Dietary Alpha-Lipoic Acid Alleviated Hepatic Glycogen Deposition and Improved Inflammation Response of Largemouth Bass (Micropterus salmoides) Fed on High Dietary Carbohydrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation for Diets
2.2. Experiment Design
2.3. Sample Collection
2.4. Chemical Analysis
2.5. Relative Gene Expression Analysis
2.6. Calculation and Statistical Analysis
3. Results
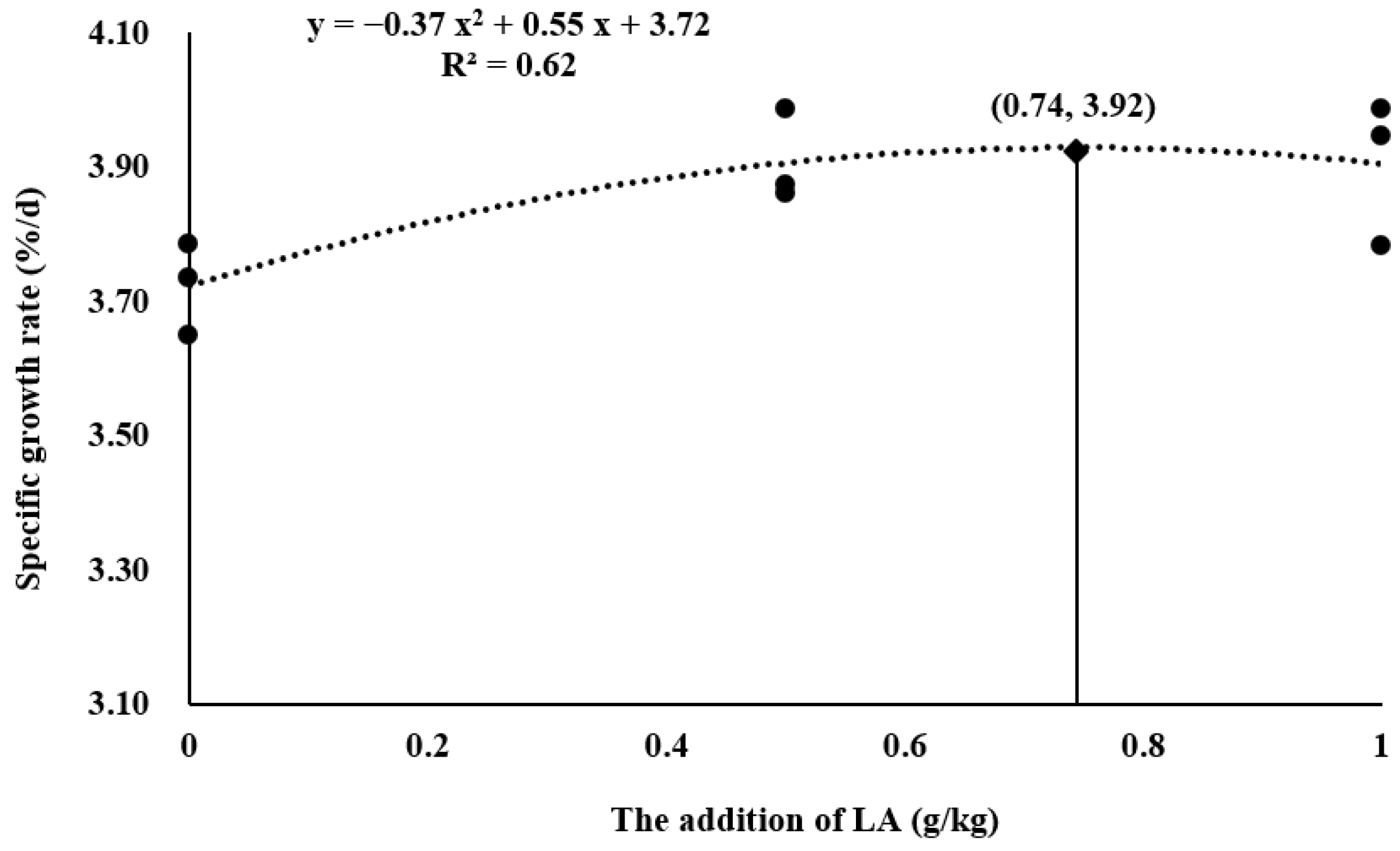
3.1. Growth Performance
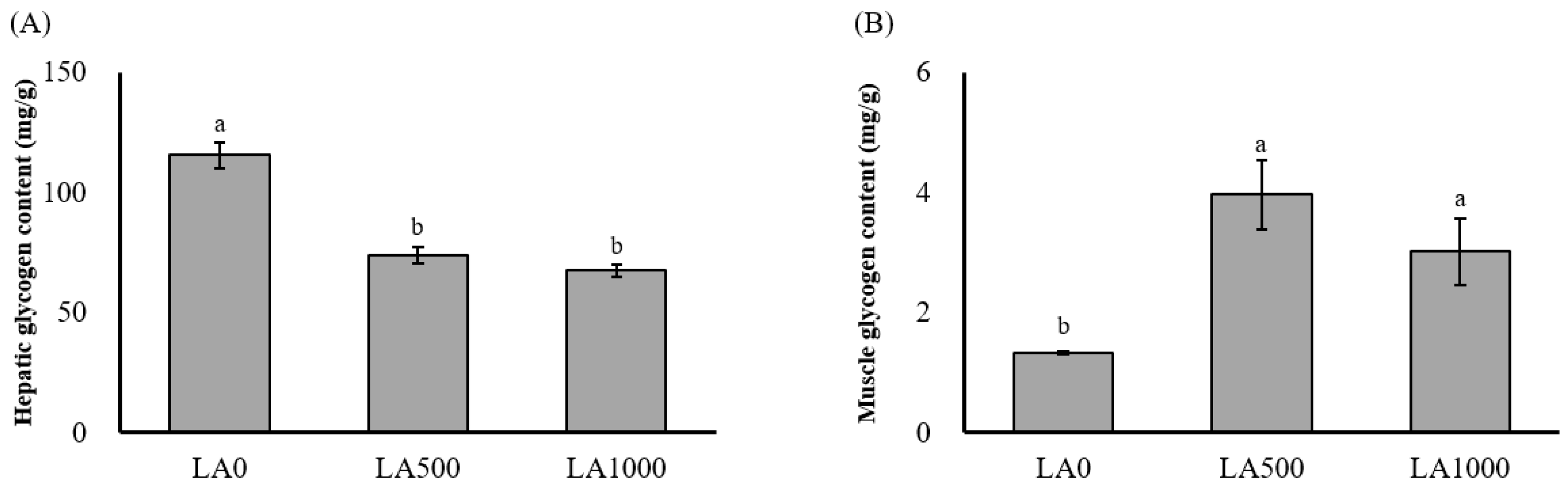
3.2. Body Composition and Glycogen Content Analysis
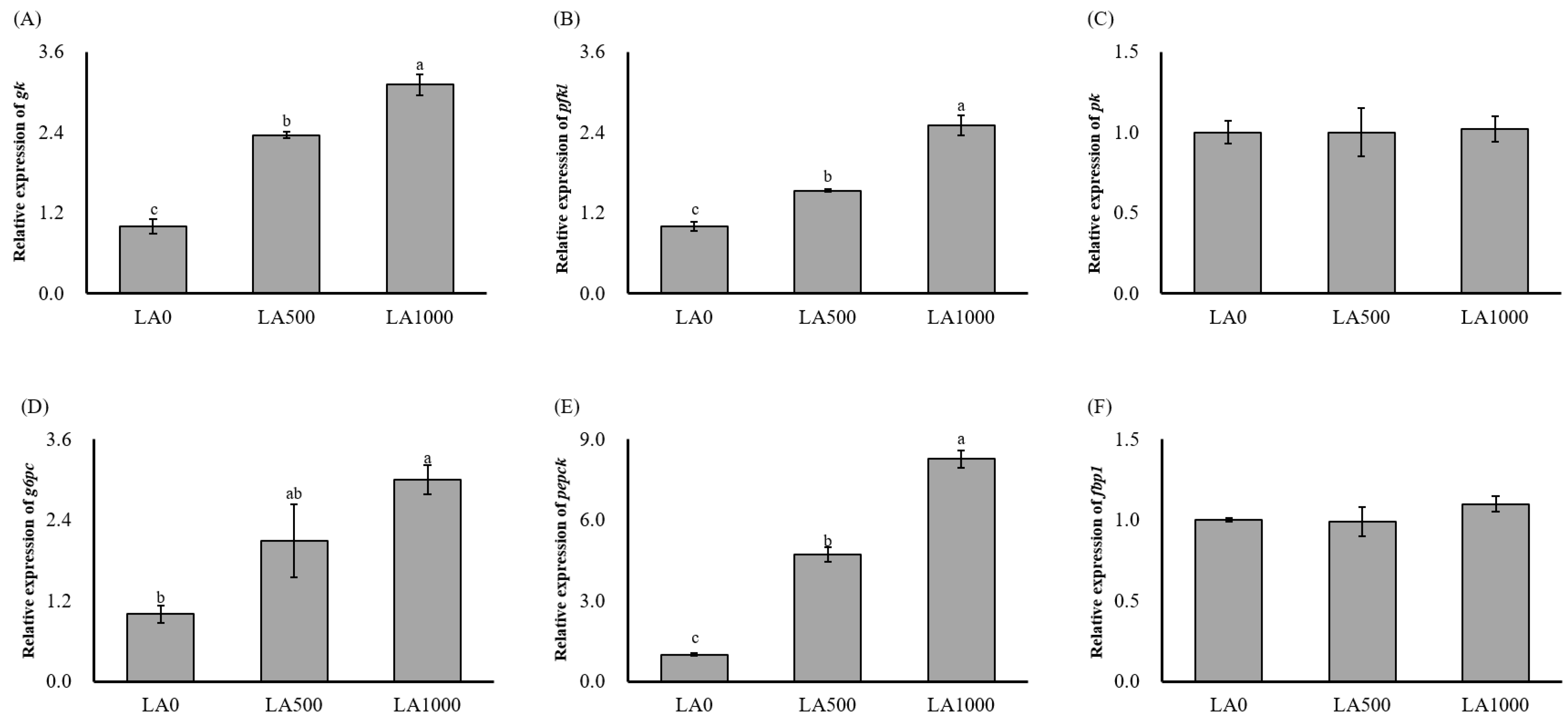
3.3. Relative Expression of Genes Related to Insulin Pathway and Glucose Metabolism
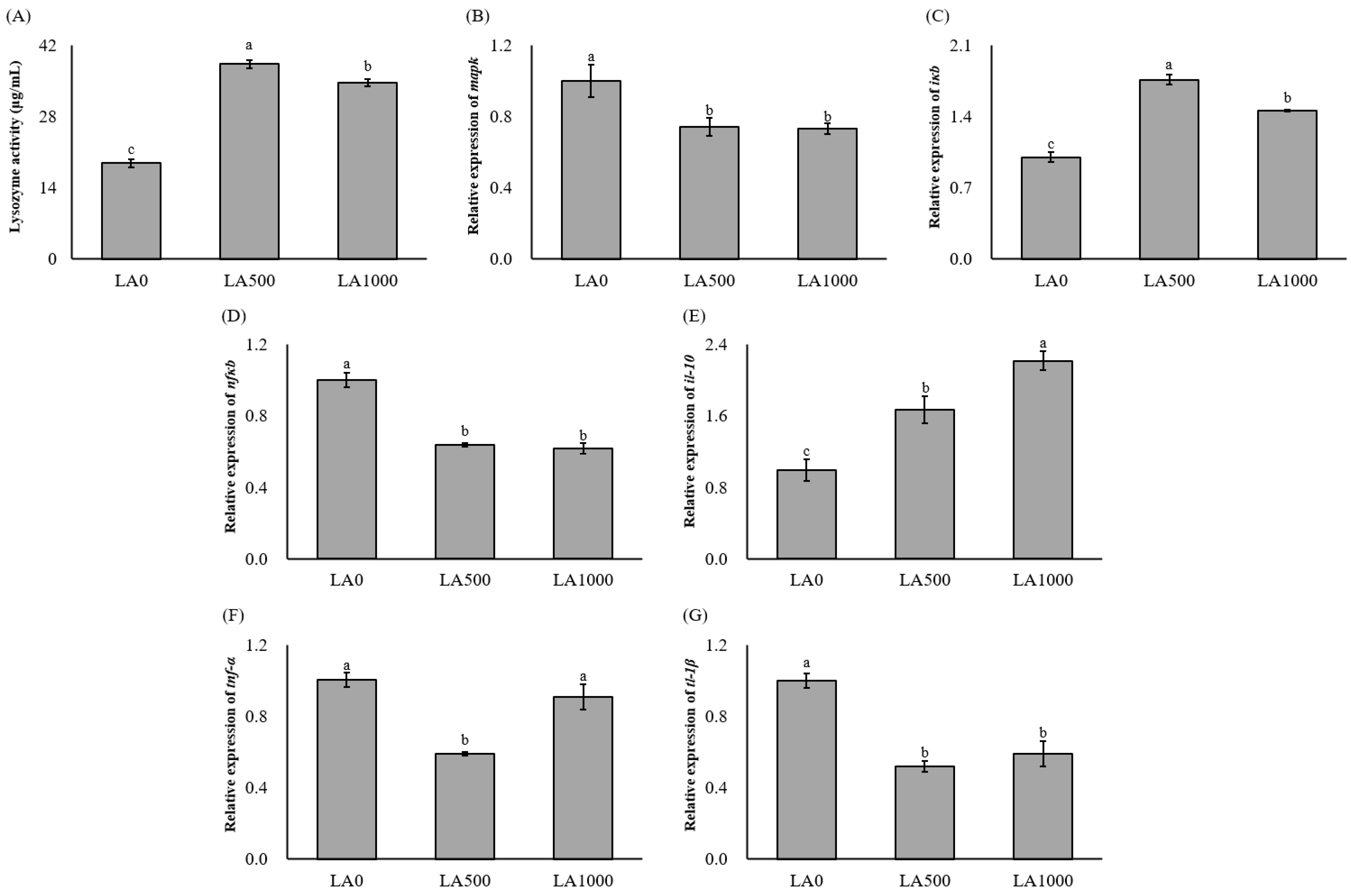
3.4. Lysozyme Activity and Inflammation Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NRC. Carbohydrates and Fibre, in Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011; pp. 135–162. [Google Scholar]
- Ma, H.J.; Mou, M.M.; Pu, D.C.; Lin, S.M.; Chen, Y.J.; Luo, L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass Micropterus salmoides. Aquaculture 2019, 498, 482–487. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Wang, A.; Chen, N.; Li, S. Resveratrol inclusion alleviated highdietary-carbohydrate-induced glycogen deposition and immune response of largemouth bass, Micropterus salmoides. Br. J. Nutr. 2022, 127, 165–176. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.A. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur. J. Nutr. 2016, 55 (Suppl. S2), 17–23. [Google Scholar] [CrossRef]
- Chen, P.; Wu, X.; Gu, X.; Han, J.; Xue, M.; Liang, X. FoxO1 in Micropterus salmoides: Molecular characterization and its roles in glucose metabolism by glucose or insulin-glucose loading. Gen. Comp. Endocr. 2021, 310, 113811. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Li, W.; Liu, N.; Fang, Z.; Zhang, N.; Chen, N.; Li, S. The beneficial effects of metformin inclusion on growth performance, glucose utilization, antioxidant capacity and apoptosis of largemouth bass (Micropterus salmoides) fed with high dietary carbohydrates. Aquaculture 2024, 588, 740957. [Google Scholar] [CrossRef]
