1. Introduction
Nitrogen (N) plays a central role in aquaculture systems as a key component of proteins, enzymes, and other biological molecules essential for growth and metabolism in all aquatic organisms [
1]. In fed aquaculture systems, nitrogen primarily enters through commercial feed and, to a lesser extent, through fertilizers. However, fish and other cultured organisms typically retain only a portion of the dietary nitrogen [
2]. The surplus is not ingested, egested, or excreted and contributes to nitrogen compounds in the water, such as suspended or dissolved organic nitrogen, ammonia, nitrite, and nitrate. The unassimilated nitrogen is then passed to the aquatic nitrogen cycle, accumulating in different ecological pond compartments, circulating throughout them, or being released into the environment. Accumulated nitrogen compounds can deteriorate water quality and contribute to eutrophication, especially in stagnant ponds. Additionally, under total or partial anaerobic conditions, microbial processes convert reactive nitrogen into nitrogenous gases (N
2, N
2O, NH
3), which go up to the atmosphere [
3,
4]. Thus, understanding the nitrogen budget and the influence of the farmed species on the nitrogen cycle is essential to optimize the use of this nutrient from feed and fertilizers, while reducing the pollution potential [
5].
Integrated Multi-Trophic Aquaculture (IMTA) has emerged as a promising cultivation model to enhance nutrient recycling and minimize waste accumulation in aquaculture. These systems typically consist of one primary fed species and one or more secondary species, also of economic importance, that utilize metabolic waste, uneaten feed, and the aquatic biota. This approach enhances nutrient retention within the system and reduces the release of pollutants [
6,
7]. However, improving the efficiency of integrated systems requires detailed knowledge of nutrient cycling. It is essential to understand how nutrients are distributed in the various ecological compartments within ponds to improve the system and to direct their accumulation to cultivated species [
8]. Only a few studies have mapped the nitrogen distribution in freshwater ponds in neotropical regions. They have demonstrated that most nitrogen accumulates on the pond bottom or is released to the atmosphere as nitrogen gas [
8,
9]. To our knowledge, no nitrogen budgets have been established for neotropical earthen pond production systems that have more than one secondary species. The addition of a third species may take advantage of another complementary characteristic and enhance the utilization of available nitrogen.
Marques et al. [
10] observed that the integrated culture of the fish species yellow-tail lambari (
Astyanax lacustris) and curimbata (striped prochilod,
Prochilodus lineatus) and the Amazon River prawn (
Macrobrachium amazonicum) is more effective and productive than the lambari monoculture and the integrated culture of lambari and prawns. The annual production per hectare increased from 9 t in lambari monoculture to 12 t in the lambari/prawn integrated culture to 16 t in the integrated culture of lambari/prawn/ curimbata, using the same space, water and feed [
10]. The authors hypothesized that the system was efficient in recovering the nutrients dispersed inside the pond, and thus, it should be investigated to confirm the proposition. Therefore, this system represents an interesting model to study the shifts in the nutrient distribution in the pond ecological compartments when one, two or three species with complementary functions are farmed together.
Therefore, the objective of this study was to establish the nitrogen budgets in freshwater earthen ponds, quantifying their accumulation in the multiple ecological compartments to compare the distribution in monoculture of yellow-tail lambari with integrated systems, including the Amazon River prawn and curimbata. In addition, we tested the hypothesis that the efficiency of nitrogen retention in the animal biomass increases progressively with the addition of benthic species with ecological niches that complement the target species.
2. Materials and Methods
2.1. Experimental Design
The experimental cultures were carried out at São Paulo State University, Jaboticabal, Brazil (21°15′22″ S, 48°18′48″ W) from February to April 2019. There were three treatments and four replications, which were completely randomized. The factor tested was the experimental production system with three levels: (1) yellow-tail lambari monoculture (L); (2) integrated culture of the yellow-tail lambari and Amazon River prawn (LP); (3) integrated culture of the yellow-tail lambari, Amazon River prawn and curimbata (LPC). The stocking density was: lambari = 50 ind. m−2, prawn = 25 ind. m−2 and curimbata = 13 ind. m−2. The animals were harvested at 60 days. This was the time for the lambari to reach the commercial size for the live bait or human consumption markets, and for the prawn to reach the size suitable for trading as live bait. The curimbata were marketed as juveniles for restocking.
2.2. Pond Preparation and Management
Twelve earthen ponds were used as experimental units. The ponds have natural bottoms and cover an area of ~0.01–0.02 ha, with a depth of ~1 m. The ponds were drained, left to dry, and then filled with nutrient-rich water; the mean values of nitrogen and phosphorus were 0.53 ± 0.28 and 0.037 ± 0.035 mg L−1, respectively. The ponds were maintained without water renewal throughout the experimental period. Water was only supplied to compensate for loss to evaporation and seepage. The supply water was subjected to mechanical filtration and passed through a 1-mm mesh to prevent the proliferation of aquatic predators and competitors. Netting was used to cover the ponds to reduce outside predation from birds and other animals.
