Trace Element Accumulation and Oxidative Stress in Three Populations of the European Eel Anguilla anguilla L. from Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Collection
2.2. Trace Elements Analysis
2.3. Measurement of Oxidative Stress Biomarkers
2.3.1. Lipid Peroxidation
2.3.2. Protein Oxidation
2.3.3. Superoxide Dismutase (SOD) Activity
2.4. Data Analysis
3. Results
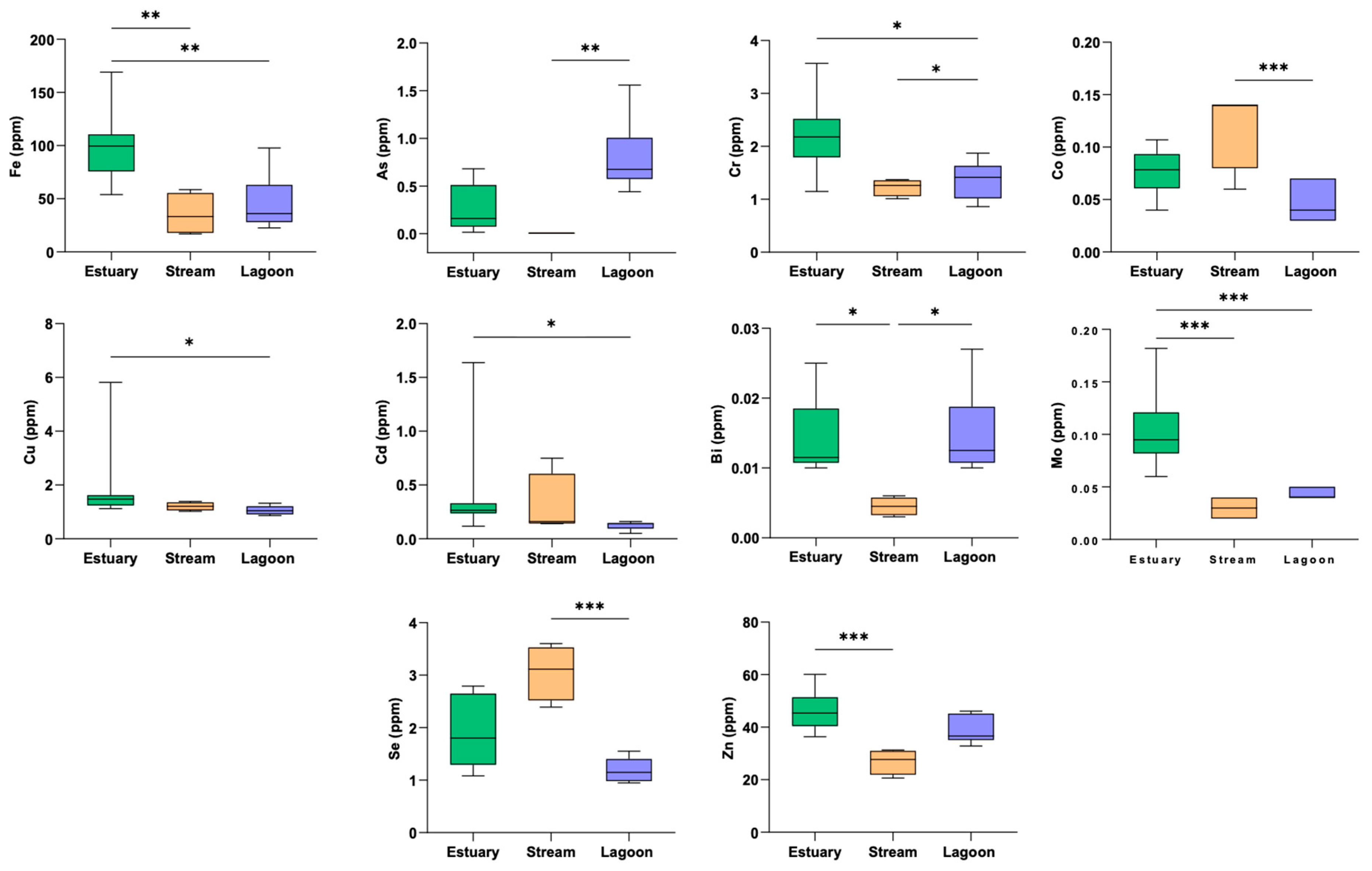
