Heart Rate Monitoring During Behavioral Stress Tests in Bold and Shy Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
2.3. Shy–Bold Categorization: First Novel Object Test
2.4. Biologger Surgery
Biologgers and Heart Rate
2.5. Second Novel Object Test
2.5.1. Confinement Test
2.5.2. Pair-Wise Contests
2.5.3. Third Novel Object Test
2.6. Statistical Analysis
3. Results
3.1. Consistency of Boldness
Differences Between Bold and Shy in Novel Object Test
3.2. Confinement Test
3.3. Pair-Wise Contest
3.4. Heart Rate
3.5. Physiological Parameters
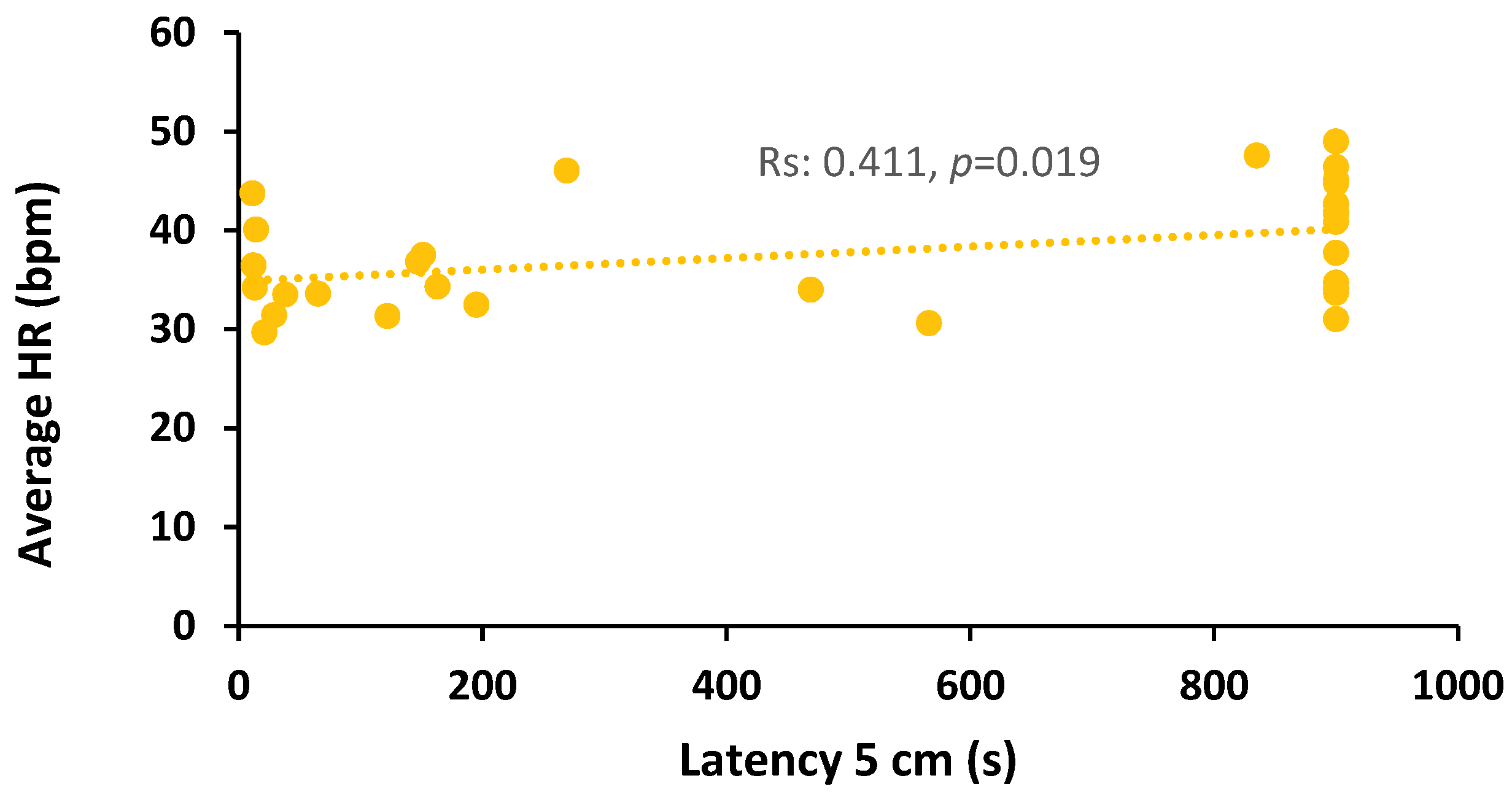
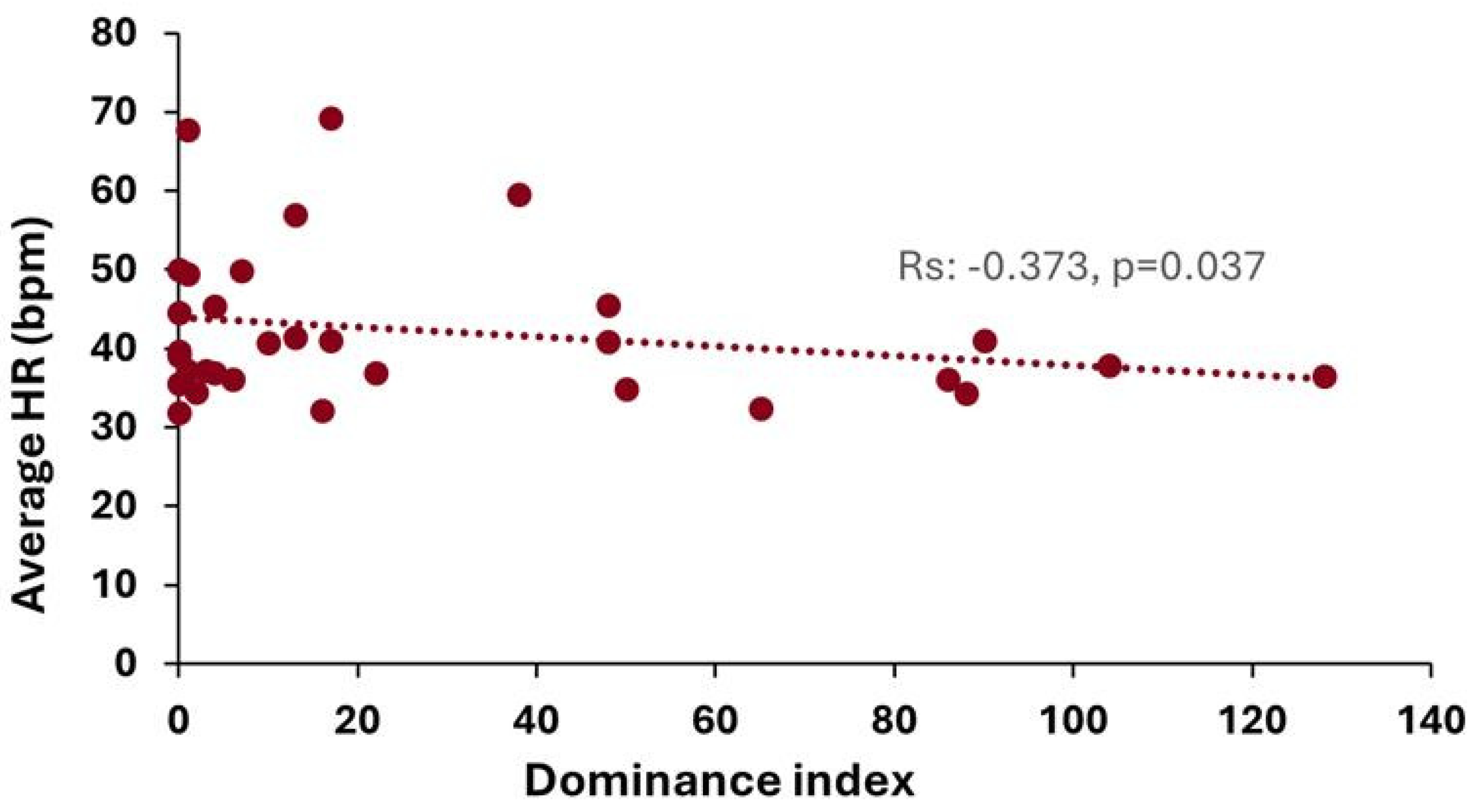
3.6. Relationships Between Parameters
4. Discussion
4.1. Behavioral Tests
4.2. Heart Rate Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browning, H. Improving welfare assessment in aquaculture. Front. Vet. Sci. 2023, 10, 1060720. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, A.M.; Hoogenboom, L.J.; Anderson, G.W. A Review of Reductionist Methods in Fish Gastrointestinal Tract Physiology. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 254, 110571. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Rosengren, M.; Sundell, K.; Berg, C.; Axelsson, M.; Gräns, A. Prospects and Pitfalls of Using Heart Rate Bio-Loggers to Assess the Welfare of Rainbow Trout (Oncorhynchus mykiss) in Aquaculture. Aquaculture 2019, 509, 188–197. [Google Scholar] [CrossRef]
- Romero, L.; Dickens, M.J.; Cyr, N.E. The Reactive Scope Model—A New Model Integrating Homeostasis, Allostasis, and Stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Düpjan, S.; Dawkins, M.S. Animal Welfare and Resistance to Disease: Interaction of Affective States and the Immune System. Front. Vet. Sci. 2022, 9, 929805. [Google Scholar] [CrossRef]
- Wilson, D.S.; Clark, A.B.; Coleman, K.; Dearstyne, T. Shyness and Boldness in Humans and Other Animals. Trends Ecol. Evol. 1994, 9, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Coe, C.; Parmigiani, S.; Koolhaas, J. Individual Differences in Behavior and Physiology: Causes and Consequences. Neurosci. Biobehav. Rev. 2005, 29, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral Syndromes: An Ecological and Evolutionary Overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Braithwaite, V.A.; Gentle, M.J. Novel Object Test: Examining Nociception and Fear in the Rainbow Trout. J. Pain 2003, 4, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F. Stress, Corticosterone Responses and Avian Personalities. J. Ornithol. 2007, 1, 169–178. [Google Scholar] [CrossRef]
