Development of a HPLC Method for Analysis of a Combination of Clofazimine, Isoniazid, Pyrazinamide, and Rifampicin Incorporated into a Dermal Self-Double-Emulsifying Drug Delivery System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Preez, J.L.; Aucamp, M.E.; Burger, C.; Gerber, M.; Viljoen, J.M.; Van Zyl, L.; Du Plessis, J. Development and Validation of the Simultaneous Determination of Artemisone, Clofazimine and Decoquinate with HPLC. Pharmazie 2018, 73, 139–142. [Google Scholar] [CrossRef]
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets—2017. Morb. Mortal. Wkly. Rep. 2019, 68, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lan, H.; Shi, H.; Wu, P.; Zhou, Y. Evaluating the Diversity of Circulating Natural Killer Cells between Active Tuberculosis and Latent Tuberculosis Infection. Tuberculosis 2022, 135, 102221. [Google Scholar] [CrossRef]
- Mashele, S.A.; Steel, H.C.; Matjokotja, M.T.; Rasehlo, S.S.M.; Anderson, R.; Cholo, M.C. Assessment of the Efficacy of Clofazimine Alone and in Combination with Primary Agents against Mycobacterium tuberculosis In Vitro. J. Glob. Antimicrob. Resist. 2022, 29, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, N.C.; Swanson, R.V.; Bautista, E.M.; Almeida, D.V.; Saini, V.; Omansen, T.F.; Guo, H.; Chang, Y.S.; Li, S.-Y.; Tapley, A.; et al. Impact of Clofazimine Dosing on Treatment Shortening of the First-Line Regimen in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, 7. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Chang, K.C.; Guglielmetti, L.; Furin, J.; Schaaf, H.S.; Chesov, D.; Esmail, A.; Lange, C. Clinical Management of Adults and Children with Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Clin. Microbiol. Infect. 2017, 23, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chen, X.; Li, Z.; Pang, Y.; Jing, W.; Liu, P.; Wu, T.; Cai, C.; Shi, J.; Qin, Z.; et al. Clofazimine Improves Clinical Outcomes in Multidrug-Resistant Tuberculosis: A Randomized Controlled Trial. Clin. Microbiol. Infect. 2019, 25, 190–195. [Google Scholar] [CrossRef]