3.1. Trace Element Concentrations in Muscle Tissue of Eels from Different Sites
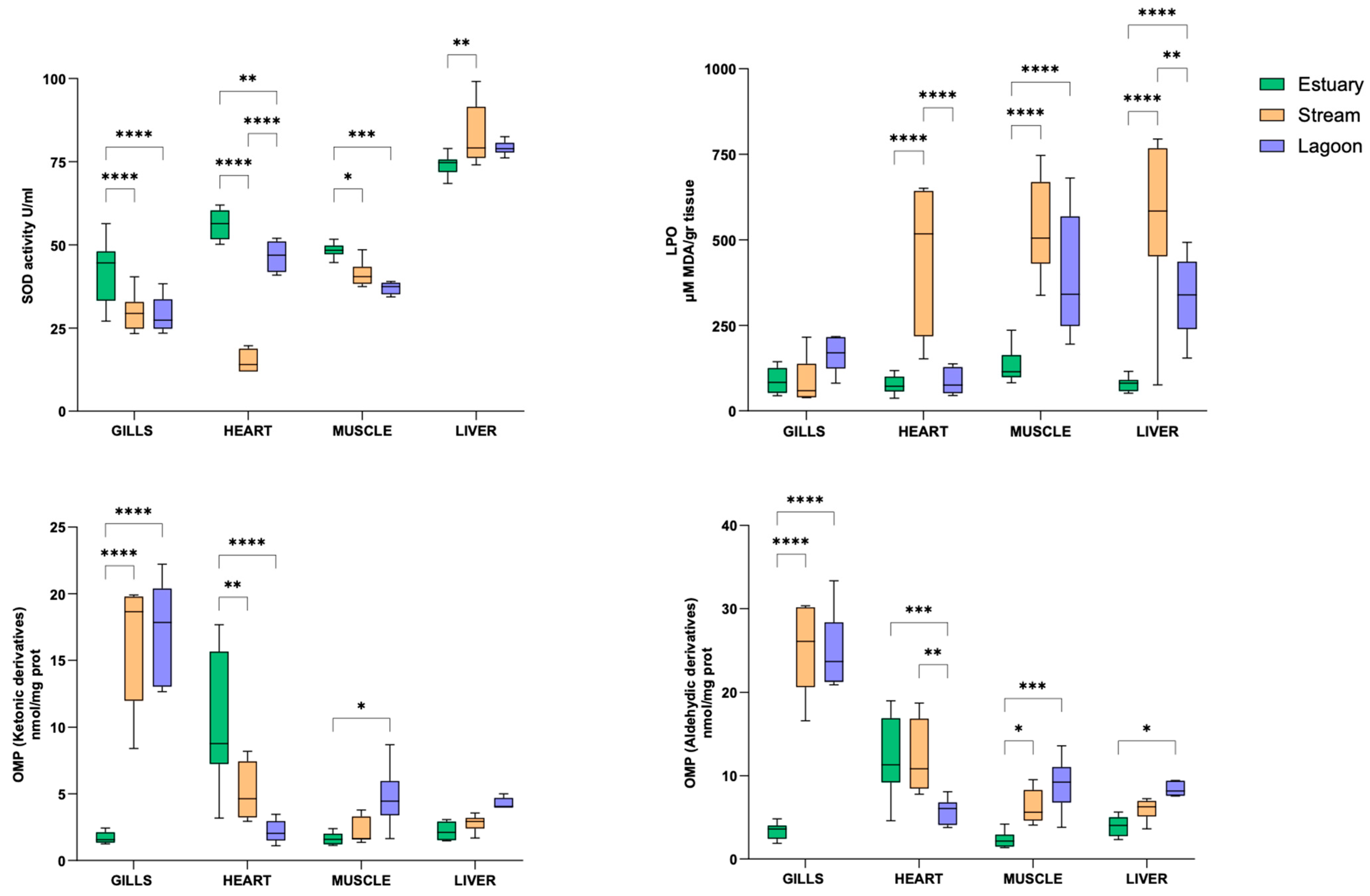
3.2. Tissue Evaluation of the Oxidative Status
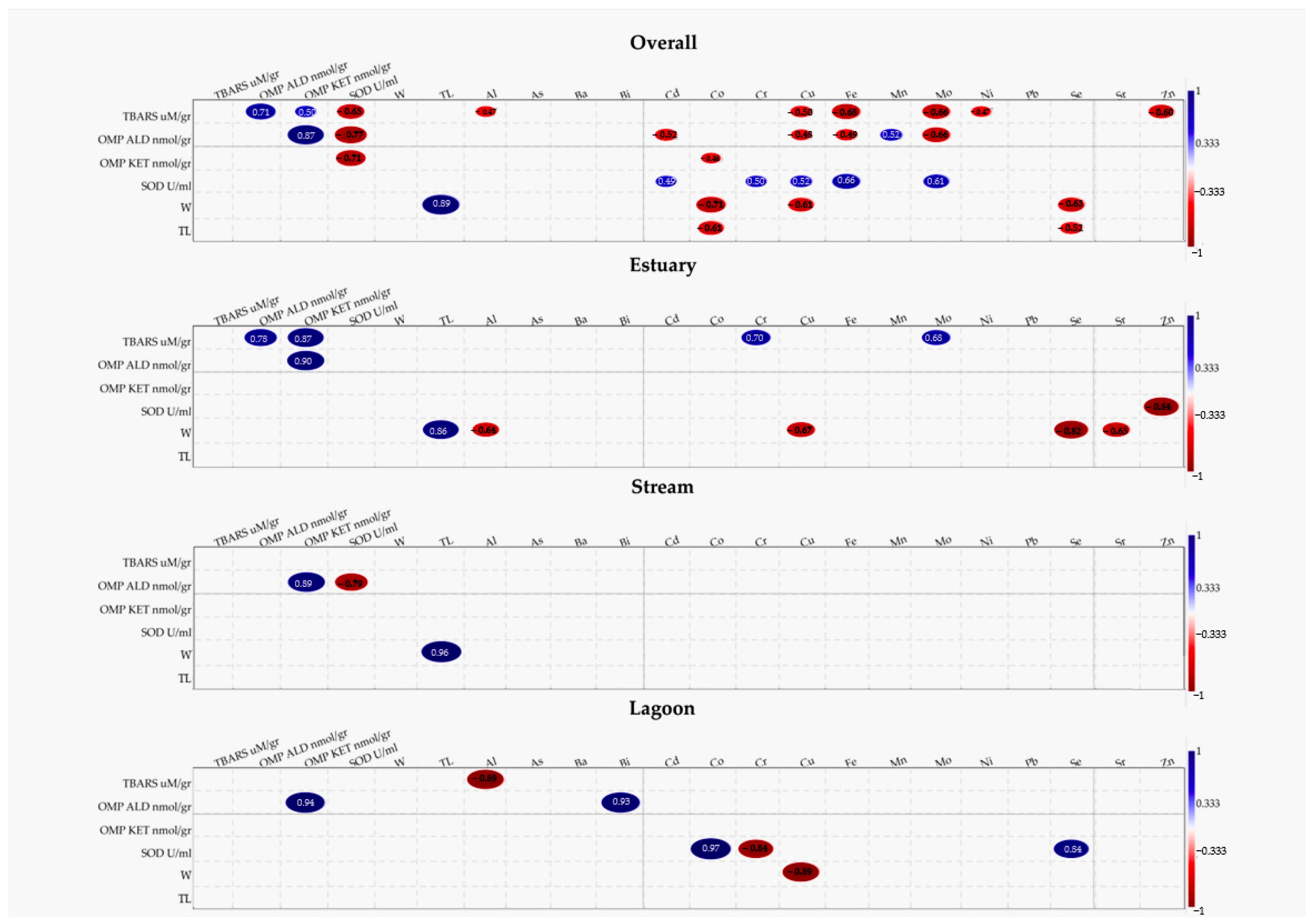
3.3. Correlation Analysis
4. Discussion
4.1. Trace Elements Accumulation
4.2. Eel Oxidative Status
4.3. Correlation Between Oxidative Markers and TE Accumulation
5. Conclusions
Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUCN | International Union for Conservation of Nature |
| LPO | Lipid Peroxidation |
| OMP | Oxidatively Modified Protein |
| TEPI | Trace Elements Pollution Index |
| SOD | Superoxide Dismutase |
References
- Dekker, W. Slipping Though Our Hands–Population Dynamics of the European Eel; Wageningen University and Research: Wageningen, The Netherlands, 2004. [Google Scholar]
- Altmayer, A. Addressing the Critical State of European Eel Stocks; European Parliament: Strasbourg, France, 2024.