2.3. Stocking and Feed Management
The animals were acclimated and stocked in the ponds according to methods recommended by farmers. A sample of 50 individuals of each species was weighed using an electronic balance (Marte—AS 2000C, Jundiai, Brazil) with a resolution of 0.01 g. The initial individual masses (mean ± standard deviation) were 0.80 ± 0.84 g for the lambari, 1.06 ± 0.24 g for the prawn, and 0.22 ± 0.08 g for the curimbata.
The yellow-tail lambari was the fed species in all of the experimental systems. Each month, a sample of 50 lambaris of each pond was weighed to determine the growth and estimate its biomass inside the ponds. Commercial diet for omnivorous fish Guabi-Aqua Onívoros QS, Guabi Tech Juvenil (Guabi, Campinas, Brazil) based on soybean, corn, and fishmeal was supplied twice daily. The proximate composition was 36% crude protein, 7% lipids, 3% fiber, and 12% mineral matter, and the pellets were 2.3 mm. The initial feeding rate was 10% of the yellow-tail lambari biomass. At 34 days, the lambari in all ponds showed a mean individual mass of 3.8 ± 0.3 g, and the feeding rate was reduced to 5% for all treatments until the end of the experiment. This diet is currently used by lambari producers in Brazil because there is no commercial feed formulated for this species. The prawn and curimbata were left to obtain nutrients from the pond ecosystem and the by-products and residues of the diet supplied to lambari.
2.4. Monitoring of Water Variables
Water quality of the ponds was monitored daily at 8:00 a.m. with a YSI 556 Professional Plus multi-parameter probe (Yellow Springs Instruments, Yellow Springs, OH, USA). The following variables were measured and expressed as mean ± standard deviation: temperature (27.0 ± 1.4 °C to 28.1 ± 1.3 °C), dissolved oxygen (4.5 ± 1.4 mg L−1 to 6.6 ± 1.8 mg L−1), pH (7.9 ± 0.5 to 8.7 ± 0.6), conductivity (133 ± 8 µS.cm−1 to 138 ± 8 µS.cm−1), and atmospheric pressure (713 ± 2 mm Hg). No significant differences were observed between treatments.
The concentration of inorganic plus dissolved organic nitrogen in the water column of each pond was determined every 15 days. Water samples were vacuum-filtered through 0.45 µm pore-size cellulose acetate membrane filters (Millipore type HA, Merck Millipore, Cork, Ireland). Nitrogen content was obtained using oxidation catalytic combustion (Elementar—Vario TOC Select, Langenselbold, Hesse, Germany).
2.5. Harvesting and Production Performance
At the end of the experiment, all the ponds were dried and harvested. The surviving fish and prawn were counted, and a random sample of 100 individuals of each species from each pond was weighed on an electronic balance (Marte—AS 2000C, Jundiai, Brazil; precision 0.1 g) to determine their mean final mass.
The production performance measures of these experimental systems are detailed in Marques et al. [
10]. Growth, survival and yield of each species in separate did not differ between treatments. Final mean individual mass of the lambari was ~7.7 g, survival ranged from ~46 to 56% and yield from ~1.8–2.1 t ha
−1 2 months
−1. The Amazon River prawn reached about 2.6 g, with survival around 80% and yield of about 0.6 t ha
−1 2 months
−1. Curimbatá grew to 6.1 g, with a survival of about 70%, and a yield of 0.5 t ha
−1 2 months
−1.
2.6. Nitrogen Budget
Sample collections occurred every 15 days throughout the experimental period. Samples were obtained from the different ecological compartments of the ponds as described below.
2.6.1. Water
Samples of inlet water during the culture and outlet water during the harvest were collected from all ponds. The pond inlet water was closed for eight hours (8:00 a.m.–4:00 p.m.) every two weeks to determine the volume of replacement water. The inlet water volume is the total volume of the replacement water added to the water used to fill the ponds. The evaporated volume was calculated using data of evaporation obtained from the UNESP Agrometeorological Station, Jaboticabal, and the area of each pond. The volume lost due to seepage was calculated by subtracting evaporation from the volume of replacement water. The nitrogen load of the inlet, outlet and seepage water was obtained by multiplying the nitrogen concentration of the relevant concentration by the total volume of each pond inlet, outlet and water column, respectively. In this way, the estimation of nitrogen loss through seepage was based on the volume of water infiltrated through the pond bottom during the entire production cycle, multiplied by the dissolved organic and inorganic nitrogen concentration in the pond water column. Nitrogen concentrations were obtained using a TOC-N—Elementar analyzer (Elementar–Vario TOC-N Select, Langenselbold, Hesse, Germany) to obtain the total nitrogen.
