Toxin Removal and Inflammatory State Modulation during Online Hemodiafiltration Using Two Different Dialyzers (TRIAD2 Study)
Abstract
:1. Introduction
- -
- serum levels of albumin, B2M, C-reactive protein (CRP), myoglobin, light chains, retinol-binding protein, homocysteine, alpha-2 microglobulin (A2M), p-cresol, indoxyl sulfate, bisphenol A (BPA), fibroblast growth factor 23 (FGF23), and inflammatory cytokines;
- -
- lymphocyte subsets, activated lymphocytes, activated monocytes, and apoptosis rate;
- -
- accumulation of advanced glycation end-products (AGEs) as an index of metabolic and oxidative stress.
- -
- the prevalence of infectious complications;
- -
- the rate of cardiovascular morbidity and mortality;
- -
- the rate of hospitalization;
- -
- patient survival;
- -
- angiogenesis and cell migration ability on in vitro models of cultured endothelial cells exposed to serum from patients treated with the two dialyzers;
- -
- the changes in arterial stiffness through pulse wave velocity (PWV).
2. Experimental Design and Methods
2.1. Study Design
- Group A: 16 patients without a previous history of hypersensitivity treated by online HDF with FX100.
- Group B: 16 patients with a previous history of hypersensitivity treated by online HDF with SOLACEA 21-H.
- -
- General features and clinical data: sex, age, weight, height, BMI, blood pressure, heart rate, and the Charlson Comorbidity Index.
- -
- Previous medical history: renal disease, age at onset, acute or acute on chronic, waiting list for a kidney transplant, and routine laboratory assays.
- -
- Physical examination: pulmonary or peripheral hyperhydration, heart rate, and vascular murmurs.
- -
- Ongoing medical therapy.
- Hemodialysis depurative treatment using online HDF technique three times a week according to regular clinical practice;
- Use of two types of high-flow dialysis filters for online HDF:
- FX100 filter (Helixone, Fresenius Medical Care), ultrafiltration coefficient 73 mL/h/mmHg, cut-off 35,000 Da, surface area 2.2 m2, steam sterilization;
- SOLACEA 21-H filter (ATA, Nipro Europe) for polyallergic patients and in particular patients allergic to polysulfone filters, ultrafiltration coefficient 76 mL/h/mmHg, cut-off 45,000 Da, surface 2.1 m2, gamma-ray sterilization;
- Convective volume during dialysis > 20 L per session will be obtained by means of two biofeedback systems, namely Fresenius Medical Care AutoSubPlus™ and Nipro Max-Sub™;
- Ultrapure dialysis water will be used, defined as <0.1 CFU/mL and <0.03 EU/mL;
- Heparinization of the extracorporeal circuit, obtained by low-molecular-weight heparin (Inhixa™, enoxaparin sodium, Techdow Pharma, Milan, Italy), at the dosage of 2000 IU in patients with body weight < 50 kg, 4000 IU in patients with weight >50 kg and <90 kg, 6000 IU in patients with weight ≥ 90 kg. Low-molecular-weight heparin will be administered in a single bolus, at the beginning of the dialysis treatment, immediately after the connection of the patient to the extracorporeal circuit;
- Administration of the appropriate medical therapies, regular laboratory, and instrumental clinical monitoring, as well as possible hospitalization of patients in case of the onset of intercurrent diseases.
- -
- monthly laboratory assays, namely circulating medium-molecular-weight uremic toxins and toxins linked to plasma proteins, inflammation markers and cytokines, analysis of lymphocyte subsets, activated lymphocytes and monocytes, and cell apoptosis rate;
- -
- monthly assessment of accumulation of AGEs as an index of metabolic and oxidative stress;
- -
- infectious complications;
- -
- cardiovascular complications;
- -
- eventual kidney transplantation;
- -
- possible interruption of online HDF and the switch to another dialysis technique;
- -
- need for hospitalization;
- -
- patient survival;
- -
- in vitro effect on endothelial cells of uremic serum collected from patients treated with the two different dialyzers on angiogenesis, cell migration, differentiation, apoptosis and proliferative potential, and gene and protein expression profile;
- -
- monthly bioimpedance analysis (BIA);
- -
- monthly measurement of changes in arterial stiffness by PWV.
