A Practical Peptide Synthesis Workflow Using Amino-Li-Resin
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Instrumentation
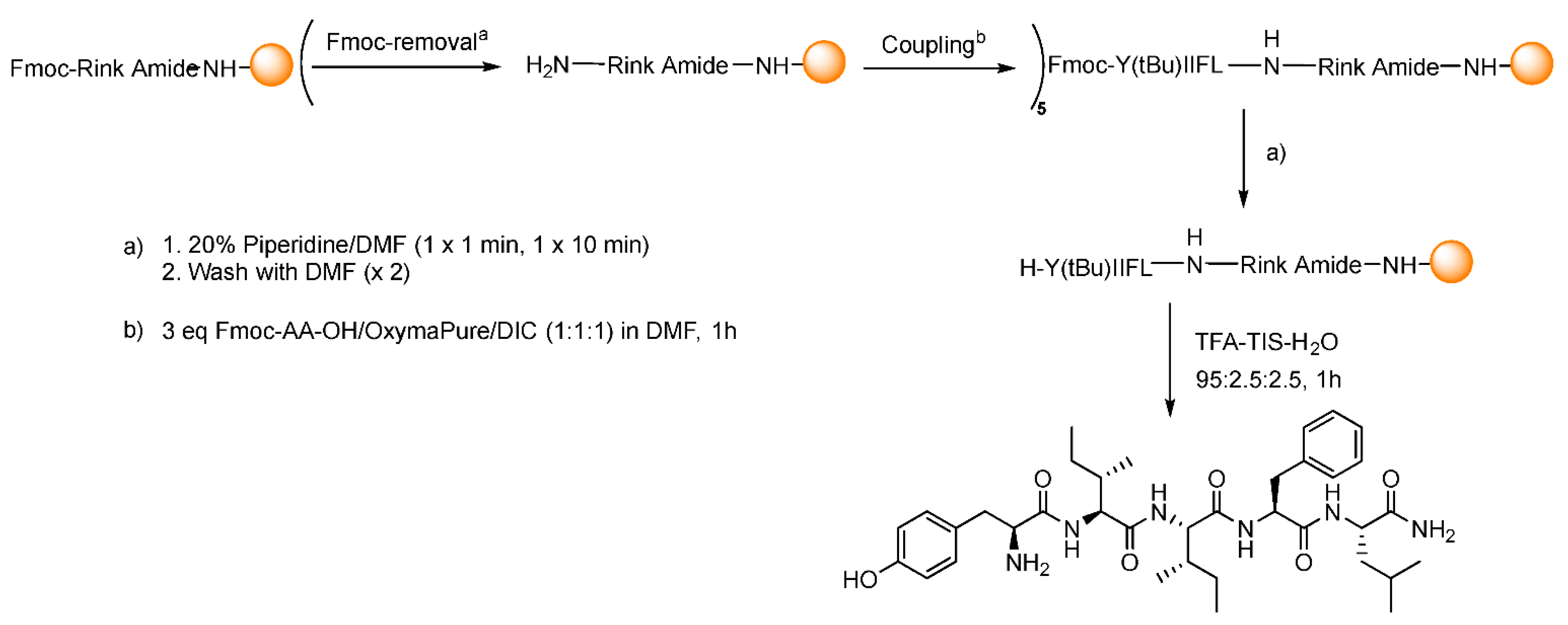
2.3. Synthesis Protocol
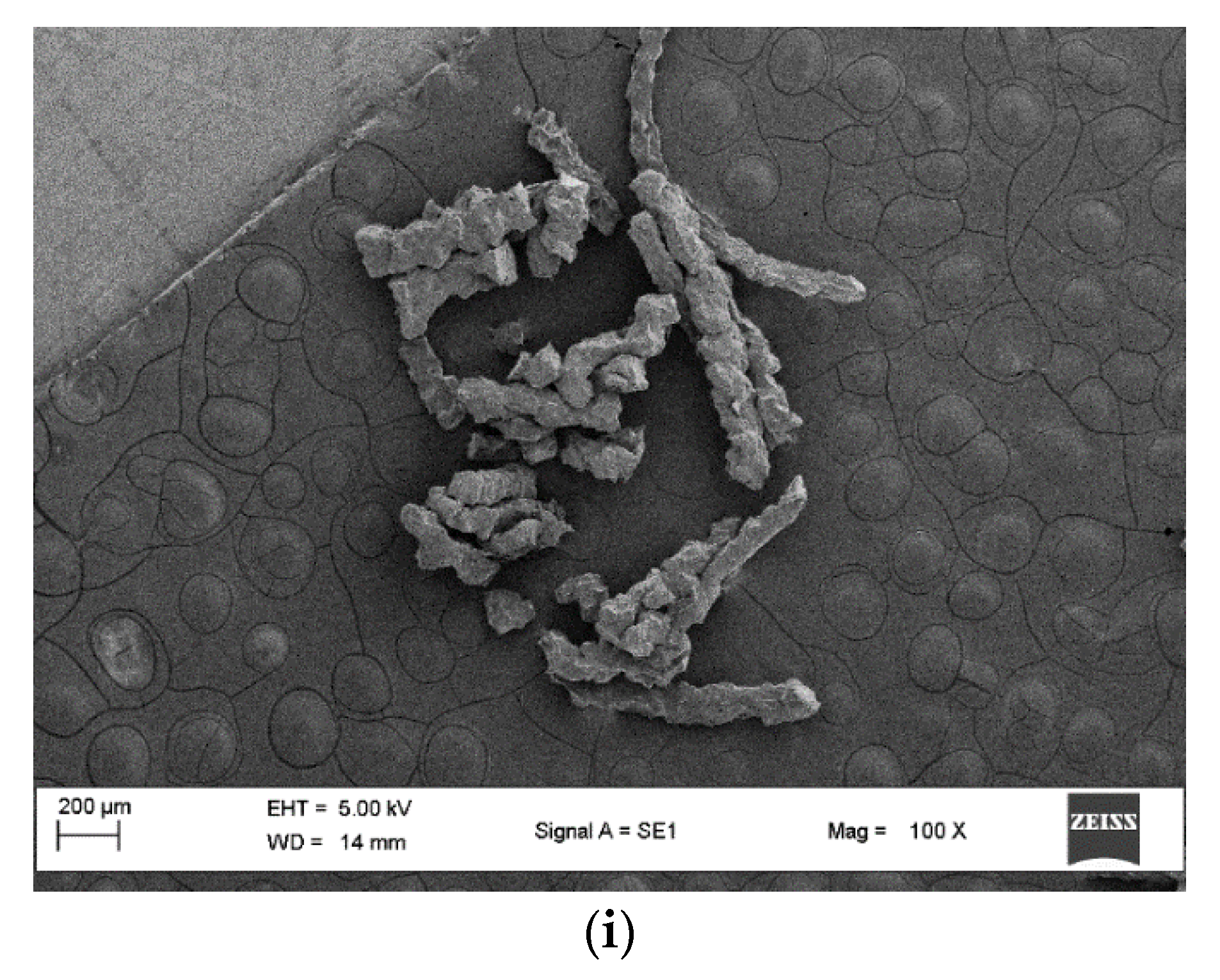
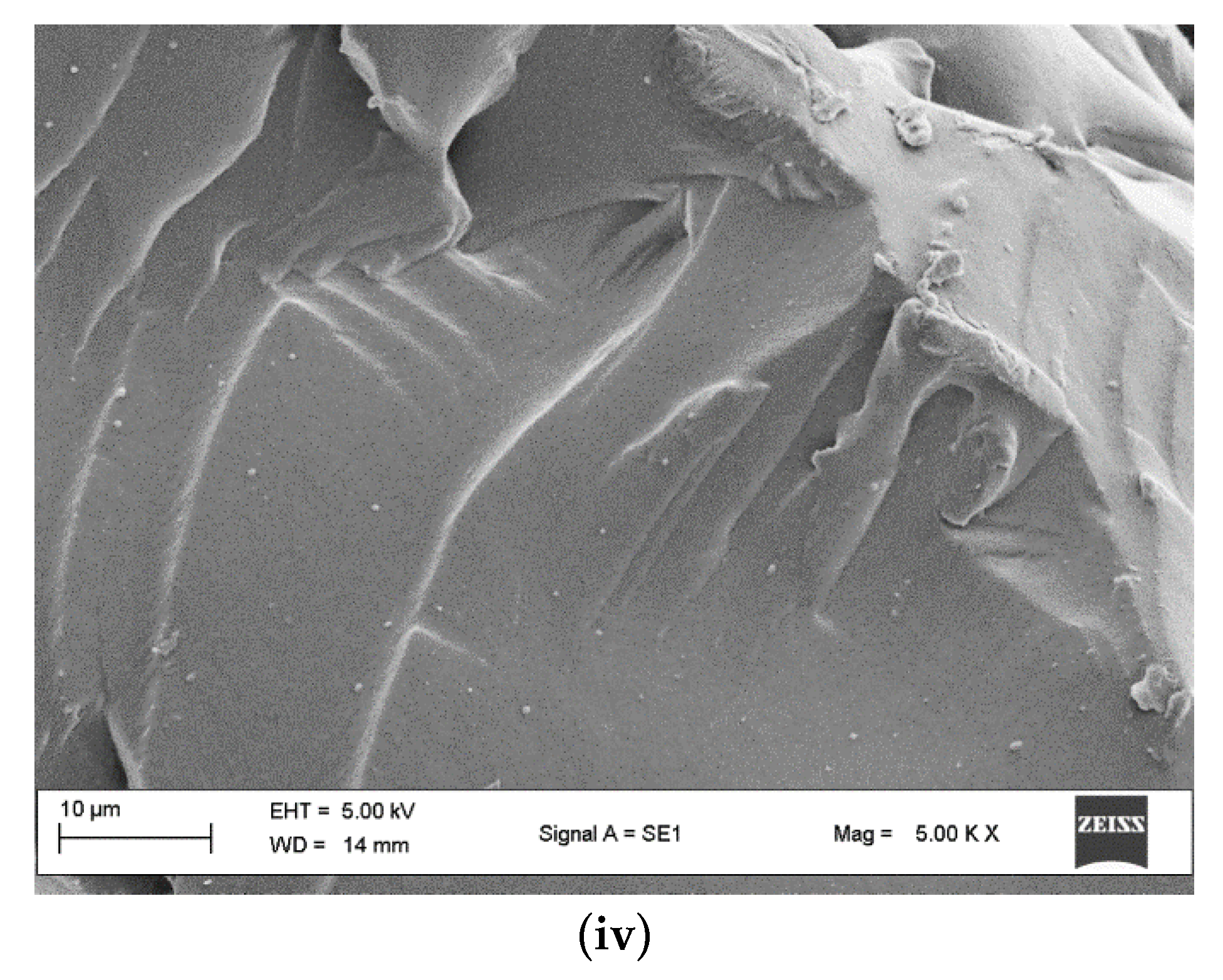
2.3.1. Loading Determination
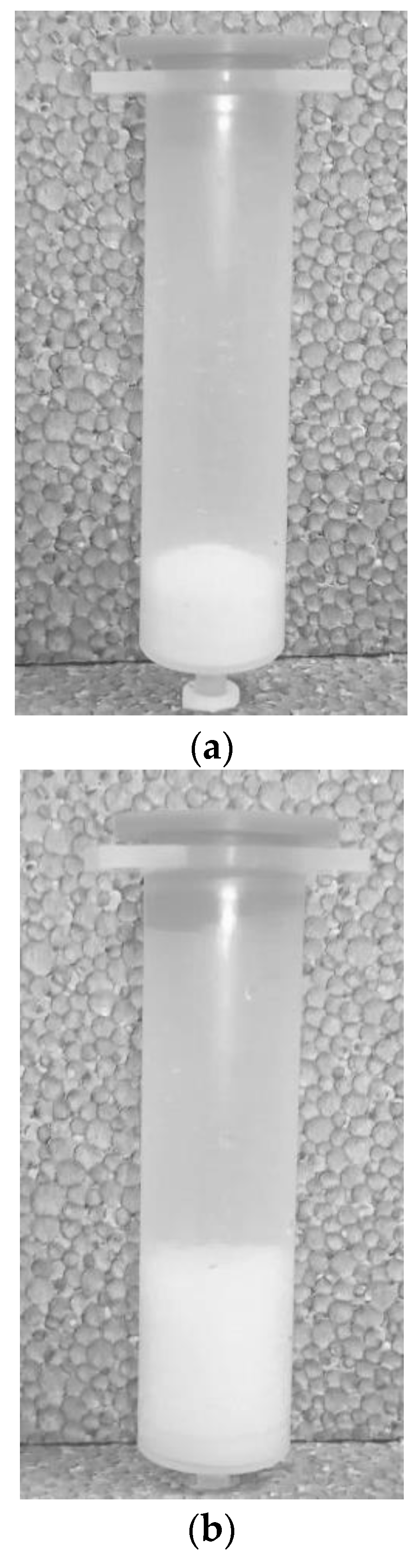
2.3.2. Resin Swelling
2.3.3. Mixing and Filtration Strategy
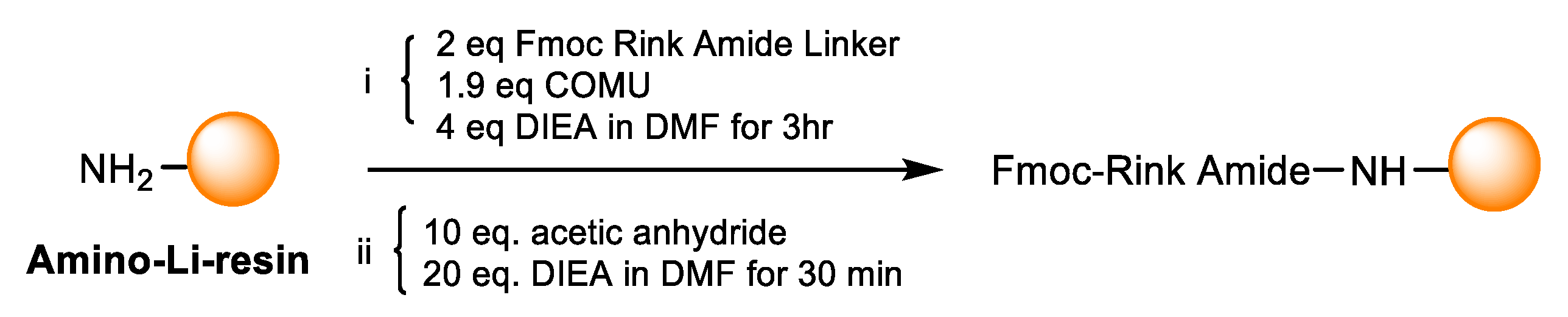
2.3.4. Addition of Fmoc Rink-Amide Linker
2.3.5. Fmoc-AA-OH Coupling
Deprotection
Coupling
Cleavage
Precipitation
3. Result
Yield and Purity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albericio, F.; Carpino, L.A. Coupling reagents and activation. Methods Enzymol. 1997, 289, 104–126. [Google Scholar] [CrossRef]
- Albericio, B.F.; Kates, S.A. Coupling methods: Solid-phase formation of amide and ester bonds. In Solid-Phase Synthesis; CRC Press: Boca Raton, FL, USA, 2000; pp. 297–352. [Google Scholar]
- Montalbetti, C.A.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Jaradat, D.s.M. Thirteen decades of peptide synthesis: Key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 2018, 50, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Biorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lath, A.; Santal, A.R.; Kaur, N.; Kumari, P.; Singh, N.P. Anti-cancer peptides: Their current trends in the development of peptide-based therapy and anti-tumor drugs. Biotechnol. Genet. Eng. Rev. 2022, 1–40. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Durak, A.; Akkus, E.; Canpolat, A.G.; Tuncay, E.; Corapcioglu, D.; Turan, B. Glucagon-like peptide-1 receptor agonist treatment of high carbohydrate intake-induced metabolic syndrome provides pleiotropic effects on cardiac dysfunction through alleviations in electrical and intracellular Ca2+ abnormalities and mitochondrial dysfunction. Clin. Exp. Pharmacol. Physiol. 2022, 49, 46–59. [Google Scholar] [CrossRef]
- Ko, S.-C.; Jeon, Y.-J. Marine peptides for preventing metabolic syndrome. Curr. Protein Pept. Sci. 2013, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, L.; He, G.; Wu, J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018, 9, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Guizy, M.; Luque-Ortega, J.R.; Guerrero, E.; Beatriz, G.; Andreu, D.; Rivas, L.; Valenzuela, C. The induction of NOS2 expression by the hybrid cecropin A–melittin antibiotic peptide CA (1–8) M (1–18) in the monocytic line RAW 264.7 is triggered by a temporary and reversible plasma membrane permeation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 110–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monaim, S.A.A.; Jad, Y.E.; El-Faham, A.; Beatriz, G.; Albericio, F. Teixobactin as a scaffold for unlimited new antimicrobial peptides: SAR study. Biorg. Med. Chem. 2018, 26, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govender, T.; Kruger, H.G.; de la Torre, B.G.; Albericio, F. Short AntiMicrobial Peptides (SAMPs) as a class of extraordinary promising therapeutic agents. J. Pept. Sci. 2016, 22, 438–451. [Google Scholar] [CrossRef]
- Seo, M.-D.; Won, H.-S.; Kim, J.-H.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017, 110, 169–187. [Google Scholar] [CrossRef]
