Enhancing Aquaculture Productivity via Polyculture with Colossoma macropomum: A Focus on Two Native Amazon Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consent
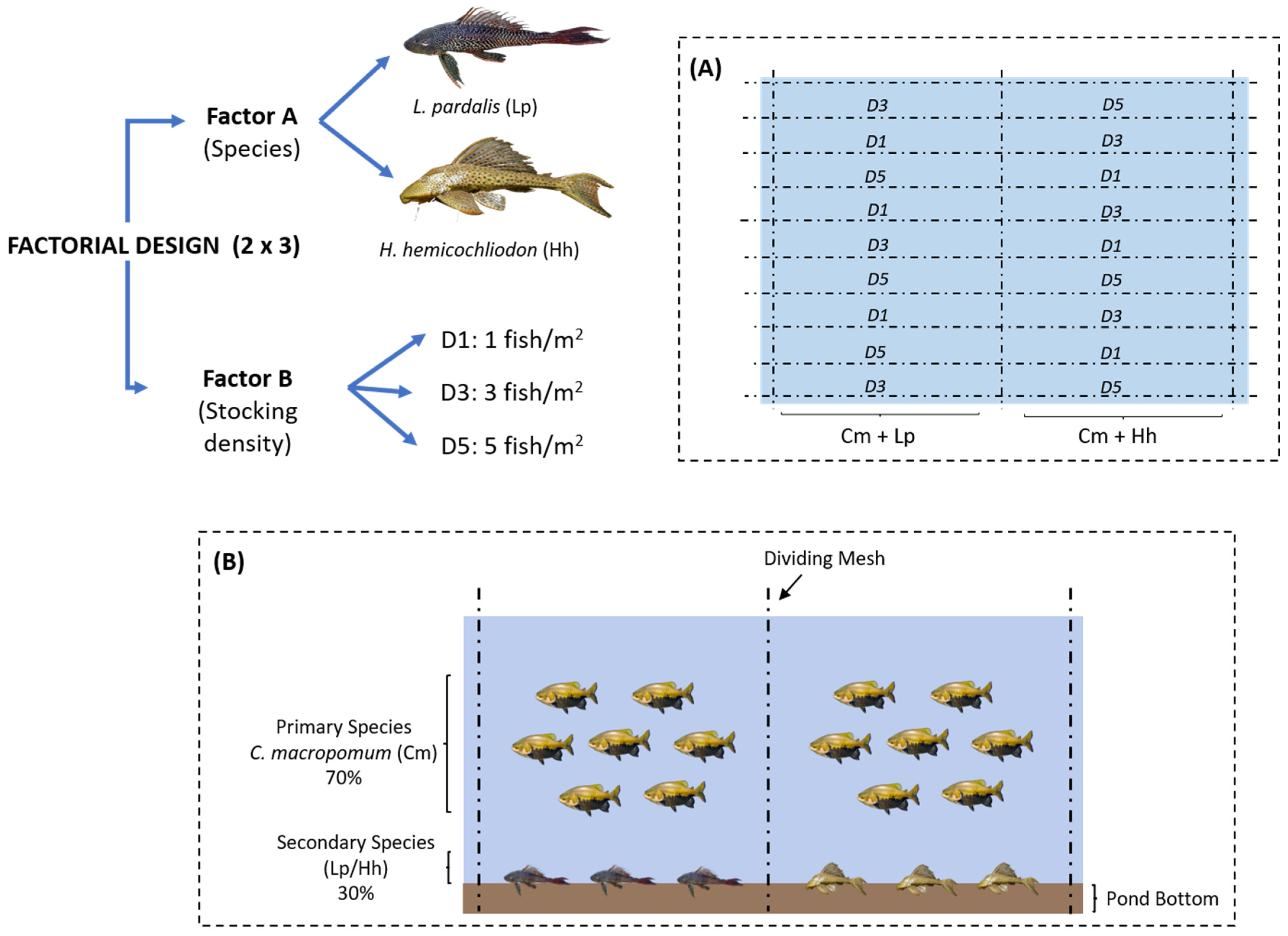
2.2. Obtaining Biological Material and Experimental Design
2.3. Stocking Densities, Feeding, and Sampling of Biological Material
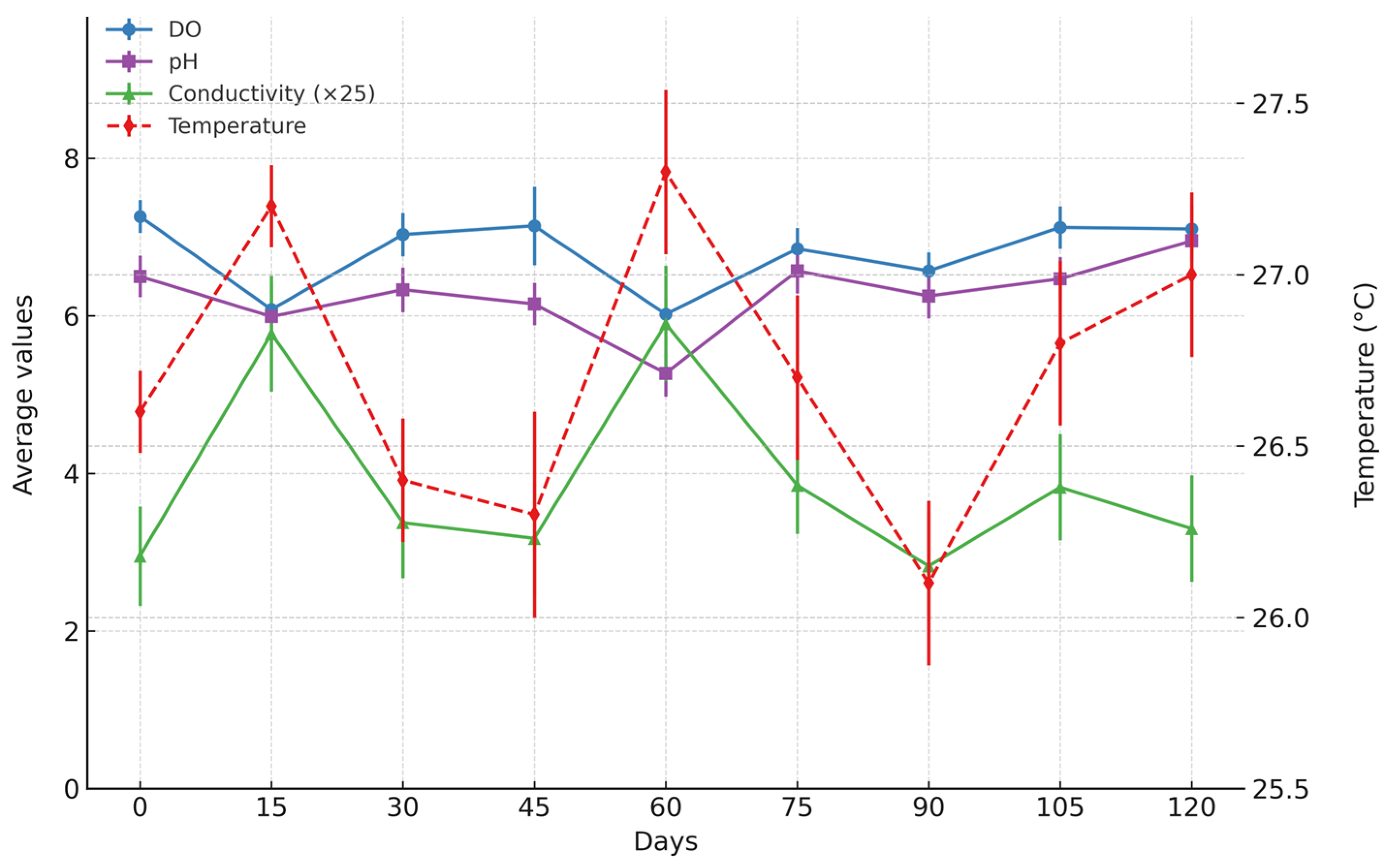
2.4. Water Physical and Chemical Parameters
2.5. Evaluation of Productive Performance
2.6. Statistical Analysis
3. Results
3.1. Water Physicochemical Parameters
3.2. Productive Performance
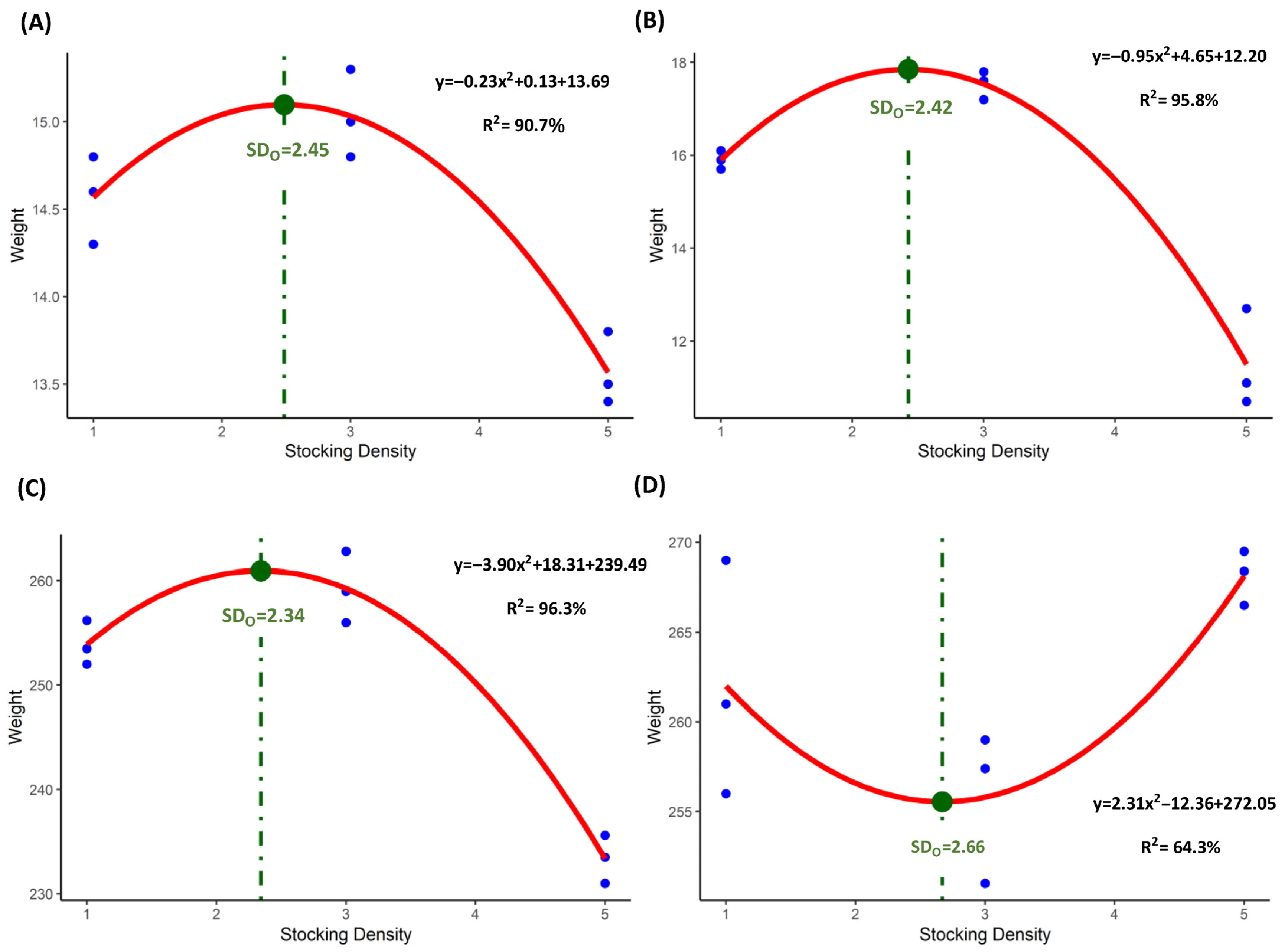
3.3. Optimum Stocking Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milstein, A. Ecological Aspects of Fish Species Interactions in Polyculture Ponds. Hydrobiologia 1992, 231, 177–186. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Quevedo, R.M.; Kreutz, L.C.; Ritter, F.; Pandolfo, A.; Hemkemeier, M.; Colla, L.; Silva, L.B.; Koakoski, G.; da Rosa, J.G.S. Comparative Analysis of Different Fish Polyculture Systems. J. World Aquac. Soc. 2012, 43, 778–789. [Google Scholar] [CrossRef]
- Nekrasova, O.; Pupins, M.; Tytar, V.; Fedorenko, L.; Potrokhov, O.; Škute, A.; Čeirāns, A.; Theissinger, K.; Georges, J.Y. Assessing Prospects of Integrating Asian Carp Polyculture in Europe: A Nature-Based Solution under Climate Change? Fishes 2024, 9, 148. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de la Pesca y la Acuicultura; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- FAO. La Sostenibilidad en Acción; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Apine, E.; Ramappa, P.; Bhatta, R.; Turner, L.M.; Rodwell, L.D. Challenges and Opportunities in Achieving Sustainable Mud Crab Aquaculture in Tropical Coastal Regions. Ocean Coast. Manag. 2023, 242, 106711. [Google Scholar] [CrossRef]
- Jhingran, V.G. Systems of Polyculture of Fishes in the Inland Waters of India. J. Fish. Board Can. 2011, 33, 905–910. [Google Scholar] [CrossRef]
- He, L.; Xi, J.; He, J.; Lin, Z. Energy Flow Analysis of in Litopenaeus Vannamei-Tegillarca Granosa Polyculture Ponds Based on EwE Model. Aquac. Rep. 2023, 33, 101790. [Google Scholar] [CrossRef]
- Wang, M.; Lu, M. Tilapia Polyculture: A Global Review. Aquac. Res. 2016, 47, 2363–2374. [Google Scholar] [CrossRef]
- New, M.B.; Valenti, W.C. Tilapia–Macrobrachium Polyculture. In Tilapia in Intensive Co-Culture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 156–185. [Google Scholar] [CrossRef]