2.2. Patients
2.3. Specimen Collection
2.4. Laboratory Assays
- -
- lymphocyte subsets through BD Simultest™ IMK-Lymphocyte, a two-color direct immunofluorescence reagent kit for enumerating percentages of the following cell types: T (CD3+) lymphocytes, B (CD19+) lymphocytes, helper/inducer T (CD3+CD4+) lymphocytes, suppressor/cytotoxic T (CD3+CD8+) lymphocytes, natural killer (NK) (CD3-CD16+ and/or CD56+) lymphocytes, and CD3+CD4+/CD3+CD8+ ratio;
- -
- activated lymphocytes CD3+HLA-DR+ and CD8+CD57+;
- -
- -
- the detection of apoptosis through the classical Annexin V method [34].
2.5. Instrumental Examinations
2.5.1. Bioimpedance Analysis
2.5.2. Measurement of AGE Accumulation through Skin Autofluorescence
2.5.3. Measurement of Arterial Stiffness through Pulse Wave Velocity
2.6. In Vitro Model
- Cell proliferation determined by bromodeoxyuridine (BrdU) integration assay. It allows the visualization of the proliferating cells in relation to the total number of cells through detection by immunofluorescence;
- Migration of endothelial cells using the modified Boyden chamber method. Endothelial cells move through a process called chemotaxis, along with a gradient of factors that induce angiogenesis. In this assay, the cells are plated on a porous surface that separates two compartments across which cells can migrate in response to an angiogenic factor placed in the chamber below;
- The ability of endothelial cells to self-organize into capillary-like structures. Cells will be grown on Matrigel matrices to assess adhesion, migration, and tubule organization. In addition, three-dimensional (3-D) matrices will also be used to mimic the in vivo angiogenic system. Initially, the endothelial cells form tubules in the horizontal plane and after 12 or more days, the endothelial tubules begin to ramify upward and penetrate the gel to form a 3-D tubule network;
- Gene expression of vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-1 (VEGFR1), vascular endothelial growth factor receptor-2 (VEGFR2), and the changes occurring in the different experimental conditions by real-time PCR using TaqMan Probes on an iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). VEGF and its receptors constitute the major regulatory system of vascular development and play a central role in vasculogenesis, as well as in both physiological and pathological angiogenesis. This system is expressed in many tissues and cells when angiogenesis is in progress, while it is restrained when the angiogenic process is inactive;
- Protein expression of VEGF and its receptors in endothelial cells and the variations they undergo under different experimental conditions evaluated by means of immunocytochemistry;
- Angiogenic growth factors, such as fibroblast growth factor 2 (FGF2), VEGF, Thrombospondin-1 (TSP-1), Troponin I, IFN-α, IFN-γ, IL-4, IL-12, and retinoic acid in the supernatant of cell cultures through Luminex MAGPIX® technology (Millipore Corp), which allows both monoplex and multiplex assays of proteins and nucleic acids.
2.7. Statistical Analysis
2.8. Rules of Good Clinical Practice
2.9. Ethics and Dissemination
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Trial Registration
References
- O’Lone, E.; Viecelli, A.K.; Craig, J.C.; Tong, A.; Sautenet, B.; Roy, D.; Herrington, W.G.; Herzog, C.A.; Jafar, T.; Jardine, M.; et al. Cardiovascular outcomes reported in hemodialysis trials. J. Am. Coll. Cardiol. 2018, 71, 2802–2810. [Google Scholar] [CrossRef]
- Longenecker, J.C.; Coresh, J.; Powe, N.R.; Levey, A.S.; Fink, N.E.; Martin, A.; Klag, M.J. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The choice study. J. Am. Soc. Nephrol. 2002, 13, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Cardiovascular Disease in Patients With CKD. Available online: https://adr.usrds.org/2020/chronic-kidney-disease/4-cardiovascular-disease-in-patients-with-ckd (accessed on 8 December 2020).