- Jacoby, D.M.P.; Gollock, M.J. Anguilla Anguilla. In The IUCN Red List of Threatened Species; Version 2014.3; IUCN: Gland, Switzerland, 2014. [Google Scholar]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels, and Country Reports 2018–2019; ICES Scientific Report; ICES: Copenhagen, Denmark, 2019. [Google Scholar]
- Geeraerts, C.; Belpaire, C. The Effects of Contaminants in European Eel: A Review. Ecotoxicology 2010, 19, 239–266. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Hall, A. Influence of Inorganic Lead on the Biochemical Blood Composition of the Eel, Anguilla anguilla L. Ecotoxicol. Environ. Saf. 1990, 20, 7–9. [Google Scholar] [CrossRef]
- Oliveira, M.; Serafim, A.; Bebianno, M.J.; Pacheco, M.; Santos, M.A. European Eel (Anguilla Anguilla L.) Metallothionein, Endocrine, Metabolic and Genotoxic Responses to Copper Exposure. Ecotoxicol. Environ. Saf. 2008, 70, 20–26. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Maffia, M.; Cappello, M.; Giordano, M.E.; Storelli, C.; Schettino, T. Effect of Cadmium on Carbonic Anhydrase and Na+-K+-ATPase in Eel, Anguilla Anguilla, Intestine and Gills. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 89–91. [Google Scholar] [CrossRef]
- Beaulieu, M. Oxidative Status: A General but Overlooked Indicator of Welfare across Animal Species? BioEssays 2024, 46, 2300205. [Google Scholar] [CrossRef]
- Ahmad, I.; Oliveira, M.; Pacheco, M.; Santos, M.A. Anguilla Anguilla L. Oxidative Stress Biomarkers Responses to Copper Exposure with or without β-Naphthoflavone Pre-Exposure. Chemosphere 2005, 61, 267–275. [Google Scholar] [CrossRef]
- Stoch, F.; Genovesi, P. Manuali per Il Monitoraggio Di Specie e Habitat Di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Specie Animali. In Serie Manuali e Linee Guida, 141/2016; ISPRA: Roma, Italy, 2016. [Google Scholar]
- Gallo, S.; Nania, G.; Caruso, V.; Zicarelli, G.; Leonetti, F.L.; Giglio, G.; Fedele, G.; Romano, C.; Bottaro, M.; Mangoni, O.; et al. Bioaccumulation of Trace Elements in the Muscle of the Blackmouth Catshark Galeus Melastomus from Mediterranean Waters. Biology 2023, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Richir, J.; Gobert, S. A Reassessment of the Use of Posidonia Oceanica and Mytilus Galloprovincialis to Biomonitor the Coastal Pollution of Trace Elements: New Tools and Tips. Mar. Pollut. Bull. 2014, 89, 390–406. [Google Scholar] [CrossRef]