- Brooks, N.A.; Pouniotis, D.S.; Tang, C.-K.; Apostolopoulos, V.; Pietersz, G.A. Cell-penetrating peptides: Application in vaccine delivery. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 25–34. [Google Scholar] [CrossRef]
- Lim, S.H.; Sun, Y.; Madanagopal, T.T.; Rosa, V.; Kang, L. Enhanced skin permeation of anti-wrinkle peptides via molecular modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.-Y. Anti-wrinkle benefits of peptides complex stimulating skin basement membrane proteins expression. Int. J. Mol. Sci. 2019, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Gorouhi, F.; Maibach, H. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.J.; Usman, M.; Zhang, C.; Mehmood, A.; Zhou, M.; Teng, C.; Li, X. An updated review on food-derived bioactive peptides: Focus on the regulatory requirements, safety, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1732–1776. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; de la Torre, B.G.; Albericio, F. In situ Fmoc removal—A sustainable solid-phase peptide synthesis approach. Green Chem. 2022, 24, 4887–4896. [Google Scholar] [CrossRef]
- Fields, G.B. Methods for removing the Fmoc group. Pept. Synth. Protoc. 1994, 35, 17–27. [Google Scholar] [CrossRef]
- Kamiński, Z.J.; Kolesińska, B.; Kolesińska, J.; Sabatino, G.; Chelli, M.; Rovero, P.; Błaszczyk, M.; Główka, M.L.; Papini, A.M. N-Triazinylammonium tetrafluoroborates. A new generation of efficient coupling reagents useful for peptide synthesis. J. Am. Chem. Soc. 2005, 127, 16912–16920. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Beatriz, G.; Albericio, F. Greening Fmoc/t Bu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Martin, V.; Jadhav, S.; Egelund, P.H.G.; Liffert, R.; Castro, H.J.; Krüger, T.; Haselmann, K.F.; Quement, S.T.L.; Albericio, F.; Dettner, F.; et al. Harnessing polarity and viscosity to identify green binary solvent mixtures as viable alternatives to DMF in solid-phase peptide synthesis. Green Chem. 2021, 23, 3295–3311. [Google Scholar] [CrossRef]
- Albericio, F.; Bofill, J.M.; El-Faham, A.; Kates, S.A. Use of Onium Salt-Based Coupling Reagents in Peptide Synthesis1. J. Org. Chem. 1998, 63, 9678–9683. [Google Scholar] [CrossRef]
- Mäde, V.; Els-Heindl, S.; Beck-Sickinger, A.G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein J. Org. Chem. 2014, 10, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Beyermann, M.; Bienert, M. Synthesis of difficult peptide sequences: A comparison of Fmoc-and Boc-technique. Tetrahedron Lett. 1992, 33, 3745–3748. [Google Scholar] [CrossRef]
- Merrifield, B. Concept and early development of solid-phase peptide synthesis. Methods Enzymol. 1997, 289, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ramkisson, S.; Jad, Y.E.; Sharma, A.; de la Torre, B.G.; Albericio, F. OctaGel Resin-A New PEG-PS-based Solid Support for Solid-Phase Peptide Synthesis. Lett. Org. Chem. 2019, 16, 935–940. [Google Scholar] [CrossRef]
- García-Martín, F.; Quintanar-Audelo, M.; García-Ramos, Y.; Cruz, L.J.; Gravel, C.; Furic, R.; Côté, S.; Tulla-Puche, J.; Albericio, F. ChemMatrix, a poly (ethylene glycol)-based support for the solid-phase synthesis of complex peptides. J. Comb. Chem. 2006, 8, 213–220. [Google Scholar] [CrossRef]