- Li, S.; Sang, C.; Wang, A.; Zhang, J.; Chen, N. The impacts of dietary carbohydrate levels on growth performance, feed utilization, glycogen accumulation and hepatic glucose metabolism in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Aquaculture 2019, 512, 734351. [Google Scholar] [CrossRef]
- Capilla, E.; M’edale, F.; Navarro, I.; Panserat, S.; Vachot, C.; Kaushik, S.; Guti’errez, J. Muscle insulin binding and plasma levels in relation to liver glucokinase activity, glucose metabolism and dietary carbohydrates in rainbow trout. Regul. Pept. 2003, 110, 123–132. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Zhang, D.D.; Cao, X.F.; Shi, H.J.; Li, X.F. Interactions between dietary carbohydrate and metformin: Implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture 2018, 483, 183–195. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Liu, H.; Gong, C.; Huang, X.; Hu, Y.; Liu, Q.; He, Z.; Zhang, X.; Yang, S.; et al. Yinchenhao Decoction ameliorates the high-carbohydrate diet induced suppression of immune response in largemouth bass (Micropterus salmoides). Fish. Shellfish. Immunol. 2022, 125, 141–151. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Vidal, S.; Lobo, C.; Prieto-Álamo, M.J.; Jurado, J.; Cordero, H.; Cerezuela, R.; Garcia de la Banda, I.; Esteban, M.A.; Balebona, M.C.; et al. The treatment with the probiotic Shewanella putrefaciens Pdp11 of specimens of Solea senegalensis exposed to high stocking densities to enhance their resistance to disease. Fish Shellfish. Immunol. 2014, 41, 209–221. [Google Scholar] [CrossRef]