2.6.2. Feed, Animals, and Sediment
Samples of 5 g of the target species were collected from each pond at the stocking and harvesting. The sampled animals were euthanized by ice and stored frozen for later analysis. For the sediment nitrogen, tripton collectors were used to sample the sediment every 15 days. The collectors comprise six 1.766 L PVC pipes, each of which was 9.7 cm in diameter and 25.4 cm in length. The collectors were filled with brackish water and set inside each pond for 24 h. Then, the accumulated sediment was removed from the tripton collectors and transferred to polyethylene bottles for later analysis. Samples of the diet were also obtained throughout the experimental period. All sampled solid material was dehydrated in a forced air oven (Nova Ética—700-7DE, Vargem Grande Paulista, Brazil) at 60 °C. Then, all material was milled for analysis. Nitrogen content of all solid samples was determined using a CHNS analyzer with thermal conductivity detection (TCD) (Elementar–Vario MACRO Cube, Langenselbold, Hesse, Germany).
2.6.3. Gases
Gas samples were obtained every 15 days of the experiment to determine the absorbed and emitted N
2 and N
2O gases by diffusion and ebullition (bubbles) using methods described in David et al. [
8] and Flickinger et al. [
9]. Diffusion at the water surface was measured using a diffusion chamber during the day and at night. Fiberglass funnels were placed on the water surface to capture gas bubbles over a period of 24 h. A graduated bottle was connected at the extremity of each funnel to trap the gas bubbles. The gas samples from both methods were transferred to pressurized gas vials for later analysis by gas chromatography (Shimadzu Instruments–GC-2014 Permanent Gas Analyzer, Kyoto, Japan) with TCD (Thermal Conductivity Detector) and FID (Flame Ionizer Detector) to measure N
2, and Electron Capture Detector (ECD) to quantify N
2O.
2.7. Budget Calculation
Nitrogen contents of the input and output compartments were summed. The total nitrogen output (TO) was subtracted from the total input (TI). The result corresponds to the unaccounted portion (UP). The equations used were
in which, IW (inlet water), CF (commercial feed), SL (stocked lambari), SA (stocked Amazon river prawns), and SC (stocked curimbata), and AG (absorbed gases), refer to the nitrogen content of the input compartments; and OW (outlet water), SW (seepage water), HL (harvested lambari), HA (harvested Amazon river prawn), HC (harvested curimbata), S (sediment), and EG (emitted gases) refer to the nitrogen content of the outlet compartments.
2.8. Statistical Analysis