- Kendrick, J.; Chonchol, M.B. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat. Clin. Pr. Nephrol. 2008, 4, 672–681. [Google Scholar] [CrossRef]
- Zoccali, C. Cardiovascular risk in uraemic patients—Is it fully explained by classical risk factors? Nephrol. Dial. Transplant. 2000, 15, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravid, J.D.; Chitalia, V.C. Molecular mechanisms underlying the cardiovascular toxicity of specific uremic solutes. Cells 2020, 9, 2024. [Google Scholar] [CrossRef] [PubMed]
- Colì, L.; Donati, G.; Cappuccilli, M.L.; Cianciolo, G.; Comai, G.; Cuna, V.; Carretta, E.; La Manna, G.; Stefoni, S. Role of the hemodialysis vascular access type in inflammation status and monocyte activation. Int. J. Artif. Organs 2011, 34, 481–488. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Cardiotoxicity of uremic toxins: A driver of cardiorenal syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2019, 1, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Martin-Malo, A.; Hannedouche, T.; Loureiro, A.; Papadimitriou, M.; Wizemann, V.; Jacobson, S.H.; Czekalski, S.; Ronco, C.; Vanholder, R.; et al. Effect of membrane permeability on survival of hemodialysis patients. J. Am. Soc. Nephrol. 2008, 20, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Georgatzakou, H.T.; Tzounakas, V.L.; Kriebardis, A.G.; Velentzas, A.D.; Kokkalis, A.C.; Antonelou, M.H.; Papassideri, I.S. Short-term effects of hemodiafiltration versus conventional hemodialysis on erythrocyte performance. Can. J. Physiol. Pharmacol. 2018, 96, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Nubé, M.J.; Peters, S.A.; Blankestijn, P.J.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Asci, G.; Locatelli, F.; Maduell, F.; Morena, M.; et al. Mortality reduction by post-dilution online-haemodiafiltration: A cause-specific analysis. Nephrol. Dial. Transplant. 2016, 32, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Marshall, M.R. Measuring the patient response to dialysis therapy: Hemodiafiltration and clinical trials. Kidney Int. 2017, 91, 1279–1282. [Google Scholar] [CrossRef]
- Chandna, S.M.; Farrington, K. Reviews: Residual renal function: Considerations on its importance and preservation in dialysis patients. Semin. Dial. 2004, 17, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Wellsted, D.; Chandna, S.M.; Greenwood, R.N.; Farrington, K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol. Dial. Transplant. 2009, 24, 2502–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potier, J.; Bowry, S.; Canaud, B. Clinical performance assessment of cordiax filters in hemodialysis and hemodiafiltration. Contrib. Nephrol. 2017, 189, 237–245. [Google Scholar] [CrossRef]
- Ward, R.A.; Beck, W.; Bernardo, A.A.; Alves, F.C.; Stenvinkel, P.; Lindholm, B. Hypoalbuminemia: A price worth paying for improved dialytic removal of middle-molecular-weight uremic toxins? Nephrol. Dial. Transplant. 2019, 34, 901–907. [Google Scholar] [CrossRef]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G.L. Biochemical and clinical impact of organic uremic retention solutes: A comprehensive update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Kerr, P.G.; Sutherland, W.H.; De Jong, S.; Vaithalingham, I.; Williams, S.M.; Walker, R.J. The impact of standard high-flux polysulfone versus novel high-flux polysulfone dialysis membranes on inflammatory markers: A randomized, single-blinded, controlled clinical trial. Am. J. Kidney Dis. 2007, 49, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Boer, W.H.; Liem, Y.; De Beus, E.; Abrahams, A.C. Acute reactions to polysulfone/polyethersulfone dialysers: Literature review and management. Neth. J. Med. 2017, 75, 4–13. [Google Scholar]