- Amoussou, N.; Thomas, M.; Pasquet, A.; Lecocq, T. Finding the Best Match: A Ranking Procedure of Fish Species Combinations for Polyculture Development. Life 2022, 12, 1315. [Google Scholar] [CrossRef]
- Hossain, M.E.; Khan, M.A.; Dey, M.M.; Alam, M.S. Insights of Freshwater Carp Polyculture in Bangladesh: Inefficiency, Yield Gap, and Yield Loss Perspectives. Aquaculture 2022, 557, 738341. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Petropoulos, G.; Barbieri, R. Polyculture Rearing of Cyprinus carpio (L.) and Oreochromis aureus (St.) Using a Closed Circulated System. Aquaculture 1992, 103, 311–320. [Google Scholar] [CrossRef]
- Orina, P.S.; Charo-Karisa, H.; Munguti, J.M.; Boera, P.; Abwao, J.; Kyule, D.; Opiyo, M.; Marcial, H.; Manyala, J.; Rasowo, J.O. A Comparative Study of Labeo victorianus (Bouelenger, 1901) and Oreochromis niloticus (Linnaeus, 1758) Grown in Polyculture Systems. Lakes Reserv. 2018, 23, 56–62. [Google Scholar] [CrossRef]
- Shrestha, M.K.; Bhandari, M.P.; Diana, J.S.; Jaiswal, R.; Mishra, R.N.; Pandit, N.P. Positive Impacts of Nile Tilapia and Predatory Sahar on Carp Polyculture Production and Profits. Aquac. Fish. 2018, 3, 204–208. [Google Scholar] [CrossRef]
- Hernández, M.; Gasca-Leyva, E.; Milstein, A. Polyculture of Mixed-Sex and Male Populations of Nile tilapia (Oreochromis niloticus) with the Mayan cichlid (Cichlasoma urophthalmus). Aquaculture 2014, 418–419, 26–31. [Google Scholar] [CrossRef]
- Hossain, M.A.; Islam, M.S. Optimization of Stocking Density of Freshwater Prawn Macrobrachium rosenbergii (de Man) in Carp Polyculture in Bangladesh. Aquac. Res. 2006, 37, 994–1000. [Google Scholar] [CrossRef]
- Uddin, M.S.; Rahman, S.M.S.; Azim, M.E.; Wahab, M.A.; Verdegem, M.C.J.; Verreth, J.A.J. Effects of Stocking Density on Production and Economics of Nile tilapia (Oreochromis niloticus) and Freshwater Prawn (Macrobrachium rosenbergii) Polyculture in Periphyton-Based Systems. Aquac. Res. 2007, 38, 1759–1769. [Google Scholar] [CrossRef]
- Rouse, D.B.; El Naggar, G.O.; Mulla, M.A. Effects of Stocking Size and Density of Tilapia on Macrobrachium rosenbergii in Polyculture. J. World Aquac. Soc. 1987, 18, 57–60. [Google Scholar] [CrossRef]