- Akintayo, D.C.; de la Torre, B.G.; Li, Y.; Albericio, F. Amino-Li-Resin—A Fiber Polyacrylamide Resin for Solid-Phase Peptide Synthesis. Polymers 2022, 14, 928. [Google Scholar] [CrossRef]
- Bruckdorfer, T.; Marder, O.; Albericio, F. From production of peptides in milligram amounts for research to multi-tons quantities for drugs of the future. Curr. Pharm. Biotechnol. 2004, 5, 29–43. [Google Scholar] [CrossRef]
- Wu, J.; An, G.; Lin, S.; Xie, J.; Zhou, W.; Sun, H.; Pan, Y.; Li, G. Solution-phase-peptide synthesis via the group-assisted purification (GAP) chemistry without using chromatography and recrystallization. Chem. Commun. 2014, 50, 1259–1261. [Google Scholar] [CrossRef]
- Kates, S.A.; McGuinness, B.F.; Blackburn, C.; Griffin, G.W.; Solé, N.A.; Barany, G.; Albericio, F. “High-load” polyethylene glycol–polystyrene (PEG–PS) graft supports for solid-phase synthesis. Pept. Sci. 1998, 47, 365–380. [Google Scholar] [CrossRef]
- Auzanneau, F.I.; Meldal, M.; Bock, K. Synthesis, characterization and biocompatibility of PEGA resins. J. Pept. Sci. 1995, 1, 31–44. [Google Scholar] [CrossRef]
- Atherton, E.; Clive, D.L.; Sheppard, R.C. Polyamide supports for polypeptide synthesis. J. Am. Chem. Soc. 1975, 97, 6584–6585. [Google Scholar] [CrossRef] [PubMed]
- Kanda, P.; Kennedy, R.C.; Sparrow, J.T. Synthesis of polyamide supports for use in peptide synthesis and as peptide-resin conjugates for antibody production. Int. J. Pept. Protein Res. 1991, 38, 385–391. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Albericio, F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Guasch-Camell, J.; Álvarez, M.; Albericio, F. p-Nitrobenzyloxycarbonyl (pNZ) as a Temporary Nα-Protecting Group in Orthogonal Solid-Phase Peptide Synthesis–Avoiding Diketopiperazine and Aspartimide Formation. Eur. J. Org. Chem. 2005, 2005, 3031–3039. [Google Scholar] [CrossRef]
