Pulse Frequency and Water Velocity Determine Crossing Probability in Pulsed Direct-Current Fish Barriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
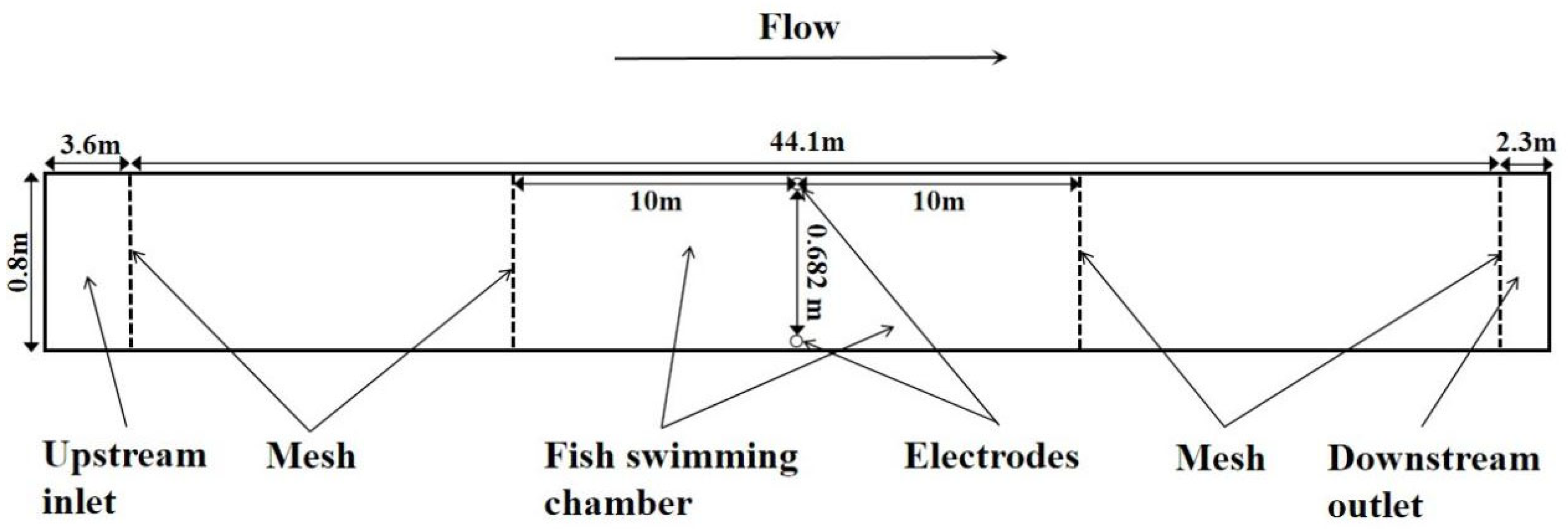
2.2. Apparatus
2.3. Fish Deterrence Testing
2.4. Data Analysis
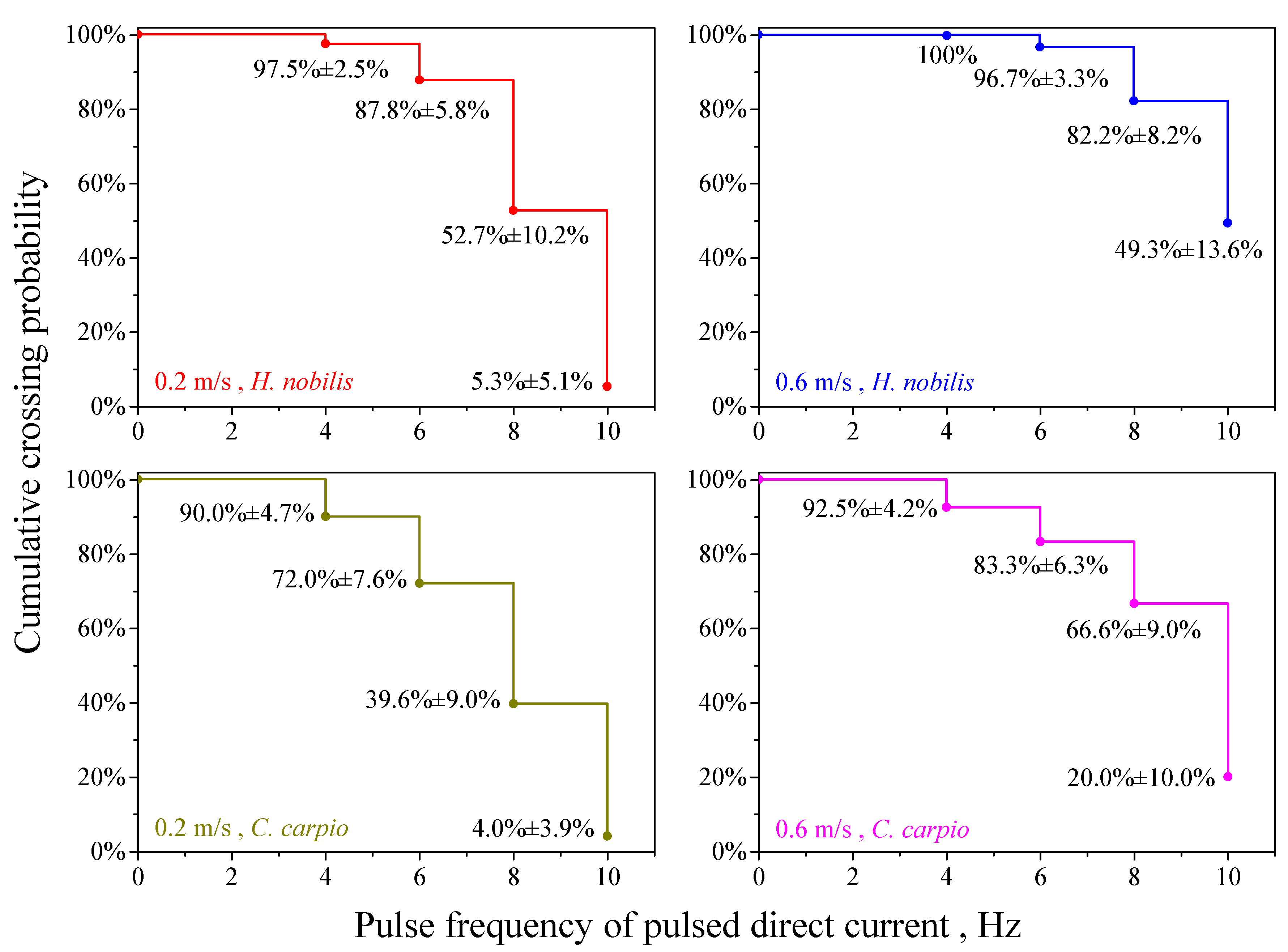
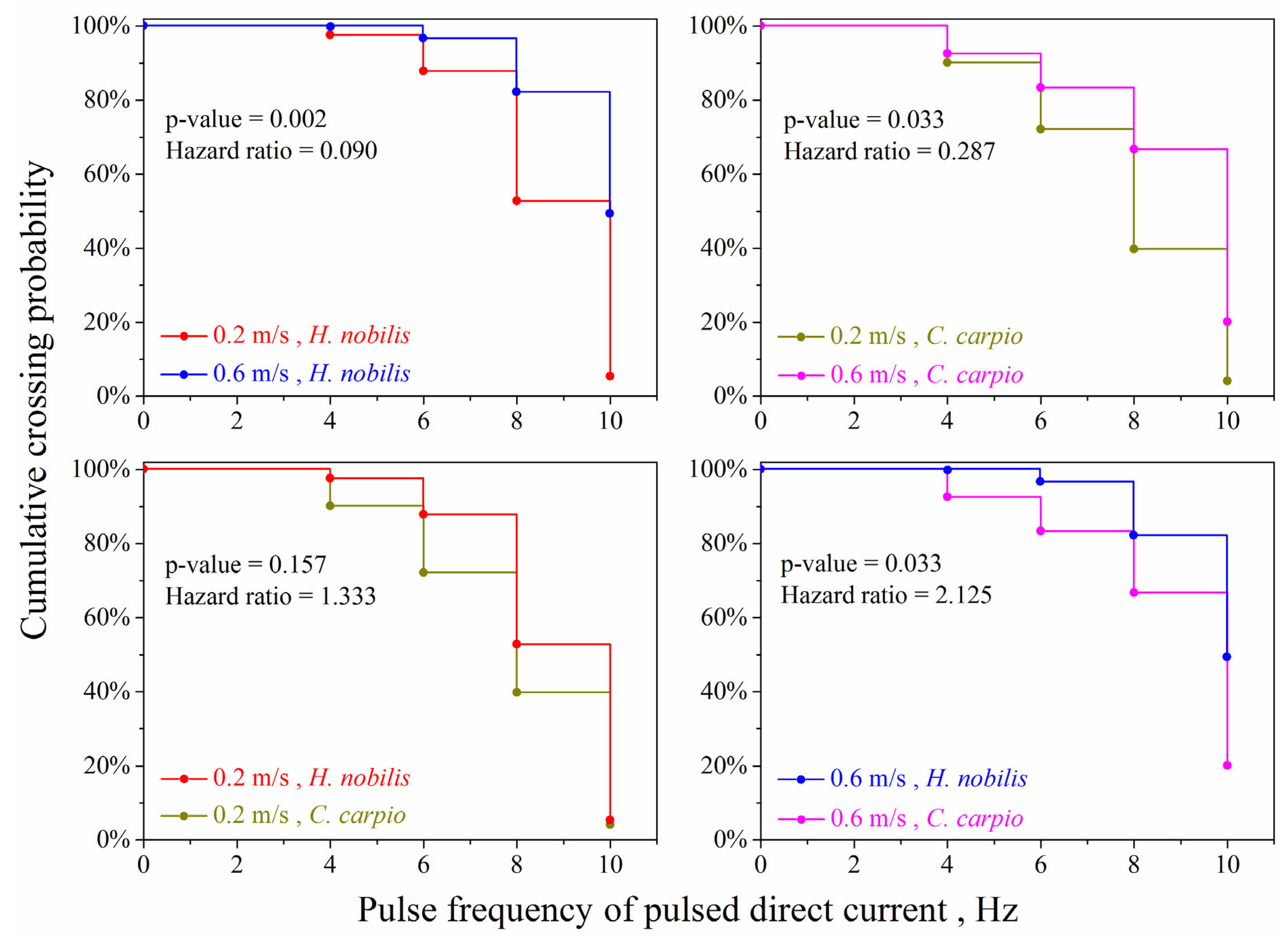
3. Results
4. Discussion
4.1. The Effect of Electric Pulse Frequency
4.2. Effect of Water Velocity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swink, W.D. Effectiveness of an electrical barrier in blocking a sea lamprey spawning migration on the Jordan River, Michigan. N. Am. J. Fish. Manag. 1999, 19, 397–405. [Google Scholar] [CrossRef]