- Pai, W.T.; Schafferer, C.; Lee, J.M.; Ho, L.M.; Lu, Y.H.; Yang, H.C.; Yeh, C.Y. Effect of Culture Period and Stocking Density on Input Demand and Scale Economies of Milkfish (Chanos chanos) Polycultures with White Shrimp (Penaeus indicus). Fishes 2022, 7, 110. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Chen, X.; Shen, L.; Chu, Y.; Qiu, L.; Hu, G.; Song, C.; Li, D.; Meng, S.; et al. Responses of Bacterial and Three Sub-Microeukaryote Communities in the Water of White Shrimp Penaeus vannamei Aquaculture Ponds in Two Polyculture Models. Can. J. Microbiol. 2023, 69, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hamidoghli, A.; Lee, S.; Torrecillas, S.; Yamamoto, F.Y.; Kathriny, D.; Dos Santos, M.; Rodrigues De Freitas, O.; Oishi, C.A.; Leão Da Fonseca, F.A.; Parisi, G.; et al. Full-Fat Black Soldier Fly Larvae Meal in Diet for Tambaqui, Colossoma macropomum: Digestibility, Growth Performance and Economic Analysis of Feeds. Animals 2023, 13, 360. [Google Scholar] [CrossRef]
- Santos, F.A.C.; Batista, F.S.; Souza, A.S.; Julio, G.S.C.; Favero, G.C.; Junior, J.F.V.; Costa, S.T.; Zeppenfeld, C.C.; Bianchini, N.H.; Heinzmann, B.M.; et al. Growth Performance and Histomorphology of Intestine, Skin, Gills and Liver of Juvenile Colossoma macropomum Fed Diets Containing Different Levels of the Essential Oil of Nectandra grandiflora. Fishes 2023, 8, 509. [Google Scholar] [CrossRef]
- Rosa García Dávila, C.; Ruiz Tafur, M.; Sánchez Riveiro, H.; Almendra Flores Silva, M.; Eduardo Mejia de Loayza, J.; Alberto Custodio Angulo Chávez, C.; Castro Ruiz, D.; Estivals, G.; García Vásquez, A.; Nolorbe Payahua, C.; et al. Guía de Identificación de Peces de Consumo de La Amazonía Peruana; Instituto de Investigaciones de la Amazonía Peruana (IIAP) and Wildlife Conservation Society: Lima, Peru, 2022. [Google Scholar] [CrossRef]
- de Ananias, I.M.C.; dos Silva, S.S.; dos Santos, F.A.C.; de Souza, A.S.; Magalhães, T.B.; Reis, P.A.R.; Favero, G.C.; Luz, R.K. Tambaqui Production at Different Stocking Densities in RAS: Growth and Physiology. Fishes 2023, 9, 19. [Google Scholar] [CrossRef]
- dos Santos, F.A.C.; dos Julio, G.S.C.; Batista, F.S.; Miranda, L.N.L.; Pedras, P.P.C.; Luz, R.K. High Stocking Densities in the Larviculture of Colossoma macropomum in a Recirculating Aquaculture System: Performance, Survival and Economic Viability. Aquaculture 2022, 552, 738016. [Google Scholar] [CrossRef]