- van Staden, D.; Haynes, R.K.; Viljoen, J.M. Adapting Clofazimine for Treatment of Cutaneous Tuberculosis by Using Self-Double-Emulsifying Drug Delivery Systems. Antibiotics 2022, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, W.J.; Glass, D.; Goode, K.; Stables, G.I.; Boorman, G.C. A Double-Blind Investigation of the Potential Systemic Absorption of Isotretinoin, When Combined with Chemical Sunscreens, Following Topical Application to Patients with Widespread Acne of the Face and Trunk. Acta Derm. Venereol. 2001, 81, 14–17. [Google Scholar] [CrossRef]
- van Staden, D.; du Plessis, J.; Viljoen, J. Development of a Self-Emulsifying Drug Delivery System for Optimized Topical Delivery of Clofazimine. Pharmaceutics 2020, 12, 523. [Google Scholar] [CrossRef]
- Becker, C.; Dressman, J.B.; Amidon, G.L.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Barends, D.M. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Isoniazid. J. Pharm. Sci. 2007, 96, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Geib, A.J.; Shannon, M.W. Isoniazid. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 919–925. [Google Scholar] [CrossRef]
- Mehta, S.K.; Jindal, N. Formulation of Tyloxapol Niosomes for Encapsulation, Stabilization and Dissolution of Anti-Tubercular Drugs. Colloids Surf. B Biointerfaces 2013, 101, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Isoniazid. Tuberculosis 2008, 88, 112–116. [CrossRef]
- Alcántara, R.; Fuentes, P.; Marin, L.; Kirwan, D.E.; Gilman, R.H.; Zimic, M.; Sheen, P. Direct Determination of Pyrazinamide (PZA) Susceptibility by Sputum Microscopic Observation Drug Susceptibility (MODS) Culture at Neutral PH: The MODS-PZA Assay. J. Clin. Microbiol. 2020, 58, 5A. [Google Scholar] [CrossRef] [PubMed]
- Pyrazinamide. Tuberculosis 2008, 88, 141–144. [CrossRef]
- Zhang, Y.; Feng, J.; McManus, S.A.; Lu, H.D.; Ristroph, K.D.; Cho, E.J.; Dobrijevic, E.L.; Chan, H.-K.; Prud’homme, R.K. Design and Solidification of Fast-Releasing Clofazimine Nanoparticles for Treatment of Cryptosporidiosis. Mol. Pharm. 2017, 14, 3480–3488. [Google Scholar] [CrossRef]
- Bodart, L.; Derlet, A.; Buol, X.; Leyssens, T.; Tumanov, N.; Wouters, J. Combining Two Antitubercular Drugs, Clofazimine and 4-Aminosalicylic Acid, in Order to Improve Clofazimine Aqueous Solubility and 4-Aminosalicylic Acid Thermal Stability. J. Pharm. Sci. 2020, 109, 3645–3652. [Google Scholar] [CrossRef]