- Eslamloo, K.; Akhavan, S.R.; Fallah, F.J.; Henry, M.A. Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress. Fish Shellfish Immunol. 2014, 37, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Tapingkae, W.; Moonmanee, T.; Seepai, A. Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 55, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological approaches for disease control in aquaculture: Advantages, limitations and challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.Y.; Feng, L.; Jiang, W.D.; Jiang, J.; Wu, P.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Methionine hydroxy analogue enhanced fish immunity via modulation of NF-κB, TOR, MLCK, MAPKs and Nrf2 signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 56, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Dong, B.; Wu, L.; Chen, Q.; Xu, W.; Li, D.; Han, D.; Zhu, X.; Liu, H.; Yang, Y.; Xie, S.; et al. Tolerance assessment of atractylodes macrocephla polysaccharide in the diet of largemouth bass (Micropterus salmoides). Antioxidants 2022, 11, 1581. [Google Scholar] [CrossRef]
- Tao, J.; Wang, S.; Qiu, H.; Xie, R.; Zhang, H.; Chen, N.; Li, S. Modulation of growth performance, antioxidant capacity, non-specific immunity and disease resistance in largemouth bass (Micropterus salmoides) upon compound probiotic cultures inclusion. Fish. Shellfish. Immunol. 2022, 127, 804–812. [Google Scholar] [CrossRef]
- Islam, M.T. Antioxidant activities of dithiol alpha-lipoic acid. Bangladesh J. Med. Sci. 2009, 8, 46. [Google Scholar] [CrossRef]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-lipoic acid and glucose metabolism: A comprehensive update on biochemical and therapeutic features. Nutrients 2023, 15, 18. [Google Scholar] [CrossRef]
- Konrad, D. Utilization of the insulin-signaling network in the metabolic actions of α-lipoic acid—Reduction or oxidation? Antioxid. Redox Signal. 2005, 7, 1032–1039. [Google Scholar] [CrossRef]