- Kim, J.; Mandrak, N.E. Effects of a vertical electric barrier on the behaviour of rainbow trout. Aquat. Ecosys. Health Manag. 2019, 22, 183–192. [Google Scholar] [CrossRef]
- Miehls, S.; Sullivan, P.; Twohey, M.; Barber, J.; McDonald, R. The future of barriers and trapping methods in the sea lamprey (Petromyzon marinus) control program in the Laurentian Great lakes. Rev. Fish Biol. Fish. 2020, 30, 1–24. [Google Scholar] [CrossRef]
- Stolbunov, I.A.; Salienko, S.N.; Barkhalov, R.M.; Rabazanov, N.I.; Mammaev, M.A.; Salienko, I.S.; Kuptsov, A.A.; Kulikova, Y.A.; Zabotkina, E.A. Influence of electric fields of a fish protection device on fish of the Dagestan coast of the Caspian Sea. Arid Ecosys. 2024, 14, 540–546. [Google Scholar] [CrossRef]
- Johnson, N.S.; Thompson, H.T.; Holbrook, C.; Tix, J.A. Blocking and guiding adult sea lamprey with pulsed direct current from vertical electrodes. Fish. Res. 2014, 150, 38–48. [Google Scholar] [CrossRef]
- Haug, J.; Auer, S.; Frees, C.; Brinkmeier, B.; Tutzer, R.; Hayes, D.S.; Aufleger, M. Retrofitting of existing bar racks with electrodes for fish protection—An experimental study assessing the effectiveness for a pilot site. Water 2022, 14, 850. [Google Scholar] [CrossRef]
- Clarkson, R.W. Effectiveness of electrical fish barriers associated with the Central Arizona Project. N. Am. J. Fish. Manag. 2004, 24, 94–105. [Google Scholar] [CrossRef]
- Conover, G.; Simmonds, R.; Whalen, M. Management and Control Plan for Bighead, Black, Grass, and Silver Carps in the United States; Asian Carp Working Group, Aquatic Nuisance Species Task Force: Washington, DC, USA, 2007; 223p.
