Oscillatory Disturbed Flow Enhances Inflammatory and Oxidative Stress Markers in Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Maintenance
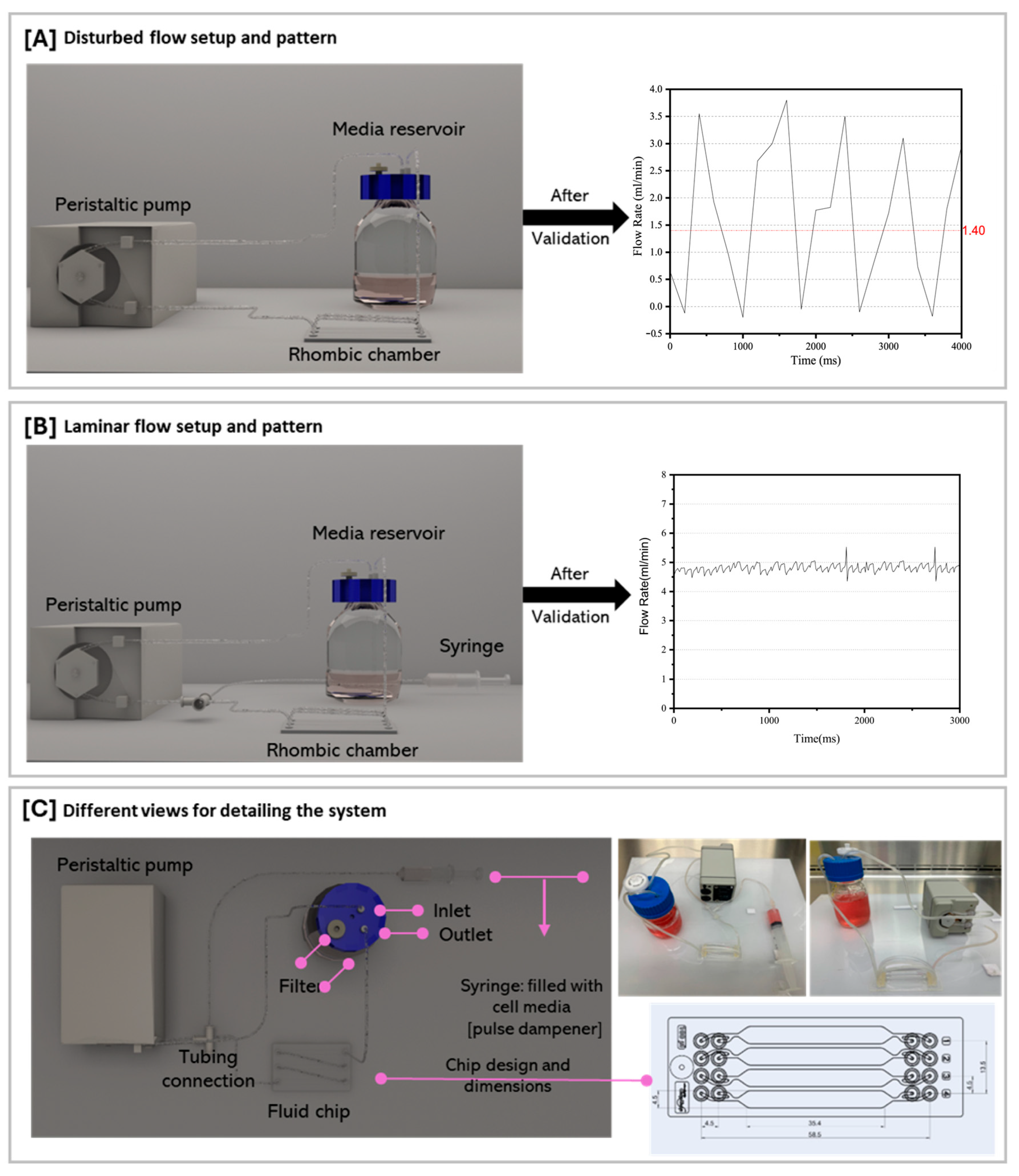
2.2. Flow Experiment Setup and Generation of Laminar and Oscillatory Flows
2.3. RNA Isolation and Gene Expression Analysis
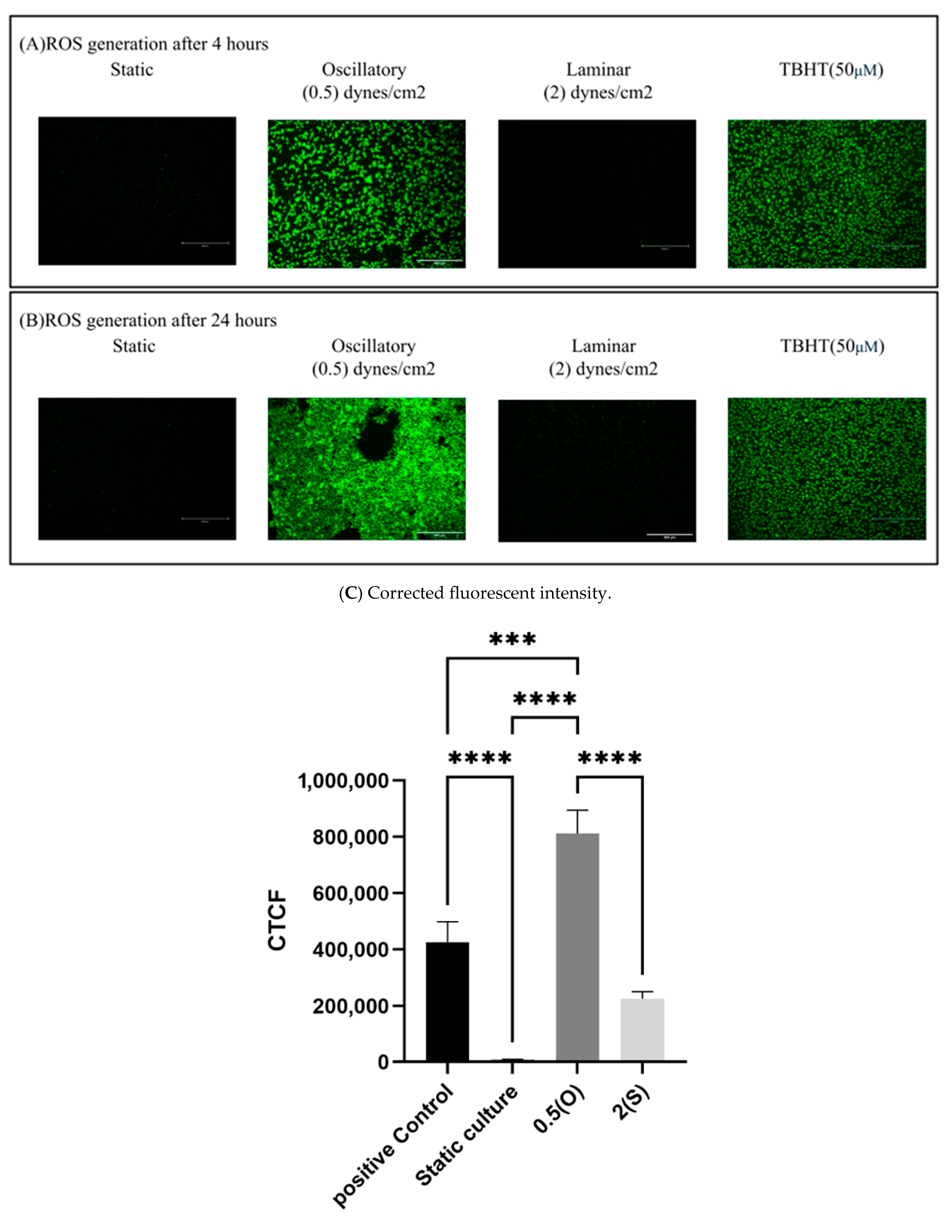
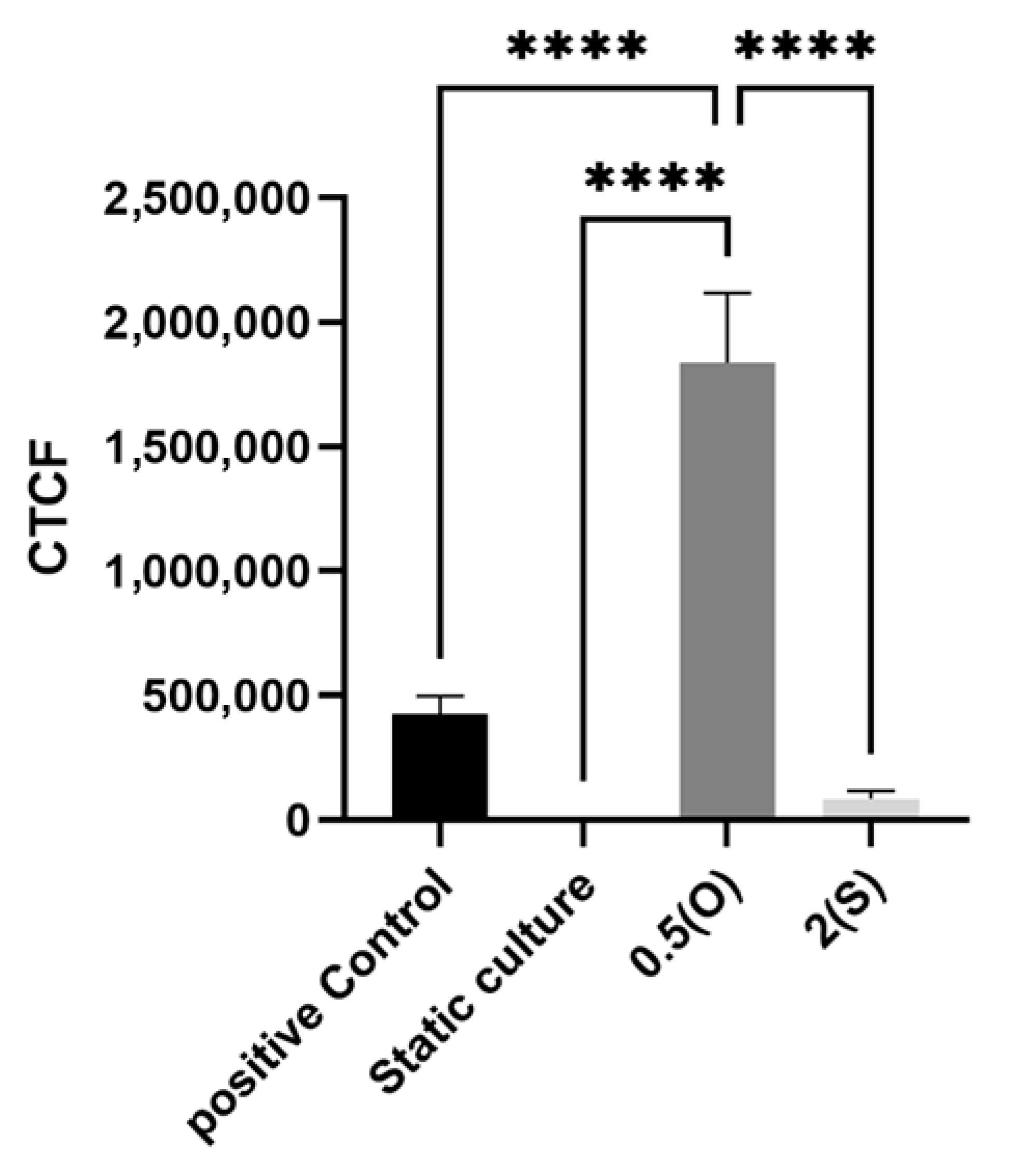
2.4. Measurement of ROS Production
2.5. Statistical Analysis
3. Results
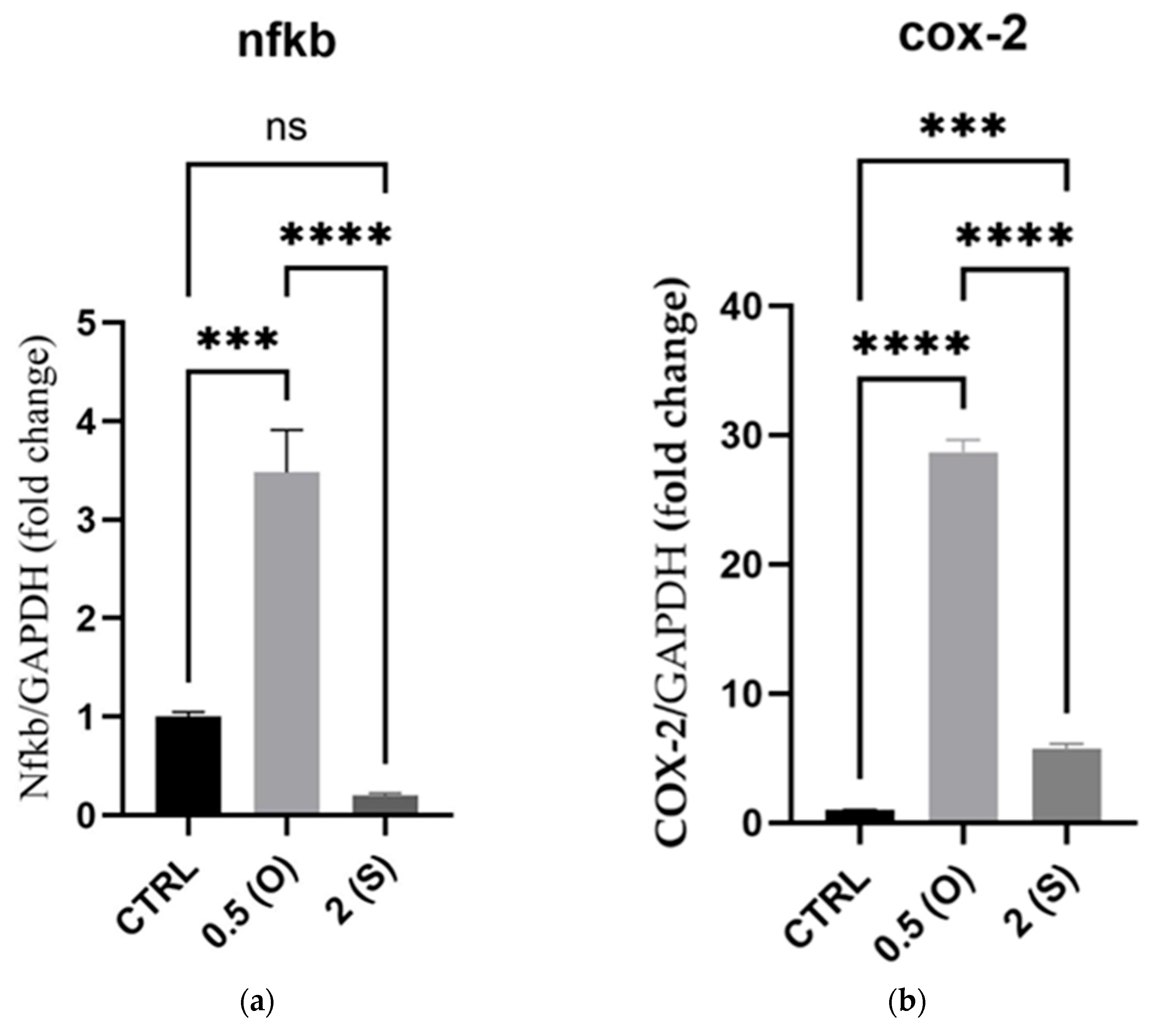
3.1. Proinflammatory Markers Gene Expression
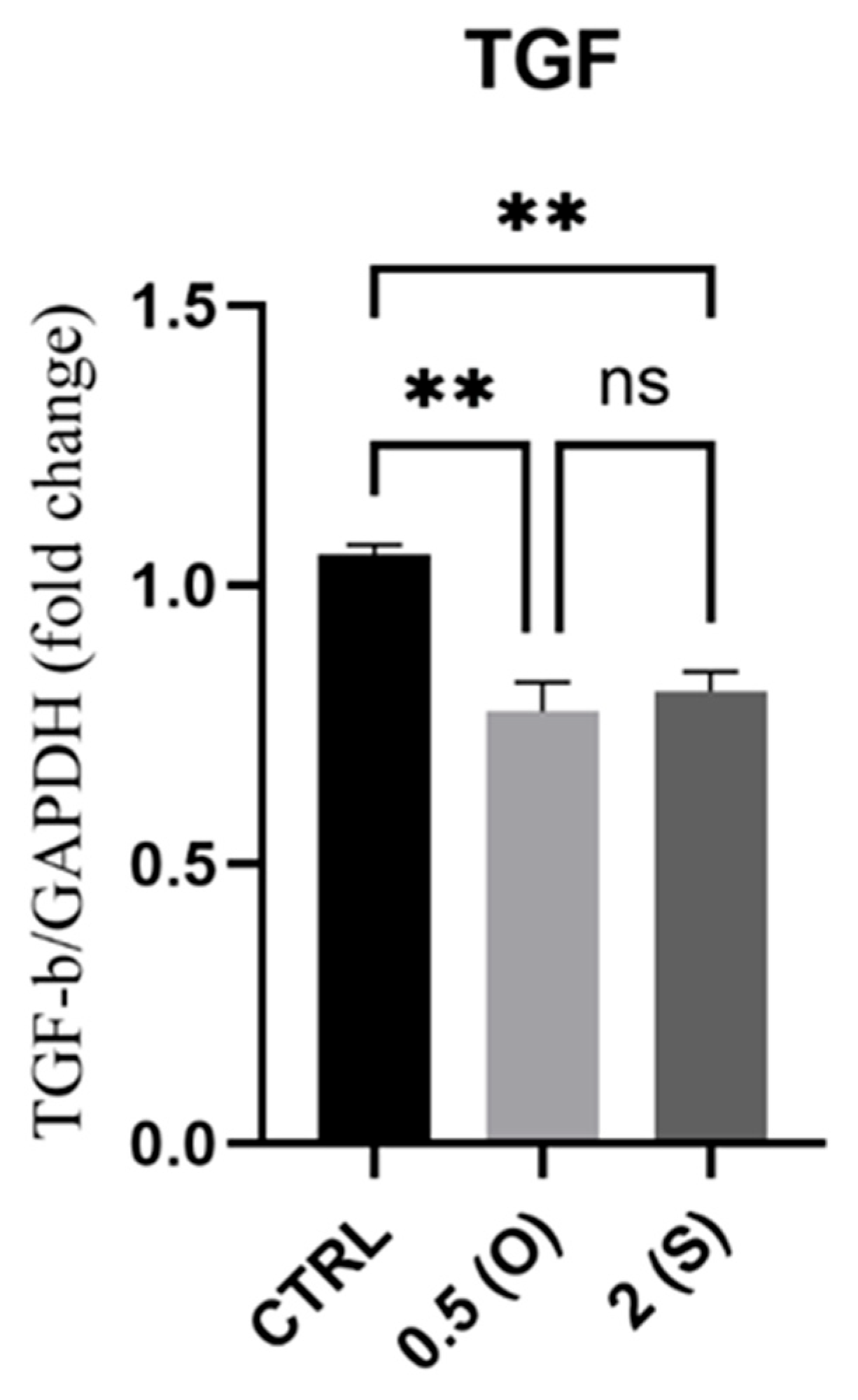
3.2. Transforming Growth Factor-Beta (TGFβ) Gene Expression
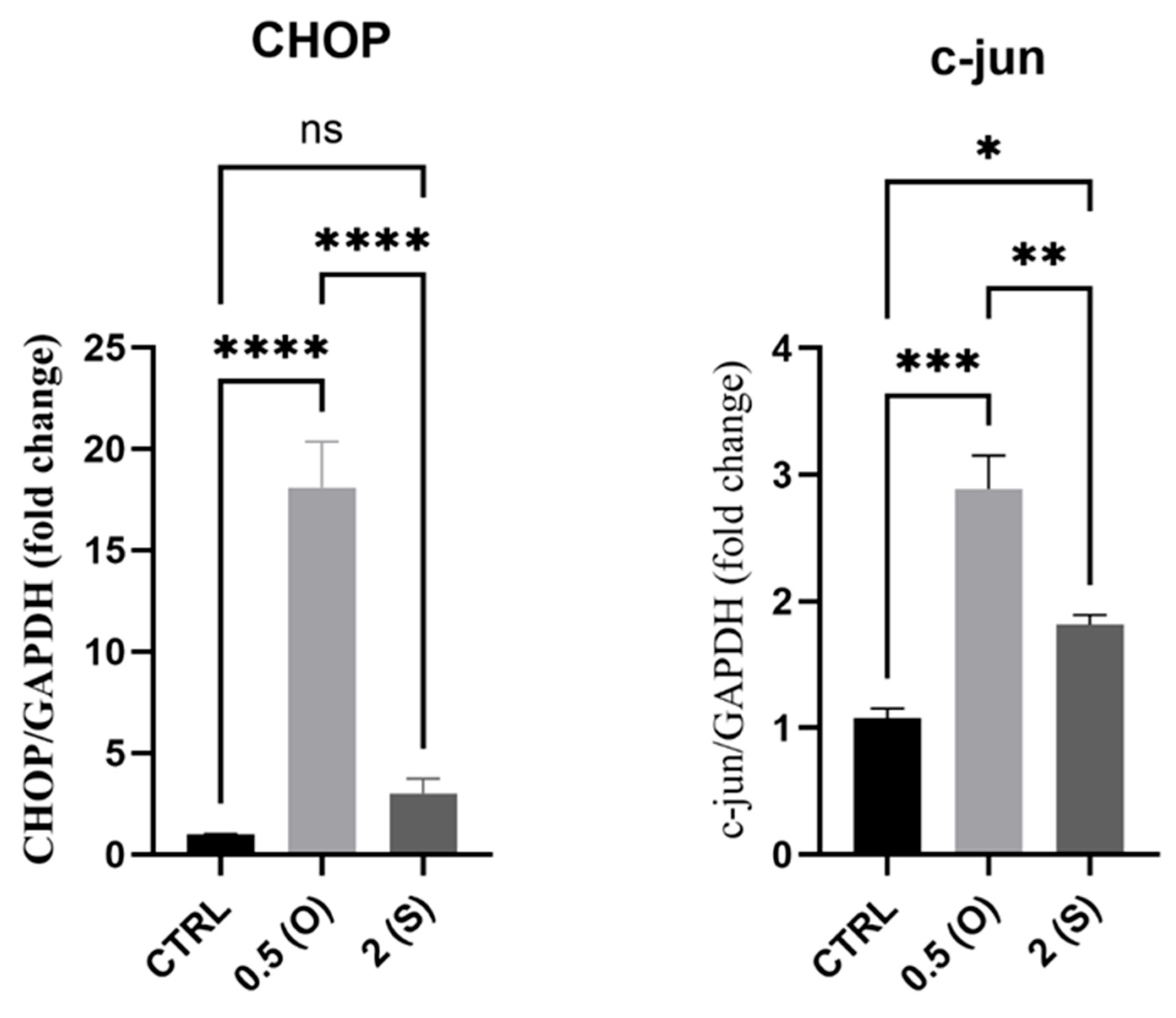
3.3. Apoptosis and Cell Death Markers Gene Expression
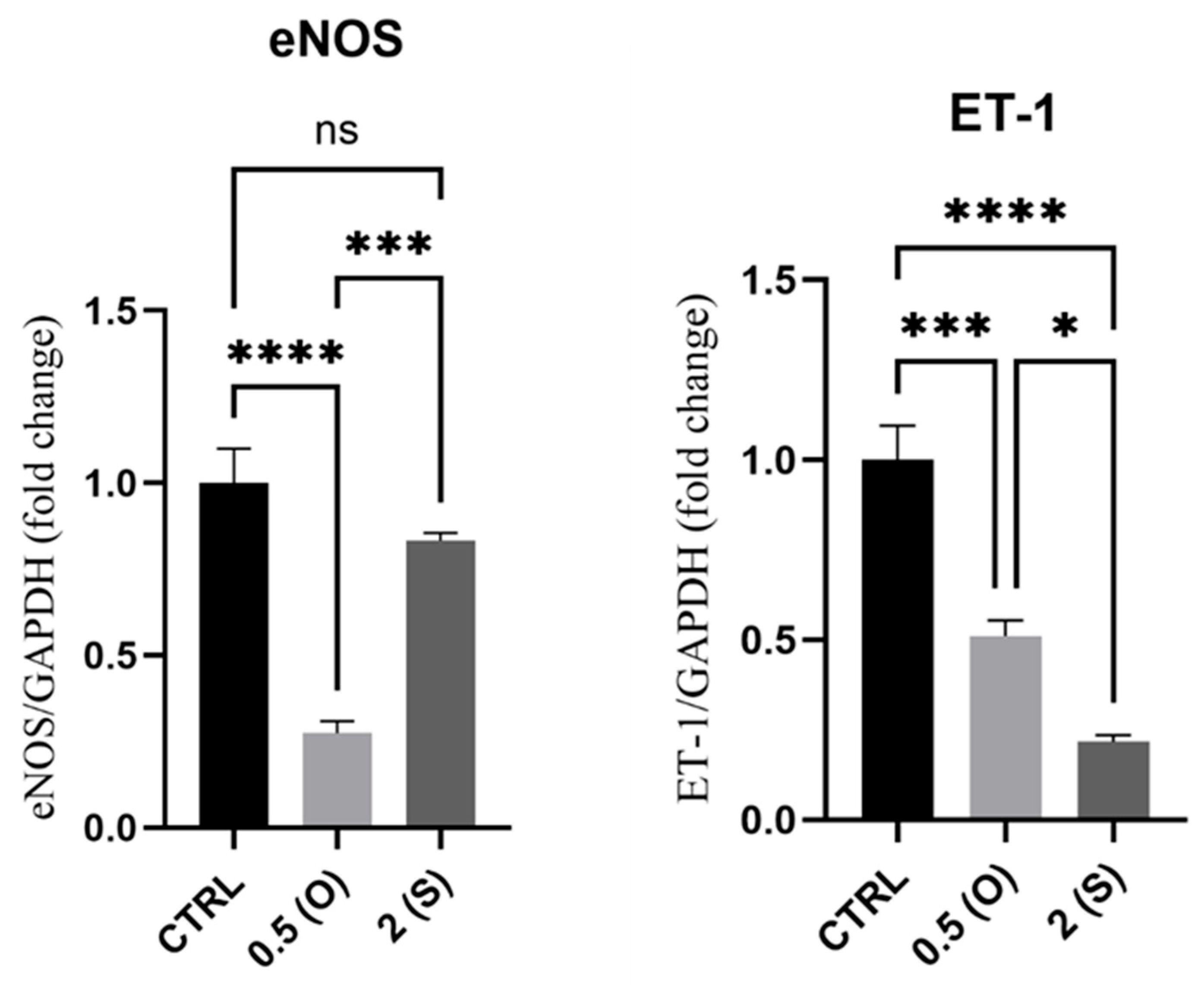
3.4. eNOS and ET-1 Gene Expression
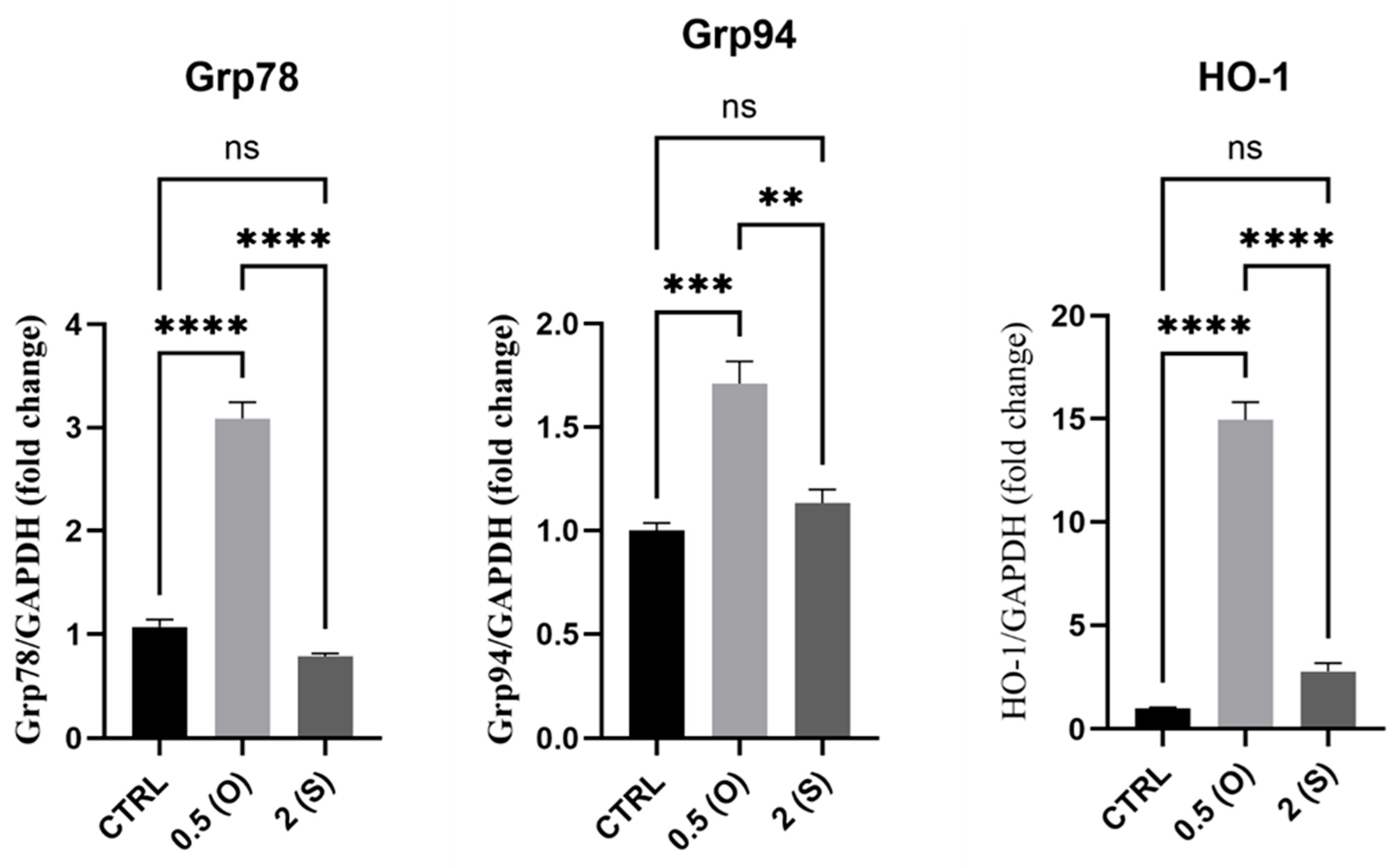
3.5. ER Stress-Associated Gene Expression
3.6. Effect of Different Flow Patterns and Shear Stresses on ROS Generation
4. Discussion
4.1. Markers of Cellular Inflammation: NF-κB and COX-2
4.2. TGF-β
4.3. Cell Death Markers: CHOP, C-Jun
4.4. eNOS and ET-1
4.5. ER Stress Markers: GRP94,GRP78, and HO-1
4.6. ROS Generation
4.7. Novelty of the Current Study and Contribution to the Existing Knowledge
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.; Al-Thani, H.; El-Menyar, A.; Zeidan, A.; Al-Thani, A.; Yalcin, H.C. Disturbed hemodynamics and oxidative stress interaction in endothelial dysfunction and AAA progression: Focus on Nrf2 pathway. Int. J. Cardiol. 2023, 389, 131238. [Google Scholar] [CrossRef]