All variables were tested for normality and homoscedasticity using the Shapiro–Wilk and Brown-Forsythe tests, respectively. No deviations from normality and homoscedasticity were detected, and thus, the data were subjected to analysis of variance (ANOVA) by F-test [
11]. Upon detecting significant differences (
p < 0.05), the means were compared using the Fisher-LSD test. Analyses were performed on the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA, version 9.0).
3. Results
Sediment accumulated on the pond bottoms was higher in the IMTA systems than in the monoculture, while seepage water was lower in the IMTA ponds (
Table 1). The mean dissolved nitrogen in the water column (about 450 µg L
−1) and the nitrogen content in the sediment (about 1%) did not differ between treatments. The nitrogen content in the body biomass of the animals did not differ between treatments; however, it increased within the culture for lambari and curimbatá. It accounted for approximately ~7% in lambari and prawn, and about ~11% in curimbatá (
Table 1).
Nitrogen loads varied among the culture systems in some input and output compartments (
Table 2). Significant differences (
p < 0.05) were observed in nitrogen from inlet water and the N
2O absorption was zero in the LPC treatment. The lambari monoculture (L) received the highest inlet water nitrogen load (76.0 ± 23.2 kg N ha
−1), whereas the LP and LPC treatments presented lower values (44.4 ± 4.2 and 47.7 ± 8.6 kg N ha
−1, respectively). N
2O absorption was null in the LPC, significantly lower than in the L (0.05 ± 0.08 kg N ha
−1) and the LP (0.07 ± 0.10 kg N ha
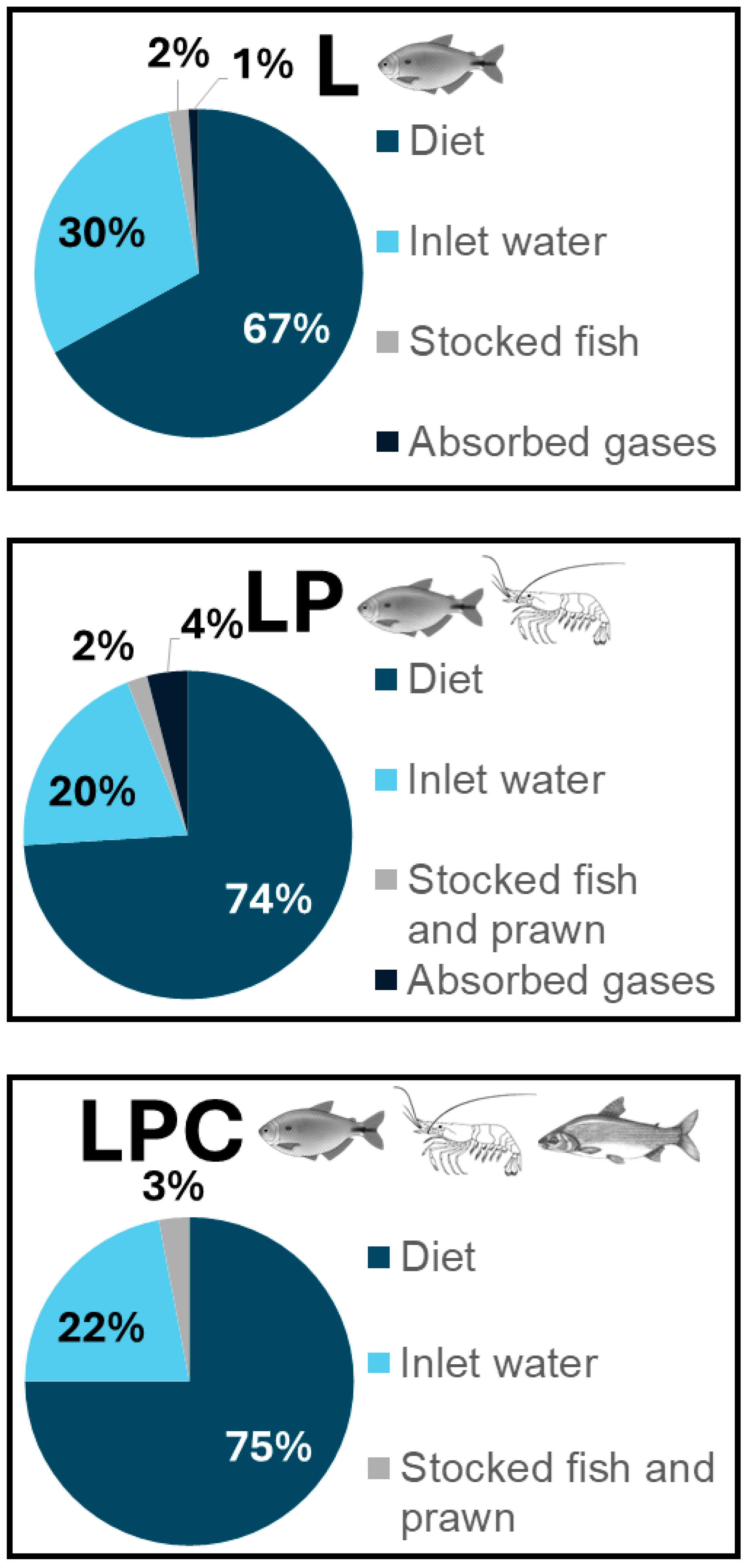
−1) treatments. Feed nitrogen represented the major input in all treatments (67% in L, 74% in LP, 75% in LPC), while inlet water contributed 30% (L), 20% (LP), and 22% (LPC) (
Figure 1). Nitrogen from stocked animals and absorption of atmospheric nitrogen was negligible, totaling less than 5% of the inputs across all systems.
Output also differed between treatments. Outlet water nitrogen loads were consistently low (5.4 ± 1.0 to 7.7 ± 2.0 kg N ha
−1) and several times lower than the nitrogen input via inlet water (
Table 1). Seepage was a more prominent pathway for nitrogen loss. The L (70.5 ± 22.9 kg N ha
−1) was significantly higher (
p < 0.05) than in the LP (39.6 ± 4.4 kg N ha
−1) but neither showed significant difference from the LPC (42.8 ± 7.9 kg N ha
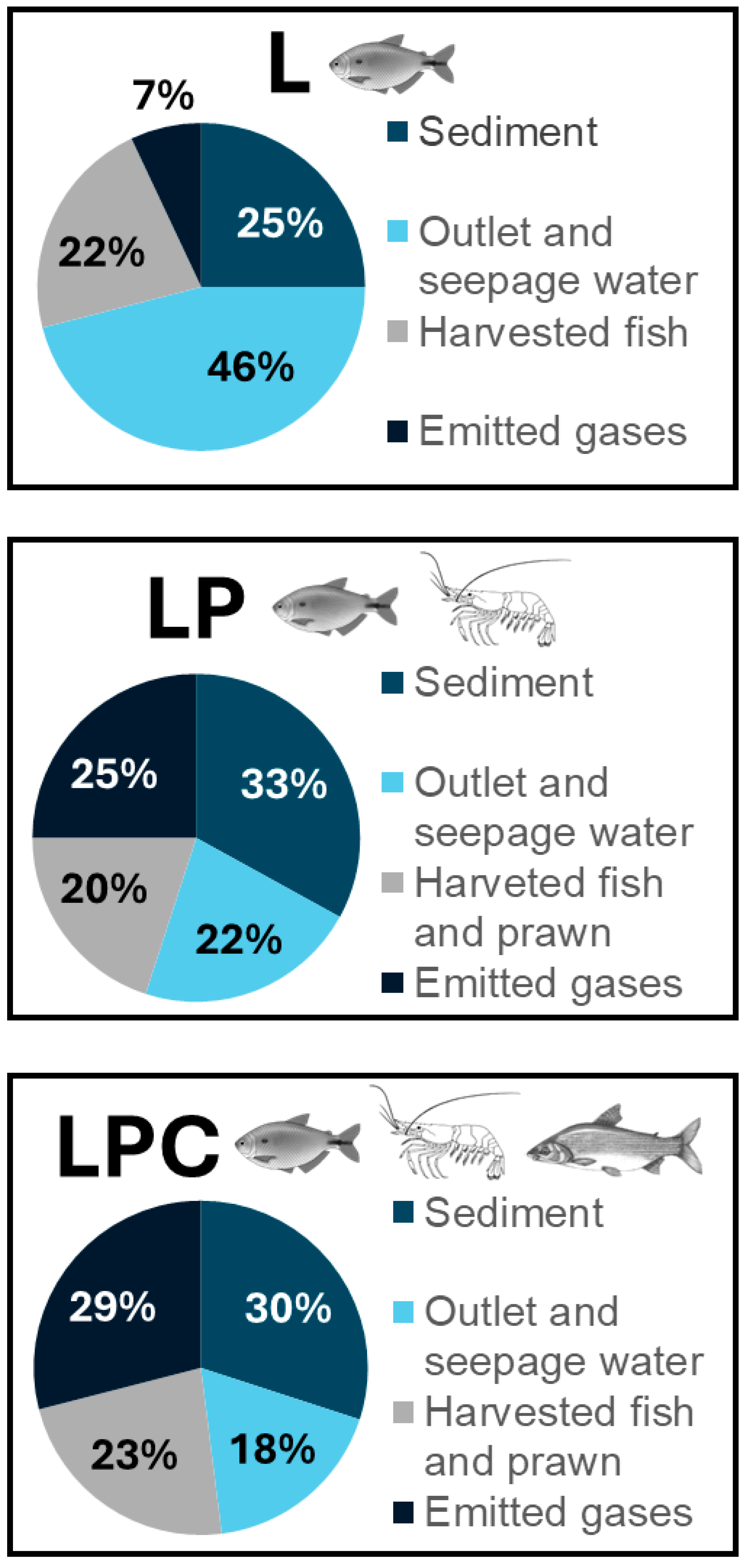
−1). Harvested biomass accounted for 22%, 20%, and 23% of the total outputs in L, LP, and LPC, respectively (
Figure 2). Nitrogen in harvested lambari was increased in the LPC (43.2 ± 7.4 kg N ha
−1) when compared to the L (36 ± 8.6 kg N ha
−1) and LP (33 ± 5.6 kg N ha
−1) treatments. Prawn biomass was 9 ± 2.3 kg N ha
−1 in LP and 7.1 ± 0.8 kg N ha
−1 in LPC, while curimbata accounted for 13.2 ± 3.4 kg N ha