- Santos, R.A.; Caldas, S.; Primel, E.G.; Tesser, M.B.; Monserrat, J.M. Effects of lipoic acid on growth and biochemical responses of common carp fed with carbohydrate diets. Fish Physiol. Biochem. 2016, 42, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Hepatic glucose metabolism and its disorders in fish. Recent Adv. Anim. Nutr. Metab. 2022, 207–236. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Lai, Y.; Wu, X.; Zhang, J.; Niu, X.; Wang, G. The effects of dietary supplementation of α-lipoic acid on the growth performance, antioxidant capacity, immune response, and disease resistance of northern snakehead, Channa argus. Fish Shellfish Immunol. 2022, 126, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Xie, R.; Huang, J.; Huang, J.; Wen, Z.; Li, Y.; Jiang, X.; Ma, Q.; Chen, G. Effects of dietary alpha-lipoic acid on growth performance, serum biochemical indexes, liver antioxidant capacity and transcriptome of juvenile hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus polyphekadion♂). Animals 2023, 13, 887. [Google Scholar] [CrossRef]
- Kütter, M.T.; Monserrat, J.M.; Primel, E.G.; Caldas, S.S.; Tesser, M.B. Effects of dietary α-lipoic acid on growth, body composition and antioxidant status in the Plata pompano Trachinotus marginatus (Pisces, Carangidae). Aquaculture 2012, 368, 29–35. [Google Scholar] [CrossRef]
- Samuki, K.; Setiawati, M.; Jusadi, D.; Agus Suprayudi, M. The evaluation of α-Lipoic acid supplementation in diet on the growth performance of giant gourami (Osphronemus goramy) juvenile. Aquacult. Res. 2021, 52, 1538–1547. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 2003. [Google Scholar]
- Seifter, S.; Dayton, S.; Novic, B. The estimation of glycogen with the anthrone reagent. Arch. Biochem. Biophys. 1950, 25, 191–200. [Google Scholar]