- Katoh, K. Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro. Int. J. Mol. Sci. 2023, 24, 16518. [Google Scholar] [CrossRef]
- Mutlu, O.; Salman, H.E.; Al-Thani, H.; El-Menyar, A.; Qidwai, U.A.; Yalcin, H.C. How does hemodynamics affect rupture tissue mechanics in abdominal aortic aneurysm: Focus on wall shear stress derived parameters, time-averaged wall shear stress, oscillatory shear index, endothelial cell activation potential, and relative residence time. Comput. Biol. Med. 2023, 154, 106609. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.E.; Yalcin, H.C. Computational Modeling of Blood Flow Hemodynamics for Biomechanical Investigation of Cardiac Development and Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 14. [Google Scholar] [CrossRef]
- Cavallero, S.; Blázquez-Medela, A.M.; Satta, S.; Hsiai, T.K. Endothelial mechanotransduction in cardiovascular development and regeneration: Emerging approaches and animal models. Curr. Top. Membr. 2021, 87, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, N.; Islam, M.S.; Raza, M.Z.; Mahin, S.K.H.; Islam, M.R.; Chowdhury, M.E.H.; Al-Ali, A.; Agouni, A.; Yalcin, H.C. Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows. Bioengineering 2024, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Warabi, E.; Wada, Y.; Kajiwara, H.; Kobayashi, M.; Koshiba, N.; Hisada, T.; Shibata, M.; Ando, J.; Tsuchiya, M.; Kodama, T.; et al. Effect on endothelial cell gene expression of shear stress, oxygen concentration, and low-density lipoprotein as studied by a novel flow cell culture system. Free Radic. Biol. Med. 2004, 37, 682–694. [Google Scholar] [CrossRef]