- Heegard, K.D.; Tilley, M.A.; Edgecombe, H.P.; Lundy, J.B.; Renz, E.M.; Chung, K.K.; Stewart, I.J. Anaphylactoid reaction during First Hemofiltration with a PUREMA® polysulfone membrane. Int. J. Artif. Organs 2013, 36, 363–366. [Google Scholar] [CrossRef]
- Ronci, M.; Leporini, L.; Felaco, P.; Sirolli, V.; Pieroni, L.; Greco, V.; Aceto, A.; Urbani, A.; Bonomini, M. Proteomic characterization of a new asymmetric cellulose triacetate membrane for hemodialysis. Proteom. Clin. Appl. 2018, 12, e1700140. [Google Scholar] [CrossRef] [PubMed]
- Sunohara, T.; Masuda, T.; Kawanishi, H.; Takemoto, Y. Fundamental characteristics of the newly developed ATA™ membrane dialyzer. Contrib. Nephrol. 2016, 189, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.A.; Miguel, P.M.; Bohorquez, L.; De Sequera, P.; Bouarich, H.; Pérez-García, R.; Puyol, D.R.; Barril, G.; Tomero, J.A.S.; Giorgi, M.; et al. El triacetato de celulosa asimétrico es una alternativa segura y eficaz para la hemodiafiltración en línea. Nefrología 2018, 38, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Karaboyas, A.; Pisoni, R.L.; Robinson, B.M.; Fort, J.; Vanholder, R.; Rayner, H.C.; Kleophas, W.; Jacobson, S.H.; Combe, C.; et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: A ‘real-world’ comparison from the DOPPS. Nephrol. Dial. Transplant. 2018, 33, 683–689. [Google Scholar] [CrossRef] [Green Version]
- La Manna, G.; Pizza, F.; Persici, E.; Baraldi, O.; Comai, G.; Cappuccilli, M.L.; Centofanti, F.; Carretta, E.; Plazzi, G.; Colì, L.; et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol. Dial. Transplant. 2010, 26, 1976–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colì, L.; La Manna, G.; Comai, G.; Ursino, M.; Ricci, D.; Piccari, M.; Locatelli, F.; Di Filippo, S.; Cristinelli, L.; Bacchi, M.; et al. Automatic adaptive system dialysis for hemodialysis-associated hypotension and intolerance: A noncontrolled multicenter trial. Am. J. Kidney Dis. 2011, 58, 93–100. [Google Scholar] [CrossRef]
- Donauer, J.; Schweiger, C.; Rumberger, B.; Krumme, B.; Böhler, J. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol. Dial. Transplant. 2003, 18, 1616–1622. [Google Scholar] [CrossRef] [Green Version]
- Hakim, R.M.; Breillatt, J.; Lazarus, J.M.; Port, F.K. Complement activation and hypersensitivity reactions to dialysis membranes. N. Engl. J. Med. 1984, 311, 878–882. [Google Scholar] [CrossRef]
- Donati, G.; Moretti, M.I.; Baraldi, O.; Spazzoli, A.; Capelli, I.; Comai, G.; Marchetti, A.; Sarma, M.; Mancini, R.; La Manna, G. Removal of free light chains in hemodialysis patients without multiple myeloma: A crossover comparison of three different dialyzers. BMC Nephrol. 2016, 17, 193. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Chen, X.; Xia, T.; Zhang, F.; Gao, S.; Chen, W. LC-MS/MS method for simultaneous determination of serump-cresyl sulfate and indoxyl sulfate in patients undergoing peritoneal dialysis. Biomed. Chromatogr. 2016, 30, 1782–1788. [Google Scholar] [CrossRef]
- Bacle, A.; Thevenot, S.; Grignon, C.; Belmouaz, S.; Bauwens, M.; Teychene, B.; Venisse, N.; Migeot, V.; Dupuis, A. Determination of bisphenol a in water and the medical devices used in hemodialysis treatment. Int. J. Pharm. 2016, 505, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Ramírez, R.; Madueño, J.A.; Soriano, S.; Rodríguez-Benot, A.; Rodríguez, M.; Martín-Malo, A.; Aljama, P. Cell apoptosis and hemodialysis-induced inflammation. Kidney Int. 2002, 61, S89–S93. [Google Scholar] [CrossRef] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef] [Green Version]