- Cui, Z.; He, F.; Li, X.; Jing, M.; Huo, C.; Zong, W.; Liu, R. Molecular insights into the binding model and response mechanisms of triclosan with lysozyme. J. Mol. Liq. 2022, 357, 119080. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Lipoic acid: A multifunctional antioxidant. Biofactors 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Siagian, D.R.; Jusadi, D.; Ekasari, J.; Setiawati, M. Dietary α-lipoic acid supplementation to improve growth, blood chemistry, and liver antioxidant status of African catfish Clarias gariepinus. Aquacult. Int. 2021, 29, 1935–1947. [Google Scholar] [CrossRef]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Long-term safety of α-lipoic acid (LA) consumption: A 2-year study. Regul. Toxicol. Pharmacol. 2006, 46, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, J.Y.; Namkoong, C.; Jang, P.G.; Ryu, J.W.; Song, H.S.; Yun, J.Y.; Namgoong, I.S.; Ha, J.; Park, I.S.; et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Avruch, J. Insulin signal transduction through protein kinase cascades. Mol. Cell Biochem. 1998, 182, 31–48. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Sang, C.; Zhang, J.; Chen, N. The variation of serum glucose, hepatic glycogen content and expression of glucose metabolism-related genes in hybrid grouper (female Epinephelus fuscoguttatus × male Epinephelus lanceolatus) in response to intraperitoneal insulin infusion. Fish. Sci. 2018, 84, 641–647. [Google Scholar] [CrossRef]
- Li, S.; Sang, C.; Turchini, G.M.; Wang, A.; Zhang, J.; Chen, N. Starch in aquafeeds: The benefits of a high amylose to amylopectin ratio and resistant starch content in diets for the carnivorous fish, largemouth bass (Micropterus salmoides). Br. J. Nutr. 2020, 124, 1145–1155. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef]
- Ding, Z.; Xiong, Y.; Zheng, J.; Zhou, D.; Kong, Y.; Qi, C.; Liu, Y.; Ye, J.; Limbu, S.M. Modulation of growth, antioxidant status, hepatopancreas morphology, and carbohydrate metabolism mediated by alpha-lipoic acid in juvenile freshwater prawns Macrobrachium nipponense under two dietary carbohydrate levels. Aquaculture 2022, 546, 737314. [Google Scholar] [CrossRef]