- Rosa García Dávila, C.; Sánchez Riveiro, H.; Almendra Flores Silva, M.; Eduardo Mejia de Loayza, J.; Alberto Custodio Angulo Chávez, C.; Castro Ruiz, D.; Estivals, G.; García Vásquez, A.; Nolorbe Payahua, C.; Vargas Dávila, G.; et al. Peces de Consumo de La Amazonía Peruana; Instituto de Investigaciones de la Amazonía Peruana: Iquitos, Peru, 2018; p. 112. [Google Scholar]
- Santana, T.M.; de Dantas, F.M.; Monteiro Dos Santos, D.K.; Kojima, J.T.; Pastrana, Y.M.; De Jesus, R.S.; Gonçalves, L.U. Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles. Fishes 2023, 8, 111. [Google Scholar] [CrossRef]
- Agudelo, J.F.G.; Mastrochirico-Filho, V.A.; de Borges, C.H.S.; Ariede, R.B.; Lira, L.V.G.; de Neto, R.R.O.; de Freitas, M.V.; Sucerquia, G.A.L.; Vera, M.; Berrocal, M.H.M.; et al. Genomic Selection Signatures in Farmed Colossoma macropomum from Tropical and Subtropical Regions in South America. Evol. Appl. 2022, 15, 679. [Google Scholar] [CrossRef]
- Artoni, R.F.; Bertollo, L.A.C. Trends in the Karyotype Evolution of Loricariidae Fish (Siluriformes). Hereditas 2001, 134, 201–210. [Google Scholar] [CrossRef]
- Kavalco, K.F.; Pazza, R.; Bertollo, L.A.C.; Moreira-Filho, O. Heterochromatin Characterization of Four Fish Species of the Family Loricariidae (Siluriformes). Hereditas 2004, 141, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, R.; Pouangcharean, S.; Yoonphand, R. Habitat, Abundance and Diet of Invasive Suckermouth Armored Catfish (Loricariidae pterygoplichthys) in the Nong Yai Canal, East Thailand. Trop. Zool. 2011, 24, 49–62. [Google Scholar]
- Wakida-Kusunoki, A.T.; Del Angel, L.E.A. New Records of the Sailfish Catfishes Pterygoplichthys pardalis (Castelnau 1855) and P. disjunctivus (Weber 1991) (Siluriformes: Loricariidae) in Southeastern Mexico [Nuevos Registros de Los Plecos Pterygoplichthys pardalis (Castelnau 1855) y P. disjunctivus (Weber 1991) (Siluriformes: Loricariidae) En El Sureste de México]. Hidrobiologica 2008, 18, 251–255. [Google Scholar]
- Armbruster, J.W. The Species of the Hypostomus cochliodon group (Siluriformes: Loricariidae). Zootaxa 2003, 249, 1–60. [Google Scholar] [CrossRef]
- Pereira, D.A.S.; Henriques, M.B. Economic Feasibility for Producing Imperial Zebra pleco (Hypancistrus zebra) in Recirculating Aquaculture Systems: An Alternative for a Critically Endangered Ornamental Fish. Aquac. Econ. Manag. 2019, 23, 428–448. [Google Scholar] [CrossRef]
- Directive of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; Official Journal of the European Union: Luxembourg, 2010.