−1 in LPC. Sediment was the second major nitrogen sink, with accumulation significantly higher (
p < 0.05) in the integrated cultures (82.7 ± 15.2 and 69.1 ± 12.3 kg N ha
−1 for LPC and LP, respectively) than in the lambari monoculture (41.8 ± 12.6 kg N ha
−1). Nitrogen gas emissions were dominated by N
2 ebullition, reaching 72.1 ± 32.4 kg N ha
−1 in LPC, significantly higher (
p < 0.05) than L (10.4 ± 10.6 kg N ha
−1), and both showed no difference to the LP (52.5 ± 40.1 kg N ha
−1). Diffusive fluxes and N
2O emissions remained minor across all systems.
Nitrogen-use efficiency in harvested biomass is presented in
Table 3. The proportion of dietary nitrogen incorporated by lambari was relatively stable across treatments, ranging from 20 ± 4.3% in L to 19 ± 4.8% in LP and 24 ± 5.4% in LPC. Although prawns and curimbata did not receive feed directly, they assimilated nitrogen from residual and autochthonous sources in the integrated systems, showing recoveries of 4.5 ± 1.5% (prawn in LP), 3.2 ± 0.7% (prawn in LPC), and 7.8 ± 1.5% (curimbata in LPC). Consequently, the total recovery of dietary nitrogen increased from 20 ± 4.3% in L to 23 ± 5.2% in LP and 35 ± 5.9% in LPC. When considering all nitrogen inputs, including feed, inlet water, stocked animals, and absorbed atmospheric gases, the total nitrogen recovery in the harvested biomass rose from 13 ± 2.2% in L to 17 ± 4.5% in LP and 26 ± 2.0% in LP.
4. Discussion
The results of this study showed that the addition of Amazon River prawn and curimbata, two bottom-feeding species, optimized the use of nitrogen available in the system. The assimilation of this nutrient into the biomass of economically valuable species steadily increased with the addition of the second and third species. In the integrated culture with three species (LPC), the mean total nitrogen incorporated into the biomass was 63 ± 9 kg ha−1, decreasing to 42 ± 6 kg ha−1 in the two-species integrated culture (LP) and to 36 ± 9 kg ha−1 in the monoculture (L). Additionally, the epibenthic species increased the escape of nitrogen into the atmosphere as N2, which is an inert gas. Nitrogen input patterns were similar in all culture systems. Feed nitrogen represented the primary source (67–75%), inlet water was the second (20–30%), while nitrogen from stocked animals and absorption of atmospheric gas accounted for less than 5% of the total inputs. The main nitrogen output route in all systems was sediment accumulation (25–33%), followed by harvested biomass (20–23%) and gas emissions (7–29%). Nitrogen loss through seepage was highest in the monoculture (70.5 ± 22.9 kg N ha−1), while outlet water remained a minor pathway across all culture systems (<3%).