- Huang, C.; Sun, J.; Ji, H.; Kaneko, G.; Xie, X.; Chang, Z.; Deng, W. Systemic effect of dietary lipid levels and α-lipoic acid supplementation on nutritional metabolism in zebrafish (Danio rerio): Focusing on the transcriptional level. Fish Physiol. Biochem. 2020, 46, 1631–1644. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Z.; Lin, H.; Ma, Z.; Wang, J.; Wang, Y.; Yu, W. Rhizoma curcumae Longae ameliorates high dietary carbohydrate-induced hepatic oxidative stress, inflammation in golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2022, 130, 31–42. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; Peng, K.; Chen, B.; Hu, J.; Huang, W.; Cao, J.; Sun, Y. The positive effects of dietary arginine on juvenile hybrid snakehead (Channa maculata♀ × Channa argus♂) fed high-carbohydrate diets: Liver inflammation antioxidant response, and glucose metabolism. Aquacult. Rep. 2023, 30, 101602. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Chen, F.; Tang, X.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef]
- Lu, D.; Limbu, S.M.; Lv, H.; Ma, Q.; Chen, L.; Zhang, M.; Du, Z. The comparisons in protective mechanisms and efficiencies among dietary α-lipoic acid, β-glucan and l-carnitine on Nile tilapia infected by Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 86, 785–793. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Ren, H.T.; Zhou, B.H.; Wu, Q.J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Opal, S.M.; De-Palo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Feng, L. Optimal α-lipoic acid strengthens immunity of young grass carp (Ctenopharyngodon idella) by enhancing immune function of head kidney, spleen and skin. Fish Shellfish Immunol. 2018, 80, 600–617. [Google Scholar] [CrossRef]
- Yu, L.L.; Yu, H.H.; Liang, X.F.; Li, N.; Wang, X.; Li, F.H.; Wu, X.F.; Zheng, Y.H.; Xue, M.; Liang, X.F. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2018, 72, 220–229. [Google Scholar] [CrossRef]