- Chen, X.L.; Varner, S.E.; Rao, A.S.; Grey, J.Y.; Thomas, S.; Cook, C.K.; Wasserman, M.A.; Medford, R.M.; Jaiswal, A.K.; Kunsch, C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J. Biol. Chem. 2003, 278, 703–711. [Google Scholar] [CrossRef]
- Goettsch, C.; Goettsch, W.; Brux, M.; Haschke, C.; Brunssen, C.; Muller, G.; Bornstein, S.R.; Duerrschmidt, N.; Wagner, A.H.; Morawietz, H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res. Cardiol. 2011, 106, 551–561. [Google Scholar] [CrossRef]
- Pech, S.; Richter, R.; Lienig, J. Peristaltic Pump with Continuous Flow and Programmable Flow Pulsation. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference (ESTC), Tønsberg, Norway, 15–18 September 2020; pp. 1–5. [Google Scholar]
- McSweeney, S.R.; Warabi, E.; Siow, R.C.M. Nrf2 as an Endothelial Mechanosensitive Transcription Factor. Hypertension 2016, 67, 20–29. [Google Scholar] [CrossRef]
- Booth, R.; Noh, S.; Kim, H. A multiple-channel, multiple-assay platform for characterization of full-range shear stress effects on vascular endothelial cells. Lab Chip 2014, 14, 1880–1890. [Google Scholar] [CrossRef]
- Orr, A.W.; Hahn, C.; Blackman, B.R.; Schwartz, M.A. p21-Activated Kinase Signaling Regulates Oxidant-Dependent NF-κB Activation by Flow. Circ. Res. 2008, 103, 671–679. [Google Scholar] [CrossRef]
- Yan, H.; Hu, Y.; Akk, A.; Wickline, S.A.; Pan, H.; Pham, C.T.N. Peptide-siRNA nanoparticles targeting NF-κB p50 mitigate experimental abdominal aortic aneurysm progression and rupture. Biomater. Adv. 2022, 139, 213009. [Google Scholar] [CrossRef]