- Costa, J.; de Freitas, R.A.; Neto, M.P.N.; de Sena, A.P.N.; Bezerra, D.B.; Nascimento, C.F.; Natividade, J.A.S.; da Silva, T.B.A. Integrated Multitrophic Aquaculture Improves the Production Performance of Amazonian Fishes in Nursery Phase. Aquaculture 2025, 600, 742240. [Google Scholar] [CrossRef]
- Wahab, M.A.; Kadir, A.; Milstein, A.; Kunda, M. Manipulation of Species Combination for Enhancing Fish Production in Polyculture Systems Involving Major Carps and Small Indigenous Fish Species. Aquaculture 2011, 321, 289–297. [Google Scholar] [CrossRef]
- Fondo Nacional de Desarrollo Pesquero Manual de Cultivo de Gamitana. Available online: https://www.gob.pe/institucion/fondepes/informes-publicaciones/2451156-manual-de-cultivo-de-gamitana (accessed on 16 January 2024).
- Boyd, C.E.; McNevin, A.A. Aquaculture, Resource Use, and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–337. [Google Scholar] [CrossRef]
- Halver, J.E.; Hardy, R.W. Fish Nutrition, 3rd ed.; Academic Press: San Diego, CA, USA, 2002; p. 588. [Google Scholar]
- Pardo-Carrasco, S.; Bru-Cordero, S.; García-Gonzáles, J.J. Es Posible Disminuir La Proteína En El Alimento Para Peces En Policultivo Con Perifiton? Orinoquia 2014, 18, 35–42. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The Effects of Replacing Fishmeal by Chlorella vulgaris and Fish Oil by Schizochytrium Sp. and Microchloropsis gaditana Blend on Growth Performance, Feed Efficiency, Muscle Fatty Acid Composition and Liver Histology of Gilthead Seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, H.; Yao, W.; Yang, H.; Xue, M.; Li, X.; Leng, X. Effects of Fish Meal Replacement by Three Protein Sources on Physical Pellet Quality and Growth Performance of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 25, 101210. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, R.X.; Zhang, D.M.; Lei, X.Y.; Wang, S.; Wan, J.W.; Liu, H.J.; Chen, Y.K.; Zhao, Y.L.; Wang, G.Q.; et al. Effects of Different Stocking Densities on Growth Performance, Nutritional Quality and Economic Benefit of Juvenile Female Chinese Mitten Crab (Eriocheir sinensis) in Rice-Crab Culture Systems. Aquaculture 2022, 553, 738111. [Google Scholar] [CrossRef]
- Tu, N.P.C.; Ha, N.N.; Linh, N.T.T.; Tri, N.N. Effect of Astaxanthin and Spirulina Levels in Black Soldier Fly Larvae-Based Diets on Growth Performance and Skin Pigmentation in Discusfish, Symphysodon Sp. Aquaculture 2022, 553, 738048. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, Q.; Zhang, X.; Zhu, T.; Guo, C.; Yang, Z.; Luo, J.; Yuan, Y.; Hu, X.; Jiao, L.; et al. Effect of Dietary Replacement of Fish Meal with Low-Gossypol Cottonseed Protein Concentrate on Growth Performance and Expressions of Genes Related to Protein Metabolism for Swimming Crab (Portunus trituberculatus). Aquaculture 2022, 549, 737820. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Zhao, Y.; Cao, Y. Effect of Stocking Density on Water Quality, Harmful Nitrogen Control, and Production Performance of Penaeus vannamei in Biofloc-Based Systems with Limited Water Exchange. Fishes 2025, 10, 326. [Google Scholar] [CrossRef]
- Pai, M.; Verma, A.K.; Krishnani, K.K.; Varghese, T.; Hittinahalli, C.M.; Verma, M.K. Stocking Density Optimization and Its Impact on Growth and Physiological Responses of Nile tilapia (Oreochromis niloticus) Reared in Hybrid Biofloc-RAS Culture System. Aquaculture 2024, 588, 740920. [Google Scholar] [CrossRef]
- Agusa, Y.; Endo, K.; Kuroda, H.; Kobayashi, S. Examination of Water Temperature Accuracy Improvement Method for Forecast. Commun. Comput. Inf. Sci. 2021, 1371 CCIS, 14–27. [Google Scholar] [CrossRef]
- Woynarovich, A.; van Anrooy, R. Field Guide to the Culture of Tambaqui (Colossoma macropomum, Cuvier, 1816); FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2019; pp. 1–121. [Google Scholar]