- Bajer, P.G.; Hundt, P.J.; Kukulski, E.; Kocian, M. Field test of an electric deterrence and guidance system during a natural spawning migration of invasive common carp. Manag. Biol. Invas. 2022, 13, 204–219. [Google Scholar] [CrossRef]
- Bullen, C.R.; Carlson, T.J. Non-physical fish barrier systems: Their development and potential applications to marine ranching. Rev. Fish Biol. Fish. 2003, 13, 201–212. [Google Scholar] [CrossRef]
- Bajer, P.; Claus, A.; Wein, J.; Kukulski, E. Field test of a low voltage, portable electric barrier to guide invasive common carp into a mock trap during seasonal migrations. Manag. Biol. Inva. 2018, 9, 291–297. [Google Scholar] [CrossRef]
- Dolan, C.R.; Miranda, L.E. Immobilization thresholds of electrofishing relative to fish size. Trans. Am. Fish. Soc. 2003, 132, 969–976. [Google Scholar] [CrossRef]
- Noatch, M.R.; Suski, C.D. Non-physical barriers to deter fish movements. Environ. Rev. 2012, 20, 71–82. [Google Scholar] [CrossRef]
- Layhee, M.J.; Sepulveda, A.J.; Shaw, A.; Smuckall, M.; Kapperman, K.; Reyes, A. Effects of electric barrier on passage and physical condition of juvenile and adult rainbow trout. J. Fish Wildl. Manag. 2016, 7, 28–35. [Google Scholar] [CrossRef]
- Pugh, J.R.; Monan, G.E.; Smith, J.R. Effect of water velocity on the fish guiding efficiency of an electrical guiding system. Fish. Bull. 1970, 68, 307–324. [Google Scholar]
- Borkholder, B.D.; Parsons, B.G. Relationship between electrofishing catch rates of age-0 walleyes and water temperature in Minnesota lakes. N. Am. J. Fish. Manag. 2001, 21, 318–325. [Google Scholar] [CrossRef]
- Johnson, N.S.; Miehls, S. Guiding out-migrating juvenile sea lamprey (Petromyzon Marinus) with pulsed direct current. River Res. Appl. 2014, 30, 1146–1156. [Google Scholar] [CrossRef]
- Parasiewicz, P.; Wisniewolski, W.; Mokwa, M.; Ziola, S.; Prus, P.; Godlewska, M. A low-voltage electric fish guidance system-NEPTUN. Fish. Res. 2016, 181, 25–33. [Google Scholar]
- Parker, A.D.; Glover, D.C.; Finney, S.T.; Rogers, P.B.; Stewart, J.G.; Simmonds, R.L. Fish distribution, abundance, and behavioral interactions within a large electric dispersal barrier designed to prevent Asian carp movement. Can. J. Fish. Aquat. Sci. 2016, 73, 1060–1071. [Google Scholar] [CrossRef]
- Utz, R.M.; Cooper, S.D.; Gido, K.B.; Stewart, J.R. Exclusion of fish and invertebrates from benthic patches of artificial aquatic environments across water conductivity levels using high-frequency (10 Hz) pulses and adjustable electrical settings. Freshw. Sci. 2017, 36, 151–161. [Google Scholar] [CrossRef]
- Soetaert, M.; Boute, P.G.; Beaumont, W.R.C. Guidelines for defining the use of electricity in marine electrotrawling. ICES J. Mar. Sci. 2019, 76, 1994–2007. [Google Scholar] [CrossRef]