- Fatima, M.T.; Hasan, M.; Abdelsalam, S.S.; Sivaraman, S.K.; El-Gamal, H.; Zahid, M.A.; Elrayess, M.A.; Korashy, H.M.; Zeidan, A.; Parray, A.S.; et al. Sestrin2 suppression aggravates oxidative stress and apoptosis in endothelial cells subjected to pharmacologically induced endoplasmic reticulum stress. Eur. J. Pharmacol. 2021, 907, 174247. [Google Scholar] [CrossRef]
- Huang, H.; Ren, P.; Zhao, Y.; Weng, H.; Jia, C.; Yu, F.; Nie, Y. Low shear stress induces inflammatory response via CX3CR1/NF-κB signal pathway in human umbilical vein endothelial cells. Tissue Cell 2023, 82, 102043. [Google Scholar] [CrossRef] [PubMed]
- Russell-Puleri, S.; Dela Paz, N.G.; Adams, D.; Chattopadhyay, M.; Cancel, L.; Ebong, E.; Orr, A.W.; Frangos, J.A.; Tarbell, J.M. Fluid shear stress induces upregulation of COX-2 and PGI(2) release in endothelial cells via a pathway involving PECAM-1, PI3K, FAK, and p38. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H485–H500. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Funk, C.D.; FitzGerald, G.A. COX-2 Inhibitors and Cardiovascular Risk. J. Cardiovasc. Pharmacol. 2007, 50, 470–479. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr. Physiol. 2019, 9, 873–904. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Pokutta-Paskaleva, A.; Kumar, S.; Timmins, L.H.; Morris, A.D.; Kang, D.