- Marengo, M.; Fullgrabe, L.; Fontaine, Q.; Boissery, P.; Cancemi, M.; Lejeune, P.; Gobert, S. Ecological and Human Health Risk Assessment of Potentially Toxic Element Contamination in Waters of a Former Asbestos Mine (Canari, Mediterranean Sea): Implications for Management. Environ. Monit. Assess. 2022, 195, 150. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Reinero, F.R.; Cerra, M.C.; Faggio, C.; Leonetti, F.L.; Micarelli, P.; Giglio, G.; Sperone, E.; Barca, D.; Imbrogno, S. Contamination by Trace Elements and Oxidative Stress in the Skeletal Muscle of Scyliorhinus Canicula from the Central Tyrrhenian Sea. Antioxidants 2023, 12, 524. [Google Scholar] [CrossRef]
- Tkachenko, H.; Grudniewska, J. Evaluation of Oxidative Stress Markers in the Heart and Liver of Rainbow Trout (Oncorhynchus Mykiss Walbaum) Exposed to the Formalin. Fish Physiol. Biochem. 2016, 42, 1819–1832. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of Carbonyl Content in Oxidatively Modified Proteins. In Methods in Enzymology; Academic Press: New York, NY, USA, 1990; Volume 186, pp. 464–478. ISBN 0076-6879. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bourillon, B.; Feunteun, E.; Acou, A.; Trancart, T.; Teichert, N.; Belpaire, C.; Dufour, S.; Bustamante, P.; Aarestrup, K.; Walker, A.; et al. Anthropogenic Contaminants Shape the Fitness of the Endangered European Eel: A Machine Learning Approach. Fishes 2022, 7, 274. [Google Scholar] [CrossRef]
- Di Duca, F.; Montuori, P.; De Rosa, E.; De Simone, B.; Russo, I.; Nubi, R.; Triassi, M. Assessing Heavy Metals in the Sele River Estuary: An Overview of Pollution Indices in Southern Italy. Toxics 2024, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Oujidi, B.; El Bouch, M.; Tahri, M.; Layachi, M.; Boutoumit, S.; Bouchnan, R.; Ouahidi, H.; Bounakhla, M.; El Ouamari, N.; Maanan, M.; et al. Seasonal and Spatial Patterns of Ecotoxicological Indices of Trace Elements in Superficial Sediments of the Marchica Lagoon Following Restoration Actions during the Last Decade. Diversity 2021, 13, 51. [Google Scholar] [CrossRef]
- Hossain, M.M.; Jahan, I.; Dar, M.A.; Dhanavade, M.J.; Mamtaz, A.F.B.; Maxwell, S.J.; Han, S.; Zhu, D. A Review of Potentially Toxic Elements in Sediment, Water, and Aquatic Species from the River Ecosystems. Toxics 2025, 13, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Liang, Y.; Cui, X.; Bu, D. Accumulation Characteristics of Trace Elements in Fish from High-Altitude Lakes and Risk Assessment: A Case Study of Nam Co Lake. Biol. Trace Elem. Res. 2025. [Google Scholar] [CrossRef]
- De Donato, C.; Barca, D.; Milazzo, C.; Santoro, R.; Giglio, G.; Tripepi, S.; Sperone, E. Is Trace Element Concentration Correlated to Parasite Abundance? A Case Study in a Population of the Green Frog Pelophylax Synkl. Hispanicus from the Neto River (Calabria, Southern Italy). Parasitol. Res. 2017, 116, 1745–1753. [Google Scholar] [CrossRef]