- Albericio, F.; El-Faham, A. Choosing the right coupling reagent for peptides: A twenty-five-year journey. Org. Process Res. Dev. 2018, 22, 760–772. [Google Scholar] [CrossRef]
- Petrou, C.; Sarigiannis, Y. Peptide synthesis. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Singapore, 2018; pp. 1–21. [Google Scholar]
- Lee, Y.S. Gram-scale preparation of C-terminal-modified Enkephalin analogues by typical liquid-phase peptide synthesis. Curr. Protoc. Protein Sci. 2019, 98, e97. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.H.; Albert, K.; Rapp, W.; Dengler, M. Peptides 1983, Proceedings of the Eighth American Peptide Symposium; Hruby, V.J., Rich, D.H., Eds.; Pierce Chemical Company: Rockford, IL, USA, 1983; pp. 87–90. [Google Scholar]
- Kim, H.; Cho, J.K.; Chung, W.-J.; Lee, Y.-S. Core−Shell-Type Resins for Solid-Phase Peptide Synthesis: Comparison with Gel-Type Resins in Solid-Phase Photolytic Cleavage Reaction. Org. Lett. 2004, 6, 3273–3276. [Google Scholar] [CrossRef]
- Kempe, M.; Barany, G. CLEAR: A novel family of highly cross-linked polymeric supports for solid-phase peptide synthesis1,2. J. Am. Chem. Soc. 1996, 118, 7083–7093. [Google Scholar] [CrossRef]
- García-Martín, F.; White, P.; Steinauer, R.; Côté, S.; Tulla-Puche, J.; Albericio, F. The synergy of ChemMatrix resin® and pseudoproline building blocks renders Rantes, a complex aggregated chemokine. Pept. Sci. 2006, 84, 566–575. [Google Scholar] [CrossRef]