- Ianes, J.; Cantoni, B.; Polesel, F.; Remigi, E.U.; Vezzaro, L.; Antonelli, M. Monitoring (Micro-)Pollutants in Wastewater Treatment Plants: Comparing Discharges in Wet- and Dry-Weather. Environ. Res. 2024, 263, 120132. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, J.; de Araújo, L.C.A.; Vasconcelos, M.D.; da Silva, I.J.S.; Motteran, F.; Rodrigues, R.H.A.; Mendes-Marques, C.L.; de Alves, R.B.O.; da Silva, H.P.; Barros, M.P.; et al. Multivariate Statistical Analysis of Surface Water Quality in the Capibaribe River (Pernambuco State, Northeast Brazil): Contributions to Water Management. Mar. Environ. Res. 2025, 204, 106876. [Google Scholar] [CrossRef]
- Krochta, M.; Chang, H. Reviewing Controls of Wetland Water Temperature Change across Scales and Typologies. Prog. Phys. Geogr. 2024, 49, 144–164. [Google Scholar] [CrossRef]
- Lee, A.C.; Bilung, L.M.; Tung, T.S.; Apun, K.; Jetom, A. Unravelling Sensitive Physicochemical Dynamics in Commercial Aquaculture Earthen Ponds Across Southern Sarawak. J. Teknol. Sci. Eng. 2025, 87, 361–371. [Google Scholar] [CrossRef]
- Mugo-Bundi, J.; Manyala, J.O.; Muchiri, M.; Matolla, G. Effects of Stocking Density and Water Flow Rate on Performance, Water Quality and Economic Benefits of African Catfish Larvae (Clarias Gariepinus Burchell, 1822) in the Aquaponic System Integrated with Azolla Fern. Aquaculture 2024, 579, 740170. [Google Scholar] [CrossRef]
- Ríos, E. Calidad Del Agua En El Cultivo de Organismos Acuáticos Amazónicos; Editorial Barreto S.A.C.: Lima, Peru, 2021; p. 90. [Google Scholar]
- Bignall, L.; Magnenet, C.; Ramsamy, C.; Lakard, S.; Vassal, S.; Lakard, B. Polyaniline-Based Flexible Sensor for PH Monitoring in Oxidizing Environments. Chemosensors 2024, 12, 97. [Google Scholar] [CrossRef]
- Garcia, F.; Martel, D.; Paiva-Peredo, E. An Automated Power of Hydrogen Controlled Filtration System for Enhanced Aquarium Fish Farming. Int. J. Electr. Comput. Eng. 2024, 14, 6265–6270. [Google Scholar] [CrossRef]
- Hardy, R.W.; Brezas, A. Diet Formulation and Manufacture. In Fish Nutrition; Academic Press: Cambridge, MA, USA, 2022; pp. 643–708. [Google Scholar] [CrossRef]
- Gilles, S.; Ismiño, R.; Sánchez, H.; David, F.; Núñez, J.; Dugué, R.; Darias, M.J.; Römer, U. An Integrated Closed System for Fish-Plankton Aquaculture in Amazonian Fresh Water. Animal 2014, 8, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.C.; Costa, G.A.; Pereira, S.A.; Valenti, W.C.; Moraes-Valenti, P. Improving Production and Diet Assimilation in Fish-Prawn Integrated Aquaculture, Using Iliophagus Species. Aquaculture 2020, 521, 735048. [Google Scholar] [CrossRef]
- Dantas, D.P.; Flickinger, D.L.; Costa, G.A.; Batlouni, S.R.; Moraes-Valenti, P.; Valenti, W.C. Technical Feasibility of Integrating Amazon River Prawn Culture during the First Phase of Tambaqui Grow-out in Stagnant Ponds, Using Nutrient-Rich Water. Aquaculture 2020, 516, 734611. [Google Scholar] [CrossRef]
- David, R. Teichert-Coddington, Effect of stocking ratio on semi-intensive polyculture of Colossoma macropomum and Oreochromis niloticus in Honduras, Central America. Aquaculture 1996, 143, 291–302. [Google Scholar] [CrossRef]
- Van Der Meer, M.B.; Faber, R.; Zamora, J.E.; Verdegem, M.C.J. Effect of Feeding Level on Feed Losses and Feed Utilization of Soya and Fish Meal Diets in Colossoma macropomum (Cuvier). Aquac. Res. 1997, 28, 391–403. [Google Scholar] [CrossRef]
- Tafur-Gonzales, J.; Alcantara-Bocanegra, F.; Del Águila-Pizarro, M.; Cubas-Guerra, R.; Mori-Pinedo, L.; Chu-Koo, F.W. Paco Piaractus brachypomus Y Gamitana Colossoma macropomum Criados En Policultivo Con El Bujurqui-Tucunaré, Chaetobranchus semifasciatus (Cichlidae). Folia Amazónica 2009, 18, 97–104. [Google Scholar] [CrossRef]
- da Costa, O.T.F.; Dias, L.C.; Malmann, C.S.Y.; de Lima Ferreira, C.A.; do Carmo, I.B.; Wischneski, A.G.; de Sousa, R.L.; Cavero, B.A.S.; Lameiras, J.L.V.; Dos-Santos, M.C. The Effects of Stocking Density on the Hematology, Plasma Protein Profile and Immunoglobulin Production of Juvenile Tambaqui (Colossoma macropomum) Farmed in Brazil. Aquaculture 2019, 499, 260–268. [Google Scholar] [CrossRef]
