamese Fighting Fish, Betta splendens. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2014. Available online: https://cnu.idm.oclc.org/login?url=https://www.proquest.com/dissertations-theses/sexual-dimorphism-potential-hormonal-modulation/docview/1611782153/se-2?accountid=10100 (accessed on 20 June 2025).
- Goldstein, R.J. The Betta Handbook; Barron’s Educational Series: Hauppauge, NY, USA, 2015; ISBN 978-0764127281. [Google Scholar]
- Monvises, A.; Nuangsaeng, B.; Sriwattanarothai, N.; Panijpan, B. The Siamese fighting fish: Well-known generally but little-known scientifically. ScienceAsia 2009, 35, 8–16. [Google Scholar] [CrossRef]
- Jaroensutasinee, M.; Jaroensutansinee, K. Bubble nest habitat characteristics of wild Siamese fighting fish. J. Fish Biol. 2001, 58, 1311–1319. [Google Scholar] [CrossRef]
- Craft, B.B.; Velkey, A.J.; Szalda-Petree, A. Instrumental conditioning of choice behavior in male Siamese fighting fish (Betta splendens). Behav. Process. 2003, 63, 171–175. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Bols, R.J.; Hogan, J.A. Runway behavior of Siamese fighting fish (Betta splendens) for aggressive display and food reinforcement. Anim. Learn. Behav. 1979, 7, 537–542. [Google Scholar] [CrossRef]
- Craft, B.B.; Szalda-Petree, A.D. Effect of various discriminative stimuli on choice behavior in male Siamese Fighting Fish (Betta splendens). Percept. Mot. Ski. 2007, 104, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.A.; Bols, R.J. Priming of aggressive motivation in Betta splendens. Anim. Behav. 1980, 28, 1189–1196. [Google Scholar] [CrossRef]
- Pepperberg, I.M.; Hartsfield, L.A. A study of executive function in grey parrots (Psittacus erithacus): Experience can affect delay of gratification. J. Comp. Psychol. 2024, 138, 8–19. [Google Scholar] [CrossRef]
- Prétôt, L.; Bshary, R.; Brosnan, S.F. Factors influencing the different performance of fish and primates on a dichotomous choice task. Anim. Behav. 2016, 119, 189–199. [Google Scholar] [CrossRef]
- Alcaro, A.; Huber, R.; Panksepp, J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Res. Rev. 2007, 56, 283–321. [Google Scholar] [CrossRef]
- Diotel, N.; Lübke, L.; Strähle, U.; Rastegar, S. Common and distinct features of adult neurogenesis and regeneration in the telencephalon of zebrafish and mammals. Front. Neurosci. 2020, 14, 568930. [Google Scholar] [CrossRef]
- Dupeyron, S.; Wallace, K.J. Quantifying the neural and behavioral correlates of repeated social competition in the fighting fish Betta splendens. Fishes 2023, 8, 384. [Google Scholar] [CrossRef]
- Yamamoto, K.; Vernier, P. The evolution of dopamine systems in chordates. Front. Neuroanat. 2023, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Barandela, M.; Jiménez-López, C. The dopaminergic control of movement-evolutionary considerations. Int. J. Mol. Sci. 2021, 22, 11284. [Google Scholar] [CrossRef]
- Costa, K.M.; Schoenbaum, G. Dopamine. Curr. Biol. 2022, 32, R817–R824. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fontaine, R.; Pasqualini, C.; Vernier, P. Classification of dopamine receptor genes in vertebrates: Nine subtypes in osteichthyes. Brain Behav. Evol. 2015, 86, 164–175. [Google Scholar] [CrossRef]
- Schultz, W. A dopamine mechanism for reward maximization. Proc. Natl. Acad. Sci. USA 2024, 121, e2316658121. [Google Scholar] [CrossRef]
- Kobayashi, S.; Schultz, W. Reward contexts extend dopamine signals to unrewarded stimuli. Curr. Biol. 2014, 24, 56–62. [Google Scholar] [CrossRef]
- Lak, A.; Stauffer, W.R.; Schultz, W. Dopamine prediction error responses integrate subjective value from different reward dimensions. Proc. Natl. Acad. Sci. USA 2014, 111, 2343–2348. [Google Scholar] [CrossRef]
- Jin, F.; Yang, L.; Yang, L.; Li, J.; Li, M.; Shang, Z. Dynamics learning rate bias in pigeons: Insights from reinforcement learning and neural correlates. Animals 2024, 14, 489. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.; Cardoso, S.C.; Malato, J.T.; Messias, J.P. Can cleanerfish overcome temptation? A selective role for dopamine influence on cooperative-based decision making. Physiol. Behav. 2017, 169, 124–129. [Google Scholar] [CrossRef]