The nitrogen content of the stocked animals represented a small fraction of the total nitrogen inputs (<5% across all treatments), whereas the harvested animals accounted for a noteworthy share of the nitrogen outputs. The efficiency of nitrogen assimilation from all input sources increased progressively with the addition of species, from 13 ± 2.2% in the lambari monoculture to 17 ± 4.5% in the lambari–prawn system, reaching 26 ± 2% in the three-species polyculture. The values obtained for the two-species IMTA are slightly lower than those reported for other integrated systems using the Amazon River prawn, such as the prawn–tilapia (~21–24%) [
8] and prawn–tambaqui systems (~29%) [
9]. In the present study, prawns were stocked at an average initial weight of 1.06 ± 0.24 g and showed a nitrogen accumulation of 9 ± 2.3 kg N ha
−1 in LP at 60 days. This value is lower than in previous reports, likely due to the shorter culture period and the lower final biomass. Nonetheless, the results affirm the ability of prawns to assimilate nutrients and their contribution to the overall efficiency of the integrated system. The improved nitrogen retention observed in LPC confirmed the previous hypothesis [
8,
9] that the pond sediments of pelagic fish farming have enough nutrients to support more than one bottom-feeding species. Two species with different feeding habits, such as the Amazon River prawn and the curimbata, exploit partially distinct niches: the prawn consumes detritus and benthic invertebrates, while the curimbata grazes on organic matter and periphyton. This complementary use of resources reduces competition and enhances nutrient recovery beyond what would be expected from additive effects alone.
The efficiency of feed nitrogen utilization increased with the inclusion of epibenthic species, reaching 35 ± 5.9% in the LPC treatment, significantly higher than in the LP (23 ± 5.2%) and L (20 ± 4.3%). Although these values represent only a portion of the total nitrogen input, they demonstrate improved recovery of feed nitrogen in integrated systems. This efficiency is similar to ~28–35% observed in prawn–tilapia systems [
8] and ~30% observed in polyculture of Chinese carps (
Cyprinus carpio var.
specularis,
Aristichthys nobilis and
Hypophthalmichthys molitrix) [
12]. However, it was higher than the 15% observed in the culture of giant gourami (
Osphronemus goramy) [
13] and lower than the ~52% reported in tambaqui and Amazon River prawn IMTA [
9]. The consistent increase across treatments of the present study supports the hypothesis that benthic species enhance nutrient retention. The improved nitrogen assimilation observed in LPC is perhaps due to the prawns and curimbata feeding on organic matter and nutrients that would otherwise be lost to the sediment or water column. Thus, the integrated culture with lambari, Amazon River prawn, and curimbata represents an effective strategy to retain feed nitrogen and reduce nutrient waste in pond aquaculture.
The proportion of feed nitrogen was ~75% of all inputs and was consistent with previous findings in freshwater aquaculture, which is usually higher than 70%. Previous research showed feed nitrogen proportions of ~70% in IMTA of Nile tilapia and Amazon river prawn [
8], ~88% for catfish (
Ictalurus punctatus) [
14], 91% for tilapia (
Oreochromis niloticus) [
15], 97% for tambaqui (
Colossoma macropomum) [
16], ~95–97% for giant river prawn [
17,
18] and 93% in polyculture of Chinese carps (
Cyprinus carpio var.
specularis,
Aristichthys nobilis and
Hypophthalmichthys molitrix) [
12]. Flickinger et al. [
9] observed lower values (29–46%) and attributed this result to the high nitrogen concentration of the source water. Nevertheless, the variations observed in the literature are likely due to different concentrations of nitrogen in the source water, feeding strategies and the unaccounted fractions. The results of the present study affirm that feed is the dominant nitrogen input in fed semi-intensive aquaculture.
Inlet water was the second major nitrogen input, contributing ~20–30% of the nitrogen inputs for all the treatments. The inlet water showed elevated nitrogen concentrations (0.53 ± 0.28 mg L−1) because it was sourced from a reservoir that receives effluents from other aquaculture activities. In contrast, nitrogen losses via outlet water were similar among treatments and were considered minimal, ranging from 5.4 ± 1.0 to 7.7 ± 2.0 kg N ha−1. These findings indicate that a considerable proportion of the nitrogen introduced through inlet water and diet was retained within the ponds, in the subjacent soil or transformed into molecular nitrogen and eliminated to the atmosphere, reinforcing the role of aquaculture ponds as a tool to mitigate pollution and possible nitrogen sinks. Investigations should be conducted to assess if part of the seepage nitrogen reaches aquifers and pollutes them. Nitrogen loads from inlet water varied significantly across treatments, with the highest input in the lambari monoculture system (L; 76.0 ± 23.2 kg N ha−1), and lower values in the LP (44.4 ± 4.2 kg N ha−1) and LPC (47.7 ± 8.6 kg N ha−1) systems. The elevated inlet water nitrogen in monoculture resulted from the higher water demand to maintain pond levels due to seepage. It is likely that this load of nitrogen was lost to the underlying soil in seepage water.