- Saidon, N.B.; Szabó, R.; Budai, P.; Lehel, J. Trophic Transfer and Biomagnification Potential of Environmental Contaminants (Heavy Metals) in Aquatic Ecosystems. Environ. Pollut. 2024, 340, 122815. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Ferrão, L.; Blanes-García, M.; Pérez, L.; Asturiano, J.F.; Morini, M. Superoxidase Dismutases (SODs) in the European Eel: Gene Characterization, Expression Response to Temperature Combined with Hormonal Maturation and Possible Migratory Implications. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 290, 111590. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Ren, Q.; Yin, S.; Liang, F.; Jia, Y. Two Superoxide Dismutases (SODs) Respond to Bacterial Challenge Identified in the Marbled Eel Anguilla Marmorata. Aquaculture 2016, 451, 316–325. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-Stimulated Reactive Oxygen Species Production and Oxidative Damage in Aquatic Organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Giuliani, M.E.; Benedetti, M.; Arukwe, A. Molecular and Biochemical Biomarkers in Environmental Monitoring: A Comparison of Biotransformation and Antioxidant Defense Systems in Multiple Tissues. Aquat. Toxicol. 2011, 105, 56–66. [Google Scholar] [CrossRef]
- Guimarães, L.; Gravato, C.; Santos, J.; Monteiro, L.S.; Guilhermino, L. Yellow Eel (Anguilla Anguilla) Development in NW Portuguese Estuaries with Different Contamination Levels. Ecotoxicology 2009, 18, 385–402. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M.A. Biotransformation, Genotoxic, and Histopathological Effects of Environmental Contaminants in European Eel (Anguilla Anguilla L.). Ecotoxicol. Environ. Saf. 2002, 53, 331–347. [Google Scholar] [CrossRef]
- Fabbri, E. Pharmaceuticals in the Environment: Expected and Unexpected Effects on Aquatic Fauna. Ann. N. Y. Acad. Sci. 2015, 1340, 20–28. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Elsawy, M.; Abdel-Hay, A.-H.M.; Abozeid, A.; Mohamed, R.; Shukry, M.; Khalafalla, M. Effect of Habitat and Water Salinity on Hematological, Biochemical, Immunological and Stress Parameters in European Eels (Anguilla Anguilla). Biol. Bull. 2024, 50, S708–S716. [Google Scholar] [CrossRef]
- Farrell, A.P.; Eliason, E.J.; Sandblom, E.; Clark, T.D. Fish Cardiorespiratory Physiology in an Era of Climate changeThe Present Review Is One of a Series of Occasional Review Articles That Have Been Invited by the Editors and Will Feature the Broad Range of Disciplines and Expertise Represented in Our Editorial Advisory Board. Can. J. Zool. 2009, 87, 835–851. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Farrell, A.P. Cardiac Plasticity in Fishes: Environmental Influences and Intraspecific Differences. J. Exp. Biol. 2004, 207, 2539–2550. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the Modulation of Redox Homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; De Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Velma, V.; Tchounwou, P.B. Oxidative Stress and DNA Damage Induced by Chromium in Liver and Kidney of Goldfish, Carassius auratus. Biomark. Insights 2013, 8, BMI.S11456. [Google Scholar] [CrossRef]