- Ruiz-gomez, M.; Kittilsen, S.; Hoglund, E.; Huntingford, F.; Sorensen, C.; Pottinger, T.; Bakken, M.; Winberg, S.; Korzan, W.; Øverli, Ø. Behavioral Plasticity in Rainbow Trout (Oncorhynchus Mykiss) with Divergent Coping Styles: When Doves Become Hawks. Horm. Behav. 2008, 54, 534–538. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R. Modification of the Plasma Cortisol Response to Stress in Rainbow Trout by Selective Breeding. Gene. Comp. Endocrinol. 1999, 116, 122–132. [Google Scholar] [CrossRef]
- Øverli, Ø.; Sørensen, C.; Pulman, K.G.T.; Pottinger, T.G.; Korzan, W.; Summers, C.H.; Nilsson, G.E. Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in nonmammalian vertebrates. Neurosci. Biobehav. Rev. 2007, 31, 396–412. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S. Autonomic Nerve Function in Vertebrates; Springer: Berlin/Heidelberg, Germany, 1983; Volume 13. [Google Scholar] [CrossRef]
- Sandblom, E.; Axelsson, M. Autonomic control of circulation in fish: A comparative view. Auton. Neurosci. 2011, 165, 127–139. [Google Scholar] [CrossRef]
- Romero, L.M.; Gormally, B.M.G. How Truly Conserved Is the “Well-Conserved” Vertebrate Stress Response? Integr. Comp. Biol. 2019, 59, 273–281. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Huyben, D.; Gräns, A. The final countdown: Continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 2018, 495, 903–911. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Karaer, M.C.; Čebulj-Kadunc, N.; Snoj, T. Stress in Wildlife: Comparison of the Stress Response among Domestic, Captive, and Free-Ranging Animals. Front. Vet. Sci. 2023, 10, 1167016. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M. Physiological Stress in Ecology: Lessons from Biomedical Research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.S. Stress management and welfare. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; pp. 463–539. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Prunet, P.; Pickering, A.D. The effects of confinement stress on circulating prolactin levels in rainbow trout (Oncorhynchus mykiss) in fresh water. Gene. Comp. Endocrinol. 1992, 88, 454–460. [Google Scholar] [CrossRef]
- Øverli, Ø.; Pottinger, T.G.; Carrick, T.R.; Øverli, E.; Winberg, S. Differences in behaviour between rainbow trout selected for high- and low-stress responsiveness. J. Exp. Biol. 2002, 205, 391–395. [Google Scholar] [CrossRef]