- Frisso, R.M.; de Matos, F.T.; Moro, G.V.; de Mattos, B.O. Stocking Density of Amazon Fish (Colossoma macropomum) Farmed in a Continental Neotropical Reservoir with a Net Cages System. Aquaculture 2020, 529, 735702. [Google Scholar] [CrossRef]
- Oliva, M.; Medina, M.; Uriarte, W.; Alvis, R. Policultivo de Paco (Piaractus brachypomus) y Gamitana (Colossoma macropomum) a Diferentes Densidades En La Fase de Engorde Utilizando Estanques Circulares En Alto Saposoa—San Martín. Rev. De Investig. De Agroproducción Sustentable 2021, 5, 48–54. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; El-Tabakh, M.A.; Hendy, D.M. Plankton and Fish Nutrition in African Lakes. In Lakes of Africa—Microbial Diversity and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 139–172. [Google Scholar] [CrossRef]
- Stickney, R.R.; Perschbacher, P.W.; Parker, N. New Models and Rationales. In Tilapia Intensive Co-Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 50–70. [Google Scholar] [CrossRef]
- Zimmermann, S.; New, M.B. Grow-out Systems—Polyculture and Integrated Culture. In Freshwater Prawn Culture: The Farming of Macrobrachium rosenbergii; Blackwell Science: Bognor Regis, UK, 2007; pp. 187–202. [Google Scholar] [CrossRef]
- Colt, J.; Semmens, K. Computation of Feed Conversion Ratio (FCRplant) and Plant-Fish Mass Ratio (PFRM) for Aquaponic Systems. Aquac. Eng. 2022, 98, 102260. [Google Scholar] [CrossRef]
- Della Rosa, P.; Ortiz, J.C.; de la Cáceres, A.C.; Sánchez, S.; Roux, J.P. Performance of Sabalo Prochilodus lineatus in Polyculture with Pacu Piaractus mesopotamicus. Lat. Am. J. Aquat. Res. 2016, 44, 336–341. [Google Scholar] [CrossRef]
- de Matos Dantas, F.; de Souza, Y.M.; Santana, T.M.; dos Santos, D.K.M.; da Fonseca, F.A.L.; Gonçalves, L.U. A Sustainable Diet for Tambaqui Farming in the Amazon: Growth Performance, Hematological Parameters, Whole-Body Composition and Fillet Color. Animals 2024, 14, 1165. [Google Scholar] [CrossRef]
- Melo, R.M.C.; Nunes, D.M.F.; Moreira, D.P.; Weber, A.A.; Bazzoli, N.; Rizzo, E. Comparative Reproductive Biology of Two Sympatric Hypostomus in a Neotropical River. Zoology 2023, 156, 126065. [Google Scholar] [CrossRef] [PubMed]
- Debnath, C. Effect of Stocking Density on Growth, Production, Ecosystem Dynamics, and Economic Viability of Common Carp (Cyprinus carpio) Cage Culture in a Forest Water Body of Northeast India. Aquac. Int. 2025, 33, 245. [Google Scholar] [CrossRef]
- Ruby, P.; Ahilan, B.; Antony, C.; Manikandavelu, D.; Selvaraj, S.; Moses, T.L.S.S. Evaluation of Effect of the Different Stocking Densities on Growth Performance, Survival, Water Quality and Body Indices of Pearlspot (Etroplus suratensis) Fingerlings in Biofloc Technology. Indian J. Anim. Res. 2022, 56, 1034–1040. [Google Scholar] [CrossRef]
| Nutrients | Content (%) |
|---|---|
| Proteins | 28 |
| Fats | 5 |
| Fiber | 8 |
| Ashes | 10 |
| Humidity | 12 |
| Response | Factors | Coefficient | p-Value | Model Fitting |
|---|---|---|---|---|
| Wf (g) | FA-Lp | −0.304 | 0.021 | R2 = 93.93% |
| FB-D1 | 0.599 | 0.005 | ||
| FB-D3 | 1.589 | 0.000 | ||
| FA × FB-Lp × D1 | −0.363 | 0.045 | ||
| FA × FB-Lp × D5 | −0.971 | 0.000 | ||
| Lf (cm) | FA-Lp | −0.189 | 0.040 | R2 = 96.90% |
| FB-D3 | 2.083 | 0.000 | ||
| FA × FB-Lp × D1 | −0.361 | 0.009 | ||
| FA × FB-Lp × D5 | −0.564 | 0.000 | ||
| RGR (g/days) | FA-Lp | −0.538 | 0.008 | R2 = 61.19% |
| Survival (%) | FB-D1 | 1.933 | 0.000 | R2 = 97.75% |
| FB-D5 | −1.266 | 0.000 |
| Species (FA) | Density (FB) | Wi (g) | Wf (g) | Li | Lf | AWGR (g/day) | ALGR (cm/day) | RGR (%/day) | Survival (%) |
|---|---|---|---|---|---|---|---|---|---|
| Liposarcus pardalis (Lp) | D1 | 0.3 ± 0.0 | 14.8 ± 0.3 | 2.7 ± 0.1 | 16.7 ± 0.2 | 0.11 ± 0.02 | 0.11 ± 0.02 | 2.95 ± 0.25 | 100 ± 0.0 |