- Sparrow, J.T.; Knieb-Cordonier, N.G.; Obeyseskere, N.U.; McMurray, J.S. Large-pore polydimethylacrylamide resin for solid-phase peptide synthesis: Applications in Fmoc chemistry. Pept. Res. 1996, 9, 297–304. [Google Scholar]




| S/N | Chemical Structure | Name | Ref |
|---|---|---|---|
| 1 | Cross-linked Polystyrene | Polystyrene | [34] |
| 2 | Polyethylene glycol-polystyrene (PEG-PS) graft | PEG-PS, TentaGel, Octagel, HiCore | [35,40,49,50] |
| 3 | Crosslinked Polyethylene glycol | ChemMatrix | [36,52] |
| 4 | Crosslinked Polyacrylate PEG | CLEAR | [51] |
| 5 | Polyacrylamide | Amino-Li-resin, SPAR50 | [37,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintayo, D.C.; Manne, S.R.; de la Torre, B.G.; Li, Y.; Albericio, F. A Practical Peptide Synthesis Workflow Using Amino-Li-Resin. Methods Protoc. 2022, 5, 72. https://doi.org/10.3390/mps5050072
Akintayo DC, Manne SR, de la Torre BG, Li Y, Albericio F. A Practical Peptide Synthesis Workflow Using Amino-Li-Resin. Methods and Protocols. 2022; 5(5):72. https://doi.org/10.3390/mps5050072
Chicago/Turabian StyleAkintayo, Damilola Caleb, Srinivasa Rao Manne, Beatriz G. de la Torre, Yongfu Li, and Fernando Albericio. 2022. "A Practical Peptide Synthesis Workflow Using Amino-Li-Resin" Methods and Protocols 5, no. 5: 72. https://doi.org/10.3390/mps5050072
APA StyleAkintayo, D. C., Manne, S. R., de la Torre, B. G., Li, Y., & Albericio, F. (2022). A Practical Peptide Synthesis Workflow Using Amino-Li-Resin. Methods and Protocols, 5(5), 72. https://doi.org/10.3390/mps5050072