- Mersereau, E.J.; Boyle, C.A.; Poitra, S.; Espinoza, A.; Seiler, J.; Longie, R.; Delvo, L.; Szarkowski, M.; Maliske, J.; Chalmers, S.; et al. Longitudinal effects of embryonic exposure to cocaine on morphology, cardiovascular physiology, and behavior in zebrafish. Int. J. Mol. Sci. 2016, 17, 847. [Google Scholar] [CrossRef]
- Naderi, M.; Jamwal, A.; Chivers, D.P.; Niyogi, S. Modulatory effects of dopamine receptors on associative learning performance in zebrafish (Danio rerio). Behav. Brain Res. 2016, 303, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Silva, P.I.M.; Vindas, M.A.; Øverli, Ø. Contrasting coping styles meet the wall: A dopamine driven dichotomy in behavior and cognition. Front. Neurosci. 2017, 17, 383. [Google Scholar] [CrossRef]
- Vindas, M.A.; Sørensen, C.; Johansen, I.B.; Folkedal, O.; Höglund, E.; Khan, U.W.; Stien, L.H.; Kristiansen, T.S.; Braastad, B.O.; Øverli, Ø. Coping with unpredictability: Dopaminergic and neurotrophic responses to omission of expected reward in Atlantic salmon (Salmo salar L.). PLoS ONE 2014, 9, e85543. [Google Scholar] [CrossRef]
- Collins, A.G.; Frank, M.J. Opponent actor learning (OpAL): Modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychol. Rev. 2014, 121, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Rink, E.; Wullimann, M.F. Development of the catecholaminergic system in the early zebrafish brain: An immunohistochemical study. Dev. Brain Res. 2002, 137, 89–100. [Google Scholar] [CrossRef]
- O’Connell, L.A.; Hofmann, H.A. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J. Comp. Neurol. 2011, 519, 3599–3639. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T. What is the thalamus in zebrafish? Front. Neurosci. 2012, 6, 64. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Rodríguez, F. Evolution of forebrain and spatial cognition in vertebrates: Conservation across diversity. Brain Behav. Evol. 2003, 62, 72–82. [Google Scholar] [CrossRef]
- Rutledge, R.B.; Skandali, N.; Dayan, P.; Dolan, R.J. Dopaminergic modulation of decision making and subjective well-being. J. Neurosci. 2015, 35, 9811–9822. [Google Scholar] [CrossRef]
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R.J. Dopamine, time, and impulsivity in humans. J. Neurosci. 2010, 30, 8888–8896. [Google Scholar] [CrossRef]
- Santangelo, G.; Barone, P.; Trojano, L.; Vitale, C. Pathological gambling in Parkinson’s disease. A comprehensive review. Park. Relat. Disord. 2013, 19, 645–653. [Google Scholar] [CrossRef]
- Culicetto, L.; Impellizzeri, F.; Lo Buono, V.; Marafioti, G.; Di Lorenzo, G.; Sorbera, C.; Brigandì, A.; Quartarone, A.; Marino, S. Gambling disorder in Parkinson’s disease: A scoping review on the challenge of rehabilitation strategies. J. Clin. Med. 2025, 14, 737. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Hassan, K.; Zurowski, M.; Duff-Canning, S.; De Souza, M.; Fox, S.; Lang, A.; Miyasaki, J. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology 2006, 66, 1750–1752. [Google Scholar] [CrossRef]
- Pirritano, D.; Plastino, M.; Bosco, D.; Gallelli, L.; Siniscalchi, A.; De Sarro, G. Gambling disorder during dopamine replacement treatment in Parkinson’s disease: A comprehensive review. BioMed Res. Int. 2014, 2014, 728038. [Google Scholar] [CrossRef]
- Pietras, C.J.; Cherek, D.R.; Lane, S.D.; Tcheremissine, O.V.; Steinberg, J.L. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology 2003, 170, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Arkell, T.R.; Bradshaw, K.; Downey, L.A.; Hayley, A.C. Acute effects of amphetamine and related psychostimulants on impulsivity: A systematic review of clinical trials. Addict. Biol. 2022, 27, e13128. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.; Gutiérrez-Casares, J.R.; Álamo, C. Molecular characterisation of the mechanism of action of stimulant drugs lisdexamfetamine and methylphenidate on ADHD neurobiology: A review. Neurol. Ther. 2022, 11, 1489–1517. [Google Scholar] [CrossRef]