- Schjolden, J.; Backström, T.; Pulman, K.G.; Pottinger, T.G.; Winberg, S. Divergence in behavioural responses to stress in two strains of rainbow trout (Oncorhynchus mykiss) with contrasting stress responsiveness. Horm. Behav. 2005, 48, 537–544. [Google Scholar] [CrossRef][Green Version]
- Johnsson, J.I.; Åkerman, A. Watch and learn: Preview of the fighting ability of opponents alters contest behaviour in rainbow trout. Anim. Behav. 1998, 56, 771–777. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R. Stress responsiveness affects dominant– subordinate relationships in rainbow trout. Horm. Behav. 2001, 40, 419–427. [Google Scholar] [CrossRef]
- Øverli, Ø.; Harris, C.A.; Winberg, S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 1999, 54, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.J. The Corticotropin-Releasing Factor System as a Mediator of the Appetite-Suppressing Effects of Stress in Fish. Gen. Comp. Endocrinol. 2006, 146, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Coleman, K.; Clark, A.B.; Biederman, L. Shy-Bold Continuum in Pumpkinseed Sunfish (Lepomis Gibbosus): An Ecological Study of a Psychological Trait. J. Comp. Psychol. 1993, 107, 250–260. [Google Scholar] [CrossRef]
- Frost, A.J.; Winrow-Giffen, A.; Ashley, P.J.; Sneddon, L.U. Plasticity in Animal Personality Traits: Does Prior Experience Alter the Degree of Boldness? Proc. R. Soc. B 2007, 274, 333–339. [Google Scholar] [CrossRef]
- Thomson, J.S.; Watts, P.C.; Pottinger, T.G.; Sneddon, L.U. Physiological and Genetic Correlates of Boldness: Characterising the Mechanisms of Behavioural Variation in Rainbow Trout, Oncorhynchus Mykiss. Horm. Behav. 2011, 59, 67–74. [Google Scholar] [CrossRef]
- Larcombe, E.; Alexander, M.E.; Snellgrove, D.; Henriquez, F.L.; Sloman, K.A. Current disease treatments for the ornamental pet fish trade and their associated problems. Rev. Aquac. 2025, 17, e12948. [Google Scholar] [CrossRef]
- McGlade, C.L.O.; Dickey, J.W.E.; Kennedy, R.; Donnelly, S.; Nelson, C.A.; Dick, J.T.A.; Arnott, G. Behavioural Traits of Rainbow Trout and Brown Trout May Help Explain Their Differing Invasion Success and Impacts. Sci. Rep. 2022, 12, 1757. [Google Scholar] [CrossRef]
- Dall, S.R.X.; Houston, A.I.; McNamara, J.M. The Behavioural Ecology of Personality: Consistent Individual Differences from an Adaptive Perspective. Ecol. Lett. 2004, 7, 734–739. [Google Scholar] [CrossRef]