-W.; Dalal, S.; Chadid, T.; Kuo, K.M.; Raykin, J.; et al. Disturbed Flow Promotes Arterial Stiffening Through Thrombospondin-1. Circulation 2017, 136, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Cuhlmann, S.; Heiden, K.V.d.; Saliba, D.; Tremoleda, J.L.; Khalil, M.; Zakkar, M.; Chaudhury, H.; Luong, L.A.; Mason, J.C.; Udalova, I.; et al. Disturbed Blood Flow Induces RelA Expression via c-Jun N-Terminal Kinase 1. Circ. Res. 2011, 108, 950–959. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, J.; Kim, K.H.; An, S.H.; Kim, M.; Park, J.; Kwon, K. Fluid shear stress regulates the expression of Lectin-like oxidized low density lipoprotein receptor-1 via KLF2-AP-1 pathway depending on its intensity and pattern in endothelial cells. Atherosclerosis 2018, 270, 76–88. [Google Scholar] [CrossRef]
- Wang, J.; An, F.S.; Zhang, W.; Gong, L.; Wei, S.J.; Qin, W.D.; Wang, X.P.; Zhao, Y.X.; Zhang, Y.; Zhang, C.; et al. Inhibition of c-Jun N-terminal kinase attenuates low shear stress-induced atherogenesis in apolipoprotein E-deficient mice. Mol. Med. 2011, 17, 990–999. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, G.; Surapisitchat, J.; Berk, B.C.; Min, W. Laminar flow inhibits TNF-induced ASK1 activation by preventing dissociation of ASK1 from its inhibitor 14-3-3. J. Clin. Invest. 2001, 107, 917–923. [Google Scholar] [CrossRef][Green Version]
- Chung, J.; Kim, K.H.; Lee, S.C.; An, S.H.; Kwon, K. Ursodeoxycholic Acid (UDCA) Exerts Anti-Atherogenic Effects by Inhibiting Endoplasmic Reticulum (ER) Stress Induced by Disturbed Flow. Mol. Cells 2015, 38, 851–858. [Google Scholar] [CrossRef]