- Pearce, R.; Heikkilä, M.; Lindén, I.B.; Jenner, P. L-dopa induces dyskinesia in normal monkeys: Behavioural and pharmacokinetic observations. Psychopharmacology 2001, 156, 402–409. [Google Scholar] [CrossRef]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257 (Suppl. 2), S253–S261. [Google Scholar] [CrossRef]
- Greene, S.M.; Szalda-Petree, A.D. Fins of fury or fainéant: Fluoxetine impacts the aggressive behavior of fighting fish (Betta splendens). Behav. Process. 2022, 194, 104544. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, B.R.; Greene, S.; Szalda-Petree, A. Of fish and mirrors: Fluoxetine disrupts aggression and learning for social rewards. Physiol. Behav. 2017, 173, 258–262. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Chapter 4. [Google Scholar]
- Yuan, M.; Fang, Q.; Lu, W.; Wang, X.; Hao, T.; Chong, C.M.; Chen, S. Stress in fish: Neuroendocrine and neurotransmitter responses. Fishes 2025, 10, 307. [Google Scholar] [CrossRef]
- Fizet, J.; Cassel, J.C.; Kelche, C.; Meunier, H. A review of the 5-Choice Serial Reaction Time (5-CSRT) task in different vertebrate models. Neurosci. Biobehav. Rev. 2016, 71, 135–153. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Vranken, N.; Hoge, C.; Lichak, M.R.; Norovich, A.L.; Francis, K.X.; Camacho-Garcia, J.; Bista, I.; Wood, J.; McCarthy, S.; et al. Genomic consequences of domestication of the Siamese fighting fish. Sci. Adv. 2022, 8, eabm4950. [Google Scholar] [CrossRef] [PubMed]
- Lauwereyns, J.; Bajramovic, J.; Bert, B.; Camenzind, S.; De Kock, J.; Elezović, A.; Erden, S.; Gonzalez-Uarquin, F.; Ulman, Y.I.; Hoffmann, O.I.; et al. Toward a common interpretation of the 3Rs principles in animal research. Lab Anim. 2024, 53, 347–350. [Google Scholar] [CrossRef] [PubMed]

| Mammalian Brain Region | Function | Fish Homolog | Evidence |
|---|---|---|---|
| Ventral Tegmental Area (VTA) | Source of dopaminergic projections to forebrain (reward, motivation) | Posterior tuberculum (TPp) | Dopaminergic neurons in TPp project to telencephalic targets, functionally similar to VTA [61] |
| Nucleus Accumbens (NAcc) | Integrates dopaminergic signals (reward processing) | Ventral part of the ventral telencephalon (Vv/Vd) | Gene expression (e.g., dopamine receptors, neuropeptides), connectivity, behavioral roles [62] |
| Amygdala | Emotion, social behavior, fear processing | Medial and dorsal parts of the ventral telencephalon (Dm) | Dm is involved in fear, aggression, and social learning in fish [63] |
| Hippocampus | Learning and memory | Dorsolateral telencephalon (Dl) | Homologous by gene expression (e.g., zic1, emx), lesion studies, and spatial tasks [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velkey, A.; Watson, K.; White, N.; Agi, A.; Doebler-Alligood, G.; Tilmont, I.; Williams Sweeten, B.; Kinslow, K. Oral L-Dopa Disrupts Behavioral Self-Control in Male Fighting Fish (Betta splendens). Fishes 2025, 10, 518. https://doi.org/10.3390/fishes10100518
Velkey A, Watson K, White N, Agi A, Doebler-Alligood G, Tilmont I, Williams Sweeten B, Kinslow K. Oral L-Dopa Disrupts Behavioral Self-Control in Male Fighting Fish (Betta splendens). Fishes. 2025; 10(10):518. https://doi.org/10.3390/fishes10100518
Chicago/Turabian StyleVelkey, Andrew, Kate Watson, Nathan White, Abigail Agi, Grace Doebler-Alligood, Isabella Tilmont, Brook Williams Sweeten, and Kaitlyn Kinslow. 2025. "Oral L-Dopa Disrupts Behavioral Self-Control in Male Fighting Fish (Betta splendens)" Fishes 10, no. 10: 518. https://doi.org/10.3390/fishes10100518
APA StyleVelkey, A., Watson, K., White, N., Agi, A., Doebler-Alligood, G., Tilmont, I., Williams Sweeten, B., & Kinslow, K. (2025). Oral L-Dopa Disrupts Behavioral Self-Control in Male Fighting Fish (Betta splendens). Fishes, 10(10), 518. https://doi.org/10.3390/fishes10100518