- Moldenhauer-Roth, A.; Selz, O.M.; Albayrak, I.; Boes, R.M. Behavioural response of chub, barbel and brown trout to pulsed direct current electric fields. J. Ecohydraul. 2025, 10, 249–264. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, X.; Xie, L.; Liu, G.; Hou, Y.; Li, W.; Zhang, Z.; Shi, X. Experimentally determined effectiveness of different electric barrier arrangements on the behavioural deterrent of silver carp (Hypophthalmichthys molitrix). Appl. Anim. Behav. Sc. 2024, 271, 106172. [Google Scholar] [CrossRef]
- Snyder, D.E. Invited overview: Conclusions from a review of electrofishing and its harmful effects on fish. Rev. Fish Biol. Fish. 2003, 13, 445–453. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Shi, X.; Haro, A. Migratory behavior of adult sea lamprey and cumulative passage performance through four fishways. Can. J. Fish. Aquat. Sci. 2017, 74, 790–800. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Johnson, D.; Tu, Z.; Huang, Y. Effect of body length on swimming capability and vertical slot fishway design. Glob. Ecol. Conserv. 2020, 22, e00990. [Google Scholar] [CrossRef]
- Miller, M.; Sharkh, S.M.; Kemp, P.S. Response of upstream migrating juvenile European eel (Anguilla anguilla) to electric fields: Application of the marginal gains concept to fish screening. PLoS ONE 2022, 17, e0270573. [Google Scholar] [CrossRef]
- Hoover, J.J.; Zielinski, D.P.; Sorensen, P.W. Swimming performance of adult bighead carp Hypophthalmichthys nobilis (Richardson, 1845) and silver carp H. molitrix (Valenciennes, 1844). J. Appl. Ichthyol. 2017, 33, 54–62. [Google Scholar] [CrossRef]
- Tudorache, C.; Viaenen, P.; Blust, R.; DeBoeck, G. Longer flumes increase critical swimming speeds by increasing burst-glide swimming duration in carp Cyprinus carpio, L.J. Fish Biol. 2007, 71, 1630–1638. [Google Scholar] [CrossRef]


| Test Number | Electric Pulse Frequency | Water Velocity | Species | Standard Length, m | Total Length, m | Weight, kg | Percentage (Total Sample = 10) | ||
|---|---|---|---|---|---|---|---|---|---|
| Stunned | Trembled >2 s | Crossed the Barrier | |||||||
| 1 | 4 Hz | 0.2 m/s | H. nobilis | 0.498 ± 0.024 | 0.588 ± 0.029 | 2.160 ± 0.288 | 0 | 0 | 90% |
| 2 | 6 Hz | 0.2 m/s | H. nobilis | 0.512 ± 0.015 | 0.610 ± 0.023 | 2.385 ± 0.199 | 0 | 0 | 70% |
| 3 | 8 Hz | 0.2 m/s | H. nobilis | 0.494 ± 0.020 | 0.583 ± 0.028 | 2.085 ± 0.194 | 0 | 0 | 20% |
| 4 | 10 Hz | 0.2 m/s | H. nobilis | 0.505 ± 0.019 | 0.596 ± 0.022 | 2.100 ± 0.227 | 0 | 20% | 10% |
| 5 | 4 Hz | 0.6 m/s | H. nobilis | 0.500 ± 0.019 | 0.603 ± 0.027 | 2.180 ± 0.255 | 0 | 0 | 100% |
| 6 | 6 Hz | 0.6 m/s | H. nobilis | 0.498 ± 0.019 | 0.594 ± 0.027 | 2.065 ± 0.183 | 0 | 0 | 90% |
| 7 | 8 Hz | 0.6 m/s | H. nobilis | 0.487 ± 0.018 | 0.575 ± 0.026 | 2.070 ± 0.230 | 0 | 0 | 70% |