- Chang, C.; Li, C.Y.; Earley, R.L.; Hsu, Y. Aggression and Related Behavioral Traits: The Impact of Winning and Losing and the Role of Hormones. Integr. Comp. Biol. 2012, 52, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.E.A. Experience Influences Male–Male Contests in the Spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 1997, 53, 913–923. [Google Scholar] [CrossRef]
- Winberg, S.; Sneddon, L.U. Impact of Intraspecific Variation in Teleost Fishes: Aggression, Dominance Status and Stress Physiology. J. Exp. Biol. 2022, 225, jeb169250. [Google Scholar] [CrossRef] [PubMed]
- Briffa, M.; Sneddon, L.U.; Wilson, A.J. Animal Personality as a Cause and Consequence of Contest Behaviour. Biol. Lett. 2015, 11, 20141007. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Schmidt, R.; Fang, Y.; Cossins, A.R. Molecular Correlates of Social Dominance: A Novel Role for Ependymin in Aggression. PLoS ONE 2011, 6, e18181. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.J.; Ringrose, S.; Edwards, K.L.; Wallington, E.; McCrohan, C.R.; Sneddon, L.U. Effect of Noxious Stimulation upon Antipredator Responses and Dominance Status in Rainbow Trout. Anim. Behav. 2009, 77, 403–410. [Google Scholar] [CrossRef]
- Höjesjö, J.; Johnsson, J.I.; Axelsson, M. Behavioural and Heart Rate Responses to Food Limitation and Predation Risk: An Experimental Study on Rainbow Trout. J. Fish Biol. 1999, 55, 1009–1019. [Google Scholar] [CrossRef]
- Gräns, A.; Sandblom, E.; Kiessling, A.; Axelsson, M. Post-Surgical Analgesia in Rainbow Trout: Is Reduced Cardioventilatory Activity a Sign of Improved Animal Welfare or the Adverse Effects of an Opioid Drug? PLoS ONE 2014, 9, e95283. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, E.; Føre, M.; Økland, F.; Gräns, A.; Hedger, R.D.; Alfredsen, J.A.; Uglem, I.; Rosten, C.M.; Frank, K.; Erikson, U.; et al. Heart Rate and Swimming Activity as Stress Indicators for Atlantic Salmon (Salmo Salar). Aquaculture 2021, 531, 735804. [Google Scholar] [CrossRef]
- Føre, M.; Svendsen, E.; Økland, F.; Gräns, A.; Alfredsen, J.A.; Finstad, B.; Hedger, R.D.; Uglem, I. Heart Rate and Swimming Activity as Indicators of Post-Surgical Recovery Time of Atlantic Salmon (Salmo Salar). Anim. Biotelem. 2021, 9, 3. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Prystay, T.S.; Dick, M.; Eliason, E.J.; Elvidge, C.K.; Hinch, S.G.; Patterson, D.A.; Lotto, A.G.; Cooke, S.J. Metabolic Constraints and Individual Variation Shape the Trade-off between Physiological Recovery and Anti-predator Responses in Adult Sockeye Salmon. J. Fish Biol. 2023, 103, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Kiessling, A.; Berg, C.; Gräns, A. Remote Physiological Monitoring Provides Unique Insights on the Cardiovascular Performance and Stress Responses of Freely Swimming Rainbow Trout in Aquaculture. Sci. Rep. 2019, 9, 9090. [Google Scholar] [CrossRef] [PubMed]
- Hvas, M.; Folkedal, O.; Oppedal, F. Heart Rate Bio-Loggers as Welfare Indicators in Atlantic Salmon (Salmo Salar) Aquaculture. Aquaculture 2020, 529, 735630. [Google Scholar] [CrossRef]
- Forin-Wiart, M.A.; Enstipp, M.R.; Le Maho, Y.; Handrich, Y. Why Implantation of Bio-loggers May Improve Our Understanding of How Animals Cope within Their Natural Environment. Integr. Zool. 2019, 14, 48–64. [Google Scholar] [CrossRef]
- Frost, A.J.; Thomson, J.S.; Smith, C.; Burton, H.C.; Davis, B.; Watts, P.C.; Sneddon, L.U. Environmental Change Alters Personality in the Rainbow Trout, Oncorhynchus Mykiss. Anim. Behav. 2013, 85, 1199–1207. [Google Scholar] [CrossRef][Green Version]
- dos Santos, C.P.; de Oliveira, M.N.; Silva, P.F.; Luchiari, A.C. Relationship between boldness and exploratory behavior in adult zebrafish. Behav. Process. 2023, 209, 104885. [Google Scholar] [CrossRef] [PubMed]
- Aubin-Horth, N.; Deschênes, M.; Cloutier, S. Natural variation in the molecular stress network correlates with a behavioural syndrome. Horm. Behav. 2012, 61, 140–146. [Google Scholar] [CrossRef]
- Cortez Ghio, S.; Boudreau Leblanc, A.; Audet, C.; Aubin-Horth, N. Effects of maternal stress and cortisol exposure at the egg stage on learning, boldness and neophobia in brook trout. Behaviour 2016, 153, 1639–1663. [Google Scholar] [CrossRef]
- Alfonso, S.; Sadoul, B.; Gesto, M.; Joassard, L.; Chatain, B.; Geffroy, B.; Bégout, M.L. Coping styles in European sea bass: The link between boldness, stress response and neurogenesis. Physiol. Behav. 2019, 207, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.N.; Røn, Ø.; Hagen, P.P.; McGurk, C. Monitoring fish welfare using heart rate bio-loggers in farmed Atlantic salmon (Salmo salar L.): An insight into the surgical recovery. Aquaculture 2022, 555, 738211. [Google Scholar] [CrossRef]