| D3 | 0.36 ± 0.01 | 14.9 ± 0.3 | 2.7 ± 0.5 | 18.4 ± 0.3 | 0.12 ± 0.02 | 0.12 ± 0.06 | 3.22 ± 0.15 | 96.8 ± 0.1 | |
| D5 | 0.34 ± 0.02 | 13.6 ± 0.2 | 2.8 ± 0.7 | 15.5 ± 0.1 | 0.11 ± 0.07 | 0.10 ± 0.07 | 3.16 ± 0.13 | 97.4 ± 0.3 | |
| Hypostomus hemicochliodon (Hh) | D1 | 0.4 ± 0.0 | 15.9 ± 0.2 | 4.10 ± 0.03 | 17.8 ± 0.4 | 0.12 ± 0.01 | 0.11 ± 0.03 | 3.00 ± 0.01 | 100 ± 0.0 |
| D3 | 0.6 ± 0.2 | 17.5 ± 0.3 | 3.8 ± 0.9 | 19.9 ± 0.4 | 0.14 ± 0.01 | 0.13 ± 0.07 | 2.93 ± 0.23 | 97.4 ± 0.4 | |
| D5 | 0.9 ± 0.1 | 14.0 ± 1.1 | 3.8 ± 1.1 | 14.0 ± 0.5 | 0.08 ± 0.09 | 0.08 ± 0.01 | 2.34 ± 0.19 | 99.8 ± 0.1 |
| Response | Factors | Coefficient | p-Value | Model Fitting |
|---|---|---|---|---|
| Wf (g) | FA-Lp | −6.567 | 0.000 | R2 = 89.55% |
| FA × FB-Lp × D5 | 8.300 | 0.000 | ||
| Lf (cm) | FA-Lp | 2.738 | 0.000 | R2 = 99.04% |
| FB-D1 | 0.344 | 0.008 | ||
| FB-D3 | 1.494 | 0.000 | ||
| FA × FB-Lp × D5 | −1.172 | 0.000 | ||
| AWGR (g/days) | FB-D3 | 0.016 | 0.000 | R2 = 83.86% |
| FA × FB-Lp × D1 | −0.005 | 0.038 | ||
| AGLR (cm/days) | FB-D3 | 0.019 | 0.000 | R2 = 82.30% |
| FA × FB-Lp × D1 | 0.002 | 0.043 | ||
| RGR (%/days) | FB-D3 | −0.065 | 0.004 | R2 = 61.77% |
| FA × FB-Lp × D1 | −0.070 | 0.002 | ||
| FA × FB-Lp × D5 | 0.050 | 0.018 | ||
| Survival (%) | FA-Lp | 0.188 | 0.020 | R2 = 97.75% |
| FB-D1 | 1.183 | 0.000 | ||
| Biomass (kg) | FA-Lp | −0.455 | 0.000 | R2 = 99.96% |
| FB-D1 | −8.322 | 0.000 | ||
| FB-D5 | 0.144 | 0.008 | ||
| FA × FB-Lp × D1 | 0.388 | 0.000 | ||
| FA × FB-Lp × D5 | 0.522 | 0.000 | ||
| FCE | FA × FB-Lp × D1 | −0.127 | 0.001 | R2 = 88.11% |
| FA × FB-Lp × D5 | −0.111 | 0.002 |
| Species (FA) | Density (FB) | Wi (g) | Wf (g) | Li | Lf | AWGR (g/day) | ALGR (cm/day) | RGR (%/day) | Survival (%) | Biomass (kg) * | FCE * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. macropomum + Lp | D1 | 7.88 ± 0.27 | 253.9 ± 2.1 | 7.7 ± 0.5 | 22.9 ± 0.1 | 2.05 ± 0.01 | 0.12 ± 0.03 | 2.89 ± 0.03 | 99.6 ± 0.3 | 4.16 ± 0.03 | 1.6 ± 0.0 |
| D3 | 7.8 ± 1.0 | 259.5 ± 3.4 | 7.5 ± 1.2 | 23.0 ± 0.2 | 2.09 ± 0.01 | 0.12 ± 0.08 | 2.92 ± 0.09 | 98.9 ± 0.2 | 12.76 ± 0.17 | 1.6 ± 0.0 | |
| D5 | 6.4 ± 0.5 | 233.4 ± 2.3 | 6.8 ± 0.3 | 22.2 ± 0.2 | 1.89 ± 0.02 | 0.12 ± 0.04 | 2.99 ± 0.07 | 97.2 ± 0.3 | 19.36 ± 0.19 | 1.9 ± 0.0 | |
| C. macropomum + Hh | D1 | 7.3 ± 0.4 | 262.0 ± 6.5 | 7.6 ± 0.5 | 22.1 ± 0.4 | 2.12 ± 0.05 | 0.13 ± 0.02 | 2.98 ± 0.03 | 99.5 ± 0.4 | 4.30 ± 0.10 | 1.8 ± 0.1 |
| D3 | 9.2 ± 0.5 | 255.8 ± 4.2 | 8.4 ± 0.3 | 21.5 ± 0.4 | 2.05 ± 0.03 | 0.10 ± 0.03 | 2.77 ± 0.05 | 98.1 ± 0.2 | 12.16 ± 0.20 | 1.8 ± 0.0 | |
| D5 | 8.3 ± 0.1 | 268.1 ± 1.5 | 8.3 ± 0.1 | 23.5 ± 0.1 | 2.16 ± 0.01 | 0.12 ± 0.08 | 2.90 ± 0.00 | 96.9 ± 0.2 | 22.11 ± 0.09 | 1.4 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Iwasaki, M.; Marcial-Ramos, R.; del Águila-Panduro, E.; Silva-Zuta, M.Z.; Cayo-Colca, I.S.; Chávez, S.G. Enhancing Aquaculture Productivity via Polyculture with Colossoma macropomum: A Focus on Two Native Amazon Species. Fishes 2025, 10, 563. https://doi.org/10.3390/fishes10110563
Flores-Iwasaki M, Marcial-Ramos R, del Águila-Panduro E, Silva-Zuta MZ, Cayo-Colca IS, Chávez SG. Enhancing Aquaculture Productivity via Polyculture with Colossoma macropomum: A Focus on Two Native Amazon Species. Fishes. 2025; 10(11):563. https://doi.org/10.3390/fishes10110563
Chicago/Turabian StyleFlores-Iwasaki, Manhiro, Ronald Marcial-Ramos, Erik del Águila-Panduro, Miguelina Z. Silva-Zuta, Ilse S. Cayo-Colca, and Segundo G. Chávez. 2025. "Enhancing Aquaculture Productivity via Polyculture with Colossoma macropomum: A Focus on Two Native Amazon Species" Fishes 10, no. 11: 563. https://doi.org/10.3390/fishes10110563
APA StyleFlores-Iwasaki, M., Marcial-Ramos, R., del Águila-Panduro, E., Silva-Zuta, M. Z., Cayo-Colca, I. S., & Chávez, S. G. (2025). Enhancing Aquaculture Productivity via Polyculture with Colossoma macropomum: A Focus on Two Native Amazon Species. Fishes, 10(11), 563. https://doi.org/10.3390/fishes10110563