- Kumari, K.; Khare, A.; Dange, S. The Applicability of Oxidative Stress Biomarkers in Assessing Chromium Induced Toxicity in the Fish Labeo Rohita. BioMed Res. Int. 2014, 2014, 782493. [Google Scholar] [CrossRef]
- Li, Z.-M.; Wang, X.-L.; Jin, X.-M.; Huang, J.-Q.; Wang, L.-S. The Effect of Selenium on Antioxidant System in Aquaculture Animals. Front. Physiol. 2023, 14, 1153511. [Google Scholar] [CrossRef] [PubMed]
- Mazza, R.; Gattuso, A.; Imbrogno, S.; Boukhzar, L.; Leo, S.; Mallouki, B.Y.; Filice, M.; Rocca, C.; Angelone, T.; Anouar, Y.; et al. Selenoprotein T as a New Positive Inotrope in the Goldfish, Carassius auratus. J. Exp. Biol. 2019, 222, jeb201202. [Google Scholar] [CrossRef] [PubMed]
- Thangarani, A.J.; Felix, N.; Suresh, A.V.; Shanmugam, S.A.; Kathirvelpandian, A.; Suresh, E.; Ramya, R.; Ranjan, A.; Prabu, E.; Suman, T.Y. Impact of Selenium Supplementation on Antioxidant Enzyme Activity and Liver Gene Expression in Juvenile Pangasius Catfish (Pangasius Pangasius). J. Fish Biol. 2025, 107, 7–22. [Google Scholar] [CrossRef]
- Kubrak, O.I.; Rovenko, B.M.; Husak, V.V.; Vasylkiv, O.Y.; Storey, K.B.; Storey, J.M.; Lushchak, V.I. Goldfish Exposure to Cobalt Enhances Hemoglobin Level and Triggers Tissue-Specific Elevation of Antioxidant Defenses in Gills, Heart and Spleen. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 325–332. [Google Scholar] [CrossRef]
- Jones, D.L.; Kochian, L.V. Aluminum Interaction with Plasma Membrane Lipids and Enzyme Metal Binding Sites and Its Potential Role in Al Cytotoxicity. FEBS Lett. 1997, 400, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Keen, C.L. Aluminum Has Both Oxidant and Antioxidant Effects in Mouse Brain Membranes. Arch. Biochem. Biophys. 1993, 300, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Rowe, M.; Butler, M.W.; McGraw, K.J. From Molecules to Living Systems: Historical and Contemporary Issues in Oxidative Stress and Antioxidant Ecology. Funct. Ecol. 2010, 24, 950–959. [Google Scholar] [CrossRef]
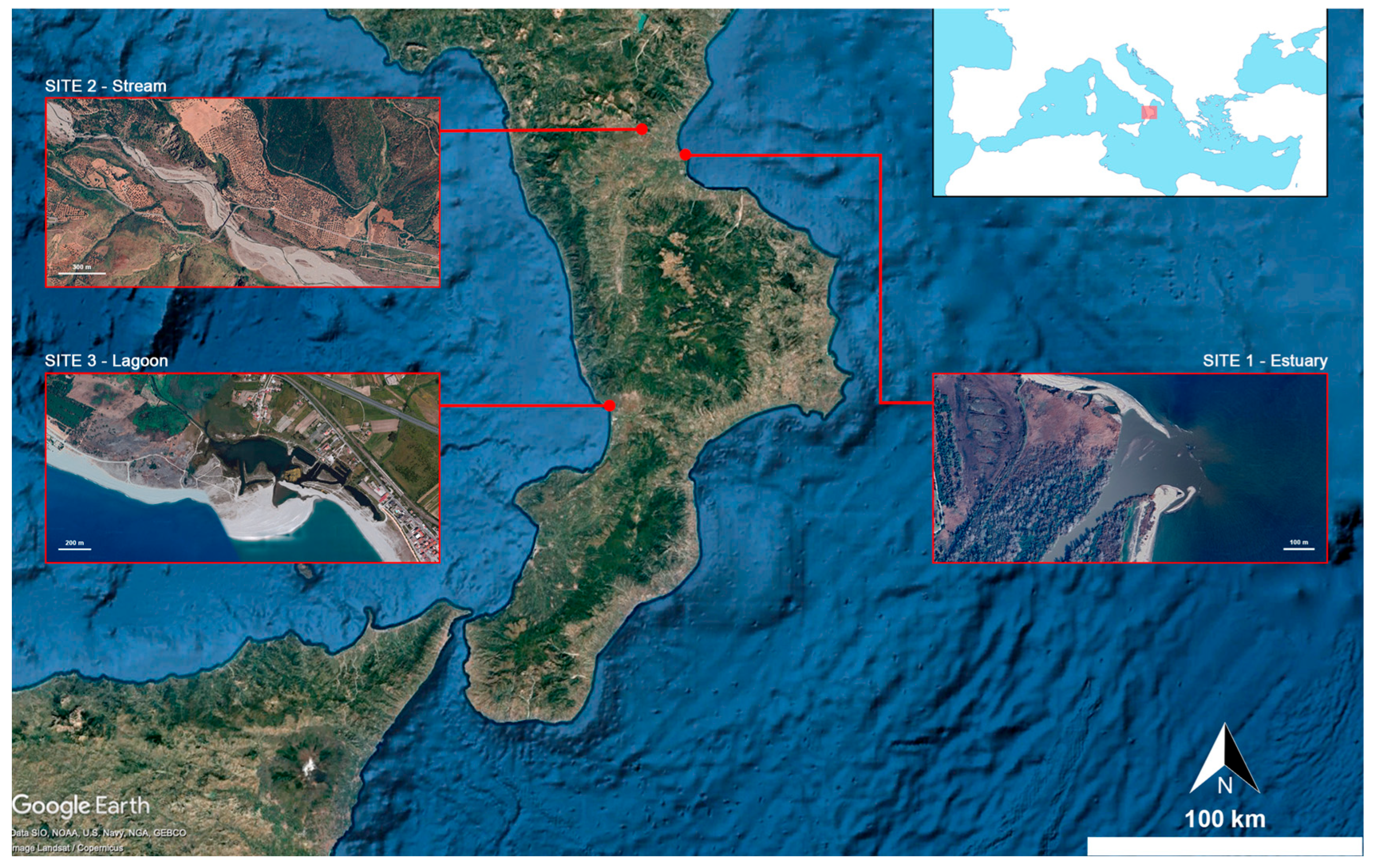
| Sites | Weight (g) Mean ± SD | Total Length (cm) Mean ± SD | N |
|---|---|---|---|
| Estuary | 145.4 ± 146.8 | 40.4 ± 11.1 | 10 |
| Stream | 119.4 ± 97.0 | 39.2 ± 12.1 | 7 |
| Lagoon | 104.3 ± 16.3 | 36.7 ± 2.7 | 6 |
| Estuary | Stream | Lagoon | KW | p | |
|---|---|---|---|---|---|
| Al | 3.68 ± 1.64 | 2.19 ± 0.24 | 2.50 ± 0.64 | 4.554 | 0.1 |