| Ingredients | LA0 | LA500 | LA1000 |
|---|---|---|---|
| White fish meal 2 | 40.00 | 40.00 | 40.00 |
| Wheat gluten meal 2 | 3.00 | 3.00 | 3.00 |
| Blood meal 2 | 3.00 | 3.00 | 3.00 |
| Shrimp meal 2 | 3.00 | 3.00 | 3.00 |
| Fermented soybean meal 2 | 12.00 | 12.00 | 12.00 |
| Corn gluten meal 2 | 12.00 | 12.00 | 12.00 |
| Brewer’s yeast meal 2 | 3.00 | 3.00 | 3.00 |
| Squid viscera meal 2 | 2.00 | 2.00 | 2.00 |
| Soybean phospholipids 2 | 2.00 | 2.00 | 2.00 |
| Fish oil 2 | 2.00 | 2.00 | 2.00 |
| Soybean oil 2 | 2.00 | 2.00 | 2.00 |
| α-lipoic acid 1 | 0.00 | 0.05 | 0.10 |
| Microcrystalline cellulose | 3.00 | 2.95 | 2.90 |
| Vitamin mixture 3 | 1.00 | 1.00 | 1.00 |
| Mineral mixture 4 | 1.00 | 1.00 | 1.00 |
| Ca(H2PO4)2 2 | 1.00 | 1.00 | 1.00 |
| α-cassava Starch 2 | 10.00 | 10.00 | 10.00 |
| Proximate analysis (Mean values, % dry weight) | |||
| Crude protein | 52.17 | 51.93 | 52.03 |
| Crude lipid | 11.84 | 10.54 | 10.73 |
| LA0 | LA500 | LA1000 | p-Value | |
|---|---|---|---|---|
| Initial body weight (g) | 5.08 ± 0.01 | 5.10 ± 0.02 | 5.09 ± 0.01 | 0.144, 0.552 |
| Final body weight (g) | 40.86 ± 0.84 b | 45.53 ± 0.96 a | 45.39 ± 1.55 a | 0.029, 0.033 |
| Specific growth rate (%/d) | 3.72 ± 0.04 b | 3.90 ± 0.04 a | 3.90 ± 0.06 a | 0.034, 0.034 |
| Condition factor (%g/cm3) | 2.29 ± 0.02 | 2.33 ± 0.02 | 2.33 ± 0.02 | 0.201, 0.201 |
| Feed intake rate (%/d·individual) | 1.91 ± 0.02 | 1.92 ± 0.03 | 1.94 ± 0.03 | 0.796, 0.403 |
| Hepatosomatic index (%) | 3.77 ± 0.04 a | 3.38 ± 0.16 b | 3.52 ± 0.06 ab | 0.034, 0.131 |
| Viscerosomatic index (%) | 8.32 ± 0.20 | 8.31 ± 0.20 | 8.20 ± 0.11 | 0.990, 0.644 |
| LA0 | LA500 | LA1000 | p-Value | |
|---|---|---|---|---|
| Whole fish | ||||
| Moisture (%) | 73.91 ± 1.16 | 74.09 ± 0.58 | 74.06 ± 0.45 | 0.877, 0.902 |
| Crude lipid (%) | 3.91 ± 0.41 | 3.92 ± 0.38 | 4.25 ± 0.37 | 0.986, 0.554 |
| Crude protein (%) | 17.26 ± 0.12 | 17.20 ± 0.14 | 17.32 ± 0.13 | 0.756, 0.730 |