The loss of water through seepage was significantly lower in ponds with the integrated cultures. Similar results were recently observed in the IMTA culture of tambaqui and curimbata [
19]. Reduction in hydraulic conductivity in water-saturated and sub-saturated soils is generally due to the physical entrainment of organic and inorganic particles in the pores, followed by the growth of slime-forming microorganisms and biofilm [
20]. Additionally, the entrapment of gas bubbles, such as methane produced by methanogenic microorganisms, may clog soil pores and contribute to decreasing hydraulic conductivity [
21,
22]. Likely, the bioturbation caused by prawns and curimbata led to a resuspension of fine particles, which then returned to the water column and settled again. This movement causes fine particles such as clay, silt, and organic matter to be redistributed and deposited in pores, crevices and channels through which water infiltrates. This process mechanically reduces sediment permeability, which slows the percolation rate into the aquifer [
23]. Thus, bioturbation and subsequent deposition can create a nearly impermeable silt layer, reducing infiltration. The movement of particles and oxygenation of sediment along the pond bottom caused by bioturbation [
24] fosters a favorable environment for the growth of biofilm composed of bacteria, algae, and fungi in the sediment and soil pores, as well as the production of gases, leading to a clogging effect.
The losses of nitrogen due to seepage were determined with the assumption that the seepage water had the same nitrogen concentration as the pond water. This approach aligns with practices in other nutrient budget studies [
14,
15,
25]. However, the decomposition of organic matter on the pond bottom surface and within soil pores can increase the amount of nitrogen compounds that can leach to deeper layers. Particulate organic nitrogen tends to settle in the upper sediment layers and is less likely to infiltrate deeper soil strata [
1,
3] but is subjected to decomposition. Liu et al. [
26] highlight that microbial activity and organic matter content near the sediment-water interface may influence nitrogen mobility. Boyd [
1] emphasizes that seepage in unlined earthen ponds can result in significant nitrogen transport, particularly of dissolved forms such as ammonia and nitrate, but also notes that particulate matter behavior varies with soil structure, particle size, and hydraulic conductivity. Muendo et al. [
27] observed that the nitrogen concentration in seepage water was significantly higher than in the pond water column in freshwater ponds. These authors stated that nitrogen loss to the soil would be underestimated by a factor of 6.5 over four months when using pond water nitrogen concentration. Considering this complexity, the values obtained in the present study, although useful, are likely to be inaccurate and may be underestimated. Research measuring the pore concentration of nitrogen directly should be conducted to better estimate the nitrogen in seepage water. Despite the limitations of the method used in the present study, it was useful to demonstrate, by empirical data, that the loss of nitrogen in seepage water is substantial and cannot be neglected.
Nitrogen accumulation in the sediment accounted for a noteworthy portion of the total output, ranging from 25% in monoculture (L) to 33% and 30% in LP and LPC, respectively. These values are lower than those reported in other freshwater culture systems, such as 57–66% in giant gourami (
Osphronemus goramy) monoculture [
13], ~47% in the integrated culture of Indian major carps (
Catla catla,
Labeo rohita, and
Cirrhinus mrigala) and giant river prawn (
Macrobrachium rosenbergii) [
25] and similar to ~24% in Nile tilapia and Amazon river prawn cultures [
8]. The values obtained in the present study remain within the expected range, given the variability in sediment dynamics and nitrogen losses through volatilization [
9]. Nitrogen retained in the sediment was significantly higher in the integrated cultures (69.1 ± 12.3 kg N ha
−1 in LP and 82.7 ± 15.2 kg N ha
−1 in LPC) when compared to the monoculture (41.8 ± 12.6 kg N ha
−1), likely due to the higher quantity of sediment deposited in those ponds. This indicates that the presence of benthic species may enhance sedimentary nitrogen, which probably results from the trophic cascade produced by the liberation of nutrients to the water column by bioturbation [
9]. These nutrients enhance photosynthesis, transforming inorganic carbon into phytoplankton biomass, which also increases the zooplankton biomass. These organisms die and settle at the pond bottom, increasing the sediment layer and the availability of energy and nutrients to the bottom-feeding species. This mechanism is supported by the higher nitrogen load observed in harvested lambari in the LPC (43.2 ± 7.4 kg N ha
−1) as compared to the L (36 ± 8.6 kg N ha
−1) and LP (33 ± 5.6 kg N ha
−1), of which the lambari consumes zooplankton. These findings underscore the potential of integrated systems to enhance nitrogen recovery within the pond ecosystem. Furthermore, sediment nitrogen accumulated in the pond bottom during production can be recovered post-harvest and reused in agriculture, supporting circular economic strategies in aquaculture [
8].