- Anderson, R.; Theron, A.J.; Nel, J.G.; Durandt, C.; Cholo, M.C.; Feldman, C.; Tintinger, G.R. Clofazimine, but Not Isoniazid or Rifampicin, Augments Platelet Activation in Vitro. Front. Pharmacol. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Henwood, S.Q.; De Villiers, M.M.; Liebenberg, W.; Lötter, A.P. Solubility and Dissolution Properties of Generic Rifampicin Raw Materials. Drug Dev. Ind. Pharm. 2000, 26, 403–408. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S. Assessment of Solubility and Hansen Solubility Parameters of Rifampicin in Various Permeation Enhancers: Experimental and Computational Approach. J. Mol. Liq. 2021, 328, 115432. [Google Scholar] [CrossRef]
- Trousil, J.; Pavliš, O.; Kubíčková, P.; Škorič, M.; Marešová, V.; Pavlova, E.; Knudsen, K.D.; Dai, Y.-S.; Zimmerman, M.; Dartois, V.; et al. Antitubercular Nanocarrier Monotherapy: Study of In Vivo Efficacy and Pharmacokinetics for Rifampicin. J. Control. Release 2020, 321, 312–323. [Google Scholar] [CrossRef]
- Sutradhar, I.; Zaman, M.H. Evaluation of the Effect of Temperature on the Stability and Antimicrobial Activity of Rifampicin Quinone. J. Pharm. Biomed. Anal. 2021, 197, 113941. [Google Scholar] [CrossRef] [PubMed]
- Chellini, P.R.; Lages, E.B.; Franco, P.H.C.; Nogueira, F.H.A.; César, I.C.; Pianetti, G.A. Development and Validation of an HPLC Method for Simultaneous Determination of Rifampicin, Isoniazid, Pyrazinamide, and Ethambutol Hydrochloride in Pharmaceutical Formulations. J. AOAC Int. 2015, 98, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Ethambutol. Tuberculosis 2008, 88, 102–105. [CrossRef] [PubMed]

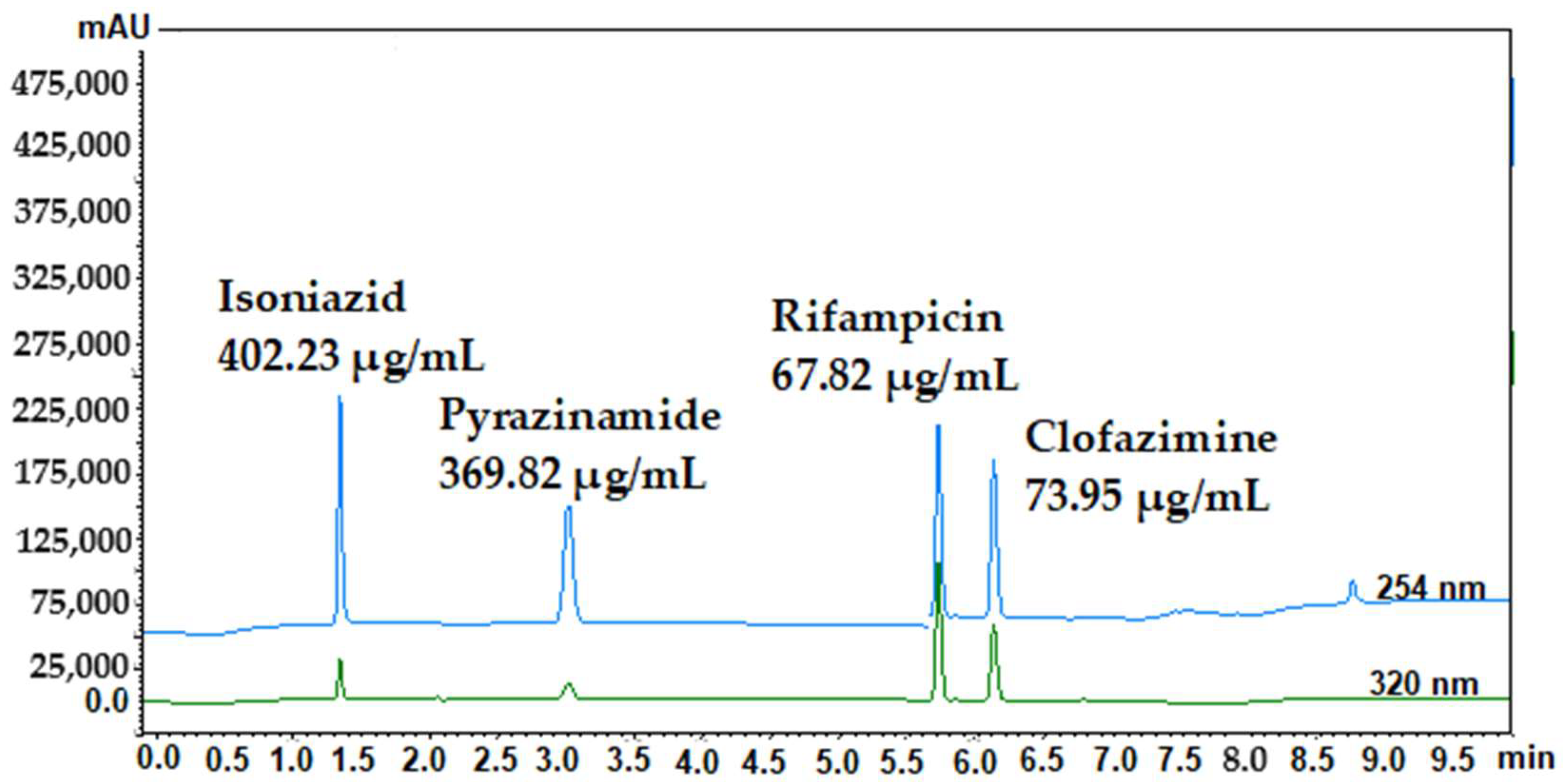
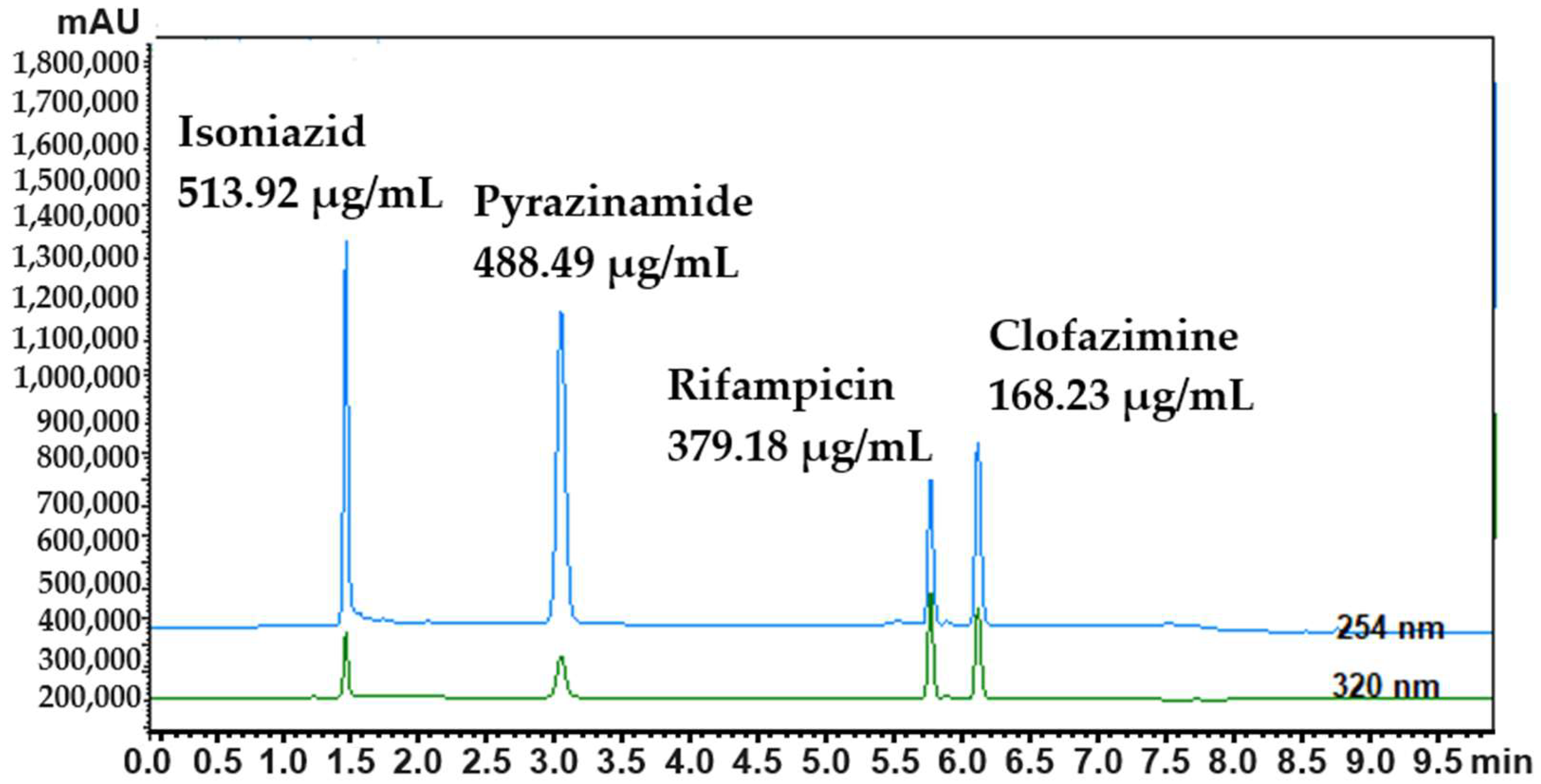
| Isoniazid | Pyrazinamide | Rifampicin | Clofazimine | |
|---|---|---|---|---|
| Concentration range (µg/mL) | 5.29–520.00 | 5.19–500.00 | 6.01–385.00 | 5.21–335.00 |
| Regression equation | y = 924.51x + 259.47 | y = 1154.40x − 1589.60 | y = 4209.90x − 7194.10 | y = 2431.60x − 624.93 |
| r2 (>0.99) 1 | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| Accuracy mean recovery (%) (%RSD) 2 (100 ± 2%) | 100.04 (±0.06) | 101.28 (±0.70) | 100.15 (±0.80) | 99.74 (±0.28) |