- Zeng, L.; Zampetaki, A.; Margariti, A.; Pepe, A.E.; Alam, S.; Martin, D.; Xiao, Q.; Wang, W.; Jin, Z.-G.; Cockerill, G.; et al. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. USA 2009, 106, 8326–8331. [Google Scholar] [CrossRef]
- Kleinert, H.; Forstermann, U. Endothelial Nitric Oxide Synthase. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–8. [Google Scholar]
- Sunderland, K.; Jiang, J.; Zhao, F. Disturbed flow’s impact on cellular changes indicative of vascular aneurysm initiation, expansion, and rupture: A pathological and methodological review. J. Cell Physiol. 2022, 237, 278–300. [Google Scholar] [CrossRef]
- Heo, K.S.; Fujiwara, K.; Abe, J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ. J. 2011, 75, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Harding, I.C.; Mitra, R.; Mensah, S.A.; Herman, I.M.; Ebong, E.E. Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation. J. Transl. Med. 2018, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Chambaz, C.; Stergiopulos, N.; Hayoz, D.; Silacci, P. Transcriptional and post-transcriptional regulation of preproendothelin-1 by plaque-prone hemodynamics. Atherosclerosis 2007, 194, 383–390. [Google Scholar] [CrossRef]
- Chatterjee, S.; Browning, E.A.; Hong, N.; DeBolt, K.; Sorokina, E.M.; Liu, W.; Birnbaum, M.J.; Fisher, A.B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H105–H114. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Liu, C.A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef]