Nitrogen gas emissions were primarily through the ebullition of N
2, especially in integrated systems, while diffusion of N
2 and N
2O were negligible for all treatments. This pattern is consistent with findings from David et al. [
8] and Flickinger et al. [
9], which also observed noteworthy N
2 ebullition. Biogeochemical and ecological factors influence nitrogen gas emissions as well as the ratio between diffusion and ebullition. Ebullition is inherently heterogeneous in the ponds because it is affected by the spatial distribution of organic matter, microbial community composition, and redox gradients within the sediment [
28,
29]. The significantly higher N
2 ebullition observed in the LPC (72.1 ± 32.4 kg N ha
−1) system when compared to the monoculture (L; 10.4 ± 10.6 kg N ha
−1) affirms that the bioturbation activities of Amazon River prawn and curimbata stimulate denitrification. The LP (52.5 ± 40.1 kg N ha
−1) showed a noteworthy increase from the monoculture as well. Bioturbation oxygenates the upper layers of sediment, favoring the nitrification, which converts the ammonia-nitrogen resulting from aerobic decomposition into nitrates. Nitrates are subjected to anaerobic microbial processes that convert them into molecular nitrogen (N
2) in the anoxic layer beneath the oxic layer. The epibenthic activities of prawn and curimbata create favorable microsites for coupled nitrification–denitrification processes thereby enhancing the transformation of reactive nitrogen into inert gas [
30,
31]. This ecosystem service plays a crucial role in mitigating the accumulation of nitrogenous compounds in pond environments and contributes directly to the improved environmental performance of integrated aquaculture systems [
3,
9].
Imbalances are generally expected in pond nutrient budgets. This may result from a combination of minor methodological errors or some overlooked compartments [
8,
9]. In the present study, the lambari monoculture (L) exhibited a positive nitrogen balance, with inputs exceeding outputs by 80 ± 13 kg N ha
−1, suggesting the occurrence of unaccounted nitrogen losses. This may result from the underestimate of the leaching nitrogen to soil, emission of ammonia to the atmosphere, the immigration of insects that have aquatic phases that leave the system after consuming nitrogen to grow, predation of the fish by birds, nitrogen uptake by other colonizing species that died at harvest but were not measured, or other unmeasured processes. In contrast, the integrated systems LP and LPC presented negative nitrogen budgets (−3.6 ± 63 and −58 ± 27 kg N ha
−1, respectively), indicating the presence of additional, unmeasured nitrogen inputs, which include the fall of leaves, flowers, and dust within the ponds; feces of aquatic birds and sediment-bound nitrogen present in the soil before ponds were filled. Several factors contributing to the imbalances mentioned above were observed during the experiment; however, they were not measured. Residual nitrogen from previous production cycles can accumulate in unlined ponds in deeper, anoxic sediment layers. The bioturbation carried out by epibenthic species may disturb these deposits, increase bioavailability of buried nitrogen and reintroduce it into the pond nitrogen cycle [
26,
32]. The LPC treatment showed a noteworthy decrease from the LP treatment. This is perhaps due to the presence of two epibenthic species and their greater potential to mobilize buried nitrogen when compared to the bioturbation of one epibenthic species. Each species has a specific niche and food habit; thus, an additive effect is expected.
The combined culture of lambari and curimbata without prawns was not assessed in the present study, which may be a limitation in fully understanding curimbata’s effect on the nitrogen budget in the absence of prawns. The objective was to evaluate whether nitrogen retention efficiency steadily increases with the addition of complementary benthic species, rather than all pairwise combinations. Additionally, there is a practical constraint due to the shortage of ponds at the research station and challenges in managing four extra ponds. Reducing the number of replicates to add another treatment could make it harder to detect treatment effects [
33,
34]. The hypothesis that curimbata alone might produce the effects seen in the LPC treatment is unlikely because its trophic niche is different from that of the prawn. This should be investigated in further studies. Even so, using three species may be more appealing to farmers because it allows diversification of production and markets, boosting economic sustainability [
35].
This study quantified nitrogen accumulation in the ecological compartments of freshwater ponds stocked with yellow-tail lambari and evaluated the impact of integrating Amazon River prawn and curimbata on nitrogen cycling. This combination of species has been shown to be advantageous, and the use in commercial farms seems to be technically feasible [
10]. Feed was the main nitrogen input, and sediment was the primary sink, but the inclusion of benthic species significantly improved nitrogen retention in harvested biomass and altered output pathways. In the three-species integrated culture (LPC), the proportion of dietary nitrogen incorporated into biomass more than doubled compared to the monoculture, while gaseous nitrogen losses, particularly via N
2 ebullition, became the dominant nitrogen output, replacing seepage as the main loss route. These shifts are attributable to bioturbation, which enhances nutrient cycling at the sediment–water interface and can mobilize previously unavailable nitrogen. Additionally, the negative nitrogen budget observed in the integrated culture with the bioturbation of two species suggests the remobilization of nitrogen from past production cycles, further contributing to nutrient recovery and denitrification. These findings reinforce the complexity of nitrogen dynamics in pond aquaculture and highlight the critical role of sediment interactions and bioturbation in shaping nutrient flows and budgets.