- Fischer, C.P.; Franco, L.A.; Romero, L.M. Are novel objects perceived as stressful? The effect of novelty on heart rate. Physiol. Behav. 2016, 161, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, L.C.; Romero, L.M. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope; Oxford University Press: Oxford, UK, 2015; Volume 3, pp. 69–114. [Google Scholar] [CrossRef]
- Watanabe-Asaka, T.; Niihori, M.; Sonobe, H.; Igarashi, K.; Oda, S.; Iwasaki, K.; Katada, Y.; Yamashita, T.; Terada, M.; Baba, S.A.; et al. Acquirement of the autonomic nervous system modulation evaluated by heart rate variability in medaka (Oryzias latipes). PLoS ONE 2022, 17, e0273064. [Google Scholar] [CrossRef] [PubMed]
- Jolles, J.W.; Briggs, H.D.; Araya-Ajoy, Y.G.; Boogert, N.J. Personality, Plasticity and Predictability in Sticklebacks: Bold Fish Are Less Plastic and More Predictable than Shy Fish. Anim. Behav. 2019, 154, 193–202. [Google Scholar] [CrossRef]







| Average (SE) | F | p-Values | ||
|---|---|---|---|---|
| Shy | Bold | |||
| Weight before | 589.82 (60.54) | 756.16 (62.44) | 0.062 | 0.033 |
| Weight after | 900.00 (44.68) | 1050.93 (34.93) | 0.212 | 0.006 |
| Length before | 33.81 (1.41) | 36.70 (1.25) | 0.912 | 0.068 |
| Length after | 39.53 (0.80) | 41.02 (0.75) | 0.005 | 0.092 |
| SGR | 1.99 (0.08) | 2.07 (0.11) | 4.062 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasiouras, E.; Riberolles, G.; Gräns, A.; Ekström, A.; Höjesjö, J.; Roques, J.A.C.; Sandblom, E.; Sneddon, L.U. Heart Rate Monitoring During Behavioral Stress Tests in Bold and Shy Rainbow Trout (Oncorhynchus mykiss). Fishes 2025, 10, 23. https://doi.org/10.3390/fishes10010023
Kasiouras E, Riberolles G, Gräns A, Ekström A, Höjesjö J, Roques JAC, Sandblom E, Sneddon LU. Heart Rate Monitoring During Behavioral Stress Tests in Bold and Shy Rainbow Trout (Oncorhynchus mykiss). Fishes. 2025; 10(1):23. https://doi.org/10.3390/fishes10010023
Chicago/Turabian StyleKasiouras, Eleftherios, Gautier Riberolles, Albin Gräns, Andreas Ekström, Johan Höjesjö, Jonathan A. C. Roques, Erik Sandblom, and Lynne U. Sneddon. 2025. "Heart Rate Monitoring During Behavioral Stress Tests in Bold and Shy Rainbow Trout (Oncorhynchus mykiss)" Fishes 10, no. 1: 23. https://doi.org/10.3390/fishes10010023
APA StyleKasiouras, E., Riberolles, G., Gräns, A., Ekström, A., Höjesjö, J., Roques, J. A. C., Sandblom, E., & Sneddon, L. U. (2025). Heart Rate Monitoring During Behavioral Stress Tests in Bold and Shy Rainbow Trout (Oncorhynchus mykiss). Fishes, 10(1), 23. https://doi.org/10.3390/fishes10010023