| Stability (%RSD) (<15%) | 1.84 | 0.78 | 0.69 | 1.06 |
| System repeatability (±SD) 3 | 1.46 (±0.18) | 3.11 (±0.04) | 5.71 (±0.16) | 5.96 (±0.34) |
| Retention time (min) %RSD | 0.34 | 0.94 | 0.88 | 0.23 |
| Isoniazid | Pyrazinamide | Rifampicin | Clofazimine | ||
|---|---|---|---|---|---|
| Concentration range (µg/mL) | 250–515 | 240–490 | 180–380 | 90–170 | |
| Repeatability (intra-day) | %RSD | 0.03 | 0.02 | 0.01 | 0.03 |
| Intermediate precision (inter-day) | Mean recovery (%) | 100.30 | 100.95 | 99.91 | 98.91 |
| %RSD (day 2) | 0.07 | 0.04 | 0.04 | 0.75 | |
| Mean recovery percentage (day 2) | 99.56 | 99.70 | 98.89 | 98.45 | |
| %RSD (day 3) | 0.02 | 0.01 | 0.17 | 0.05 | |
| Mean recovery percentage (day 3) | 101.25 | 98.70 | 99.68 | 97.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staden, D.v.; Haynes, R.K.; Van der Kooy, F.; Viljoen, J.M. Development of a HPLC Method for Analysis of a Combination of Clofazimine, Isoniazid, Pyrazinamide, and Rifampicin Incorporated into a Dermal Self-Double-Emulsifying Drug Delivery System. Methods Protoc. 2023, 6, 104. https://doi.org/10.3390/mps6060104
Staden Dv, Haynes RK, Van der Kooy F, Viljoen JM. Development of a HPLC Method for Analysis of a Combination of Clofazimine, Isoniazid, Pyrazinamide, and Rifampicin Incorporated into a Dermal Self-Double-Emulsifying Drug Delivery System. Methods and Protocols. 2023; 6(6):104. https://doi.org/10.3390/mps6060104
Chicago/Turabian StyleStaden, Daniélle van, Richard K. Haynes, Frank Van der Kooy, and Joe M. Viljoen. 2023. "Development of a HPLC Method for Analysis of a Combination of Clofazimine, Isoniazid, Pyrazinamide, and Rifampicin Incorporated into a Dermal Self-Double-Emulsifying Drug Delivery System" Methods and Protocols 6, no. 6: 104. https://doi.org/10.3390/mps6060104
APA StyleStaden, D. v., Haynes, R. K., Van der Kooy, F., & Viljoen, J. M. (2023). Development of a HPLC Method for Analysis of a Combination of Clofazimine, Isoniazid, Pyrazinamide, and Rifampicin Incorporated into a Dermal Self-Double-Emulsifying Drug Delivery System. Methods and Protocols, 6(6), 104. https://doi.org/10.3390/mps6060104