| 8 | 10 Hz | 0.6 m/s | H. nobilis | 0.494 ± 0.021 | 0.582 ± 0.028 | 2.090 ± 0.202 | 0 | 0 | 60% |
| 9 | 4 Hz | 0.2 m/s | C. carpio | 0.315 ± 0.012 | 0.381 ± 0.015 | 0.653 ± 0.063 | 0 | 0 | 60% |
| 10 | 6 Hz | 0.2 m/s | C. carpio | 0.309 ± 0.014 | 0.369 ± 0.014 | 0.624 ± 0.067 | 0 | 0 | 40% |
| 11 | 8 Hz | 0.2 m/s | C. carpio | 0.317 ± 0.009 | 0.380 ± 0.006 | 0.653 ± 0.058 | 0 | 0 | 10% |
| 12 | 10 Hz | 0.2 m/s | C. carpio | 0.324 ± 0.009 | 0.395 ± 0.011 | 0.682 ± 0.051 | 0 | 10% | 10% |
| 13 | 4 Hz | 0.6 m/s | C. carpio | 0.319 ± 0.009 | 0.389 ± 0.014 | 0.643 ± 0.062 | 0 | 0 | 70% |
| 14 | 6 Hz | 0.6 m/s | C. carpio | 0.320 ± 0.010 | 0.392 ± 0.024 | 0.673 ± 0.047 | 0 | 0 | 70% |
| 15 | 8 Hz | 0.6 m/s | C. carpio | 0.319 ± 0.011 | 0.397 ± 0.017 | 0.666 ± 0.052 | 0 | 0 | 60% |
| 16 | 10 Hz | 0.6 m/s | C. carpio | 0.322 ± 0.007 | 0.400 ± 0.021 | 0.671 ± 0.028 | 0 | 10% | 30% |
| Species | Water Velocity | SCpf | ||
|---|---|---|---|---|
| Frequency Increase from 4 Hz to 6 Hz | Frequency Increase from 6 Hz to 8 Hz | Frequency Increase from 8 Hz to 10 Hz | ||
| H. nobilis | 0.2 m/s | 1.11 | 1.67 | 9.94 |
| H. nobilis | 0.6 m/s | 1.03 | 1.18 | 1.67 |
| C. carpio | 0.2 m/s | 1.25 | 1.82 | 9.90 |
| C. carpio | 0.6 m/s | 1.11 | 1.25 | 3.33 |
| Species | Electric Stimulation Frequency | SCfv (Velocity Increased from 0.2 m/s to 0.6 m/s) |
|---|---|---|
| H. nobilis | 4 Hz | 1.03 |
| H. nobilis | 6 Hz | 1.10 |
| H. nobilis | 8 Hz | 1.56 |
| H. nobilis | 10 Hz | 9.30 |
| C. carpio | 4 Hz | 1.03 |
| C. carpio | 6 Hz | 1.16 |
| C. carpio | 8 Hz | 1.68 |
| C. carpio | 10 Hz | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, W.; Cai, L.; Tan, Y.; Xu, B.; Li, J.; Liu, L.; Xu, L.; Johnson, D.; Zhu, S.; Yang, G. Pulse Frequency and Water Velocity Determine Crossing Probability in Pulsed Direct-Current Fish Barriers. Fishes 2025, 10, 510. https://doi.org/10.3390/fishes10100510
Yi W, Cai L, Tan Y, Xu B, Li J, Liu L, Xu L, Johnson D, Zhu S, Yang G. Pulse Frequency and Water Velocity Determine Crossing Probability in Pulsed Direct-Current Fish Barriers. Fishes. 2025; 10(10):510. https://doi.org/10.3390/fishes10100510
Chicago/Turabian StyleYi, Wanshuang, Lu Cai, Yun Tan, Bo Xu, Jun Li, Lianwei Liu, Lanlan Xu, David Johnson, Shihong Zhu, and Guosheng Yang. 2025. "Pulse Frequency and Water Velocity Determine Crossing Probability in Pulsed Direct-Current Fish Barriers" Fishes 10, no. 10: 510. https://doi.org/10.3390/fishes10100510
APA StyleYi, W., Cai, L., Tan, Y., Xu, B., Li, J., Liu, L., Xu, L., Johnson, D., Zhu, S., & Yang, G. (2025). Pulse Frequency and Water Velocity Determine Crossing Probability in Pulsed Direct-Current Fish Barriers. Fishes, 10(10), 510. https://doi.org/10.3390/fishes10100510