- Ruze, A.; Zhao, Y.; Li, H.; Gulireba, X.; Li, J.; Lei, D.; Dai, H.; Wu, J.; Zhao, X.; Nie, Y. Low shear stress upregulates the expression of fractalkine through the activation of mitogen-activated protein kinases in endothelial cells. Blood Coagul. Fibrinolysis 2018, 29, 361–368. [Google Scholar] [CrossRef]
- Balaguru, U.M.; Sundaresan, L.; Manivannan, J.; Majunathan, R.; Mani, K.; Swaminathan, A.; Venkatesan, S.; Kasiviswanathan, D.; Chatterjee, S. Disturbed flow mediated modulation of shear forces on endothelial plane: A proposed model for studying endothelium around atherosclerotic plaques. Sci. Rep. 2016, 6, 27304. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chen, Y.; Pei, Y.; Long, Y.; Liu, C.; Cao, J.; Chen, P. The role of cyclooxygenase-2 in the protection against apoptosis in vascular endothelial cells induced by cigarette smoking. J. Thorac. Dis. 2017, 9, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P. Coronary Artery Wall Shear Stress Is Associated With Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients With Coronary Artery Disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Lacroix, P.; Criqui, M.H. Large and Small Vessels Atherosclerosis: Similarities and Differences. Prog. Cardiovasc. Dis. 2007, 50, 112–125. [Google Scholar] [CrossRef]
| Target | Forward | Reverse |
|---|---|---|
| GRP78 | CATCACGCCGTCCTATGTCG | CGTCAAAGACCGTGTTCTCG |
| GRP94 | GCTGACGATGAAGTTGATGTGG | CATCCGTCCTTGATCCTTCTCTA |
| CHOP | GAACGGCTCAAGCAGGAAATC | TTCACCATTCGGTCAATCAGAG |
| HO-1 | AAGACTGCGTTCCTGCTCAAC | AAAGCCCTACAGCAACTGTCG |
| c-Jun | CCTTGAAAGCTCAGAACTCGGAG | TGCTGCGTTAGCATGAGTTGGC |