| As | 0.26 ± 0.25 | 0.00 | 0.80 ± 0.39 | 13.55 | 0.001 |
| Ba | 0.27 ± 0.14 | 0.15 ± 0.04 | 0.16 ± 0.08 | 4.51 | 0.1 |
| Bi | 0.01 ± 0.00 | 0.00 | 0.01 ± 0.01 | 9.33 | 0.01 |
| Cd | 0.40 ± 0.44 | 0.30 ± 0.29 | 0.12 ± 0.04 | 9.05 | 0.01 |
| Co | 0.08 ± 0.02 | 0.12 ± 0.04 | 0.05 ± 0.02 | 8.33 | 0.015 |
| Cr | 2.22 ± 0.66 | 1.23 ± 0.16 | 1.36 ± 0.36 | 9.92 | 0.01 |
| Cu | 1.84 ± 1.41 | 1.21 ± 0.16 | 1.06 ± 0.17 | 9.05 | 0.01 |
| Fe | 98.8 ± 33.3 | 35.5 ± 20.0 | 45.7 ± 27.4 | 11.76 | 0.003 |
| Mn | 1.39 ± 0.35 | 2.19 ± 0.58 | 2.98 ± 2.24 | 7.005 | 0.03 |
| Mo | 0.10 ± 0.03 | 0.03 ± 0.01 | 0.04 ± 0.01 | 15.49 | 0.0004 |
| Ni | 17.3 ± 33.8 | 6.94 ± 11.9 | 4.61 ± 8.06 | 5.869 | 0.05 |
| Pb | 0.07 ± 0.03 | 0.04 ± 0.01 | 0.09 ± 0.09 | 3.26 | 0.2 |
| Se | 1.90 ± 0.65 | 3.05 ± 0.52 | 1.19 ± 0.24 | 11.58 | 0.003 |
| Sr | 2.30 ± 0.87 | 2.03 ± 0.52 | 4.03 ± 2.31 | 2.76 | 0.25 |
| Zn | 46.4 ± 8.01 | 26.8 ± 4.79 | 38.8 ± 5.38 | 11.89 | 0.003 |
| Site 1 | Fe > Zn > Ni > Al > Sr > Cr > Se > Cu > Mn > Cd > Ba > As > Mo > Co > Pb > Bi |
| Site 2 | Fe > Zn > Ni > Se > Al = Mn > Sr > Cr > Cu > Cd > Ba > Co > Pb > Mo > Bi > As |
| Site 3 | Fe > Zn > Ni > Sr > Mn > Al > Cr > Se > Cu > As > Cd > Ba > Pb > Co > Mo > Bi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filice, M.; Gallo, S.; Caferro, A.; Giglio, G.; Leonetti, F.L.; Milazzo, C.; Gattuso, A.; Cerra, M.C.; Barca, D.; Sperone, E.; et al. Trace Element Accumulation and Oxidative Stress in Three Populations of the European Eel Anguilla anguilla L. from Southern Italy. Fishes 2025, 10, 517. https://doi.org/10.3390/fishes10100517
Filice M, Gallo S, Caferro A, Giglio G, Leonetti FL, Milazzo C, Gattuso A, Cerra MC, Barca D, Sperone E, et al. Trace Element Accumulation and Oxidative Stress in Three Populations of the European Eel Anguilla anguilla L. from Southern Italy. Fishes. 2025; 10(10):517. https://doi.org/10.3390/fishes10100517
Chicago/Turabian StyleFilice, Mariacristina, Samira Gallo, Alessia Caferro, Gianni Giglio, Francesco Luigi Leonetti, Concetta Milazzo, Alfonsina Gattuso, Maria Carmela Cerra, Donatella Barca, Emilio Sperone, and et al. 2025. "Trace Element Accumulation and Oxidative Stress in Three Populations of the European Eel Anguilla anguilla L. from Southern Italy" Fishes 10, no. 10: 517. https://doi.org/10.3390/fishes10100517
APA StyleFilice, M., Gallo, S., Caferro, A., Giglio, G., Leonetti, F. L., Milazzo, C., Gattuso, A., Cerra, M. C., Barca, D., Sperone, E., & Imbrogno, S. (2025). Trace Element Accumulation and Oxidative Stress in Three Populations of the European Eel Anguilla anguilla L. from Southern Italy. Fishes, 10(10), 517. https://doi.org/10.3390/fishes10100517