| Ash (%) | 3.05 ± 0.13 | 2.97 ± 0.09 | 3.02 ± 0.02 | 0.588, 0.849 |
| Liver | ||||
| Moisture (%) | 74.38 ± 0.48 | 75.38 ± 0.50 | 73.62 ± 0.41 | 0.344, 0.528 |
| Crude lipid (%) | 2.78 ± 0.04 | 2.78 ± 0.03 | 2.74 ± 0.05 | 0.911, 0.543 |
| Crude protein (%) | 9.00 ± 0.04 b | 10.47 ± 0.45 a | 9.73 ± 0.19 ab | 0.030, 0.207 |
| Muscle | ||||
| Moisture (%) | 78.34 ± 0.32 | 78.00 ± 0.25 | 78.12 ± 0.17 | 0.378, 0.555 |
| Crude lipid (%) | 1.79 ± 0.14 | 1.84 ± 0.10 | 1.48 ± 0.07 | 0.735, 0.085 |
| Crude protein (%) | 19.12 ± 0.36 | 19.43 ± 0.25 | 19.39 ± 0.12 | 0.431, 0.490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Pan, X.; Gong, Y.; Zhang, N.; Chen, S.; Liu, N.; Chen, N.; Li, S. Dietary Alpha-Lipoic Acid Alleviated Hepatic Glycogen Deposition and Improved Inflammation Response of Largemouth Bass (Micropterus salmoides) Fed on High Dietary Carbohydrates. Fishes 2025, 10, 9. https://doi.org/10.3390/fishes10010009
Fang Z, Pan X, Gong Y, Zhang N, Chen S, Liu N, Chen N, Li S. Dietary Alpha-Lipoic Acid Alleviated Hepatic Glycogen Deposition and Improved Inflammation Response of Largemouth Bass (Micropterus salmoides) Fed on High Dietary Carbohydrates. Fishes. 2025; 10(1):9. https://doi.org/10.3390/fishes10010009
Chicago/Turabian StyleFang, Zishuo, Xianwei Pan, Ye Gong, Nihe Zhang, Shiwen Chen, Ning Liu, Naisong Chen, and Songlin Li. 2025. "Dietary Alpha-Lipoic Acid Alleviated Hepatic Glycogen Deposition and Improved Inflammation Response of Largemouth Bass (Micropterus salmoides) Fed on High Dietary Carbohydrates" Fishes 10, no. 1: 9. https://doi.org/10.3390/fishes10010009
APA StyleFang, Z., Pan, X., Gong, Y., Zhang, N., Chen, S., Liu, N., Chen, N., & Li, S. (2025). Dietary Alpha-Lipoic Acid Alleviated Hepatic Glycogen Deposition and Improved Inflammation Response of Largemouth Bass (Micropterus salmoides) Fed on High Dietary Carbohydrates. Fishes, 10(1), 9. https://doi.org/10.3390/fishes10010009