| NFκ-B | GCAGCACTACTTCTTGACCACC | TCTGCTCCTGAGCATTGACGTC |
| COX-2 | CGGTGAAACTCTGGCTAGACAG | GCAAACCGTAGATGCTCAGGGA |
| GAPDH | CCAAGGAGTAAGACCCCTGG | TGGTTGAGCACAGGGTACTT |
| TGFβ | CCCAGCATCTGCAAAGCTC | GTCAATGTACAGCTGCCGCA |
| eNOS | GAAGGCGACAATCCTGTATGGC | TGTTCGAGGGACACCACGTCAT |
| ET-1 | CTACTTCTGCCACCTGGACATC | TCACGGTCTGTTGCCTTTGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Mutlu, O.; Islam, M.S.; Shurbaji, S.; Sulaiman, R.; Elsharabassi, Y.; Agouni, A.; Yalcin, H.C. Oscillatory Disturbed Flow Enhances Inflammatory and Oxidative Stress Markers in Endothelial Cells. Methods Protoc. 2025, 8, 130. https://doi.org/10.3390/mps8060130
Hasan M, Mutlu O, Islam MS, Shurbaji S, Sulaiman R, Elsharabassi Y, Agouni A, Yalcin HC. Oscillatory Disturbed Flow Enhances Inflammatory and Oxidative Stress Markers in Endothelial Cells. Methods and Protocols. 2025; 8(6):130. https://doi.org/10.3390/mps8060130
Chicago/Turabian StyleHasan, Maram, Onur Mutlu, Munshi Sajidul Islam, Samar Shurbaji, Ruba Sulaiman, Yasmin Elsharabassi, Abdelali Agouni, and Huseyin C. Yalcin. 2025. "Oscillatory Disturbed Flow Enhances Inflammatory and Oxidative Stress Markers in Endothelial Cells" Methods and Protocols 8, no. 6: 130. https://doi.org/10.3390/mps8060130
APA StyleHasan, M., Mutlu, O., Islam, M. S., Shurbaji, S., Sulaiman, R., Elsharabassi, Y., Agouni, A., & Yalcin, H. C. (2025). Oscillatory Disturbed Flow Enhances Inflammatory and Oxidative Stress Markers in Endothelial Cells. Methods and Protocols, 8(6), 130. https://doi.org/10.3390/mps8060130