- Sarafidis, P.A.; Loutradis, C.; Karpetas, A.; Tzanis, G.; Piperidou, A.; Koutroumpas, G.; Raptis, V.; Syrgkanis, C.; Liakopoulos, V.; Efstratiadis, G.; et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension 2017, 70, 148–157. [Google Scholar] [CrossRef]
- Lauri, K.; Arund, J.; Holmar, J.; Tanner, R.; Kalle, S.; Luman, M.; Fridolin, I. Removal of Urea, β2-microglobulin, and indoxyl sulfate assessed by absorbance and fluorescence in the spent dialysate during hemodialysis. ASAIO J. 2019, 66, 698–705. [Google Scholar] [CrossRef]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum β-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J. Am. Soc. Nephrol. 2005, 17, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Liabeuf, S.; Lenglet, A.; Desjardins, L.; Neirynck, N.; Glorieux, G.; Lemke, H.-D.; Vanholder, R.; Diouf, M.; Choukroun, G.; Massy, Z.A. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012, 82, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, G.; Haag-Weber, M.; Mai, B.; Deicher, R.; Hörl, W.H. Effect of immunoglobulin light chains from hemodialysis and contin-uous ambulatory peritoneal dialysis patients on polymorphonuclear leukocyte functions. J. Am. Soc. Nephrol. 1995, 6, 1592–1599. [Google Scholar] [PubMed]
- Desjardins, L.; Liabeuf, S.; Lenglet, A.; Lemke, H.-D.; Vanholder, R.; Choukroun, G.; Massy, Z.A. European uremic toxin (EUTOX) work group association between free light chain levels, and disease progression and mortality in chronic kidney disease. Toxins 2013, 5, 2058–2073. [Google Scholar] [CrossRef] [Green Version]
- Cianciolo, G.; Galassi, A.; Capelli, I.; Schillaci, R.; La Manna, G.; Cozzolino, M. Klotho-FGF23, Cardiovascular disease, and vascular calcification: Black or white? Curr. Vasc. Pharmacol. 2018, 16, 143–156. [Google Scholar] [CrossRef]
- Grabner, A.; Mazzaferro, S.; Cianciolo, G.; Krick, S.; Capelli, I.; Rotondi, S.; Ronco, C.; La Manna, G.; Faul, C. Fibroblast growth factor 23: Mineral metabolism and beyond. Contrib. Nephrol. 2017, 190, 83–95. [Google Scholar] [CrossRef]
- Cianciolo, G.; Capelli, I.; Cappuccilli, M.; Schillaci, R.; Cozzolino, M.; La Manna, G. Calcifying circulating cells: An uncharted area in the setting of vascular calcification in CKD patients. Clin. Kidney J. 2016, 9, 280–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Potier, J.; Thebaud, H.E. Facteurs de risque des réactions aiguës d’hypersensibilité en hémodialyse: Enquête prospective multicentrique sur six mois dans l’Ouest de la France [Risk factors for acute hypersensitivity reactions in hemodialysis]. Nephrologie 1996, 17, 163–170. [Google Scholar] [PubMed]
- Mas, S.; Bosch-Panadero, E.; Abaigar, P.; Camarero, V.; Mahillo, I.; Civantos, E.; Sanchez-Ospina, D.; Ruiz-Priego, A.; Egido, J.; Ortiz, A.; et al. Influence of dialysis membrane composition on plasma bisphenol A levels during online hemodiafiltration. PLoS ONE 2018, 13, e0193288. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Panadero, E.; Mas, S.; Sanchez-Ospina, D.; Camarero, V.; Pérez-Gómez, M.V.; Saez-Calero, I.; Abaigar, P.; Ortiz, A.; Egido, J.; González-Parra, E. The choice of hemodialysis membrane affects bisphenol a levels in blood. J. Am. Soc. Nephrol. 2015, 27, 1566–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Ohashi, A.; Hori, H.; Hibiya, M.; Shoji, Y.; Kunisaki, M.; Akita, M.; Yagi, A.; Sugiyama, K.; Shimozato, S.; et al. Accumulation of bisphenol a in hemodialysis patients. Blood Purif. 2007, 25, 290–294. [Google Scholar] [CrossRef]
- Badding, M.A.; Vargas, J.R.; Fortney, J.; Cheng, Q.J.; Ho, C.-H. Toxicological risk assessment of bisphenol a released from dialyzers under simulated-use and exaggerated extraction conditions. Regul. Toxicol. Pharmacol. 2020, 118, 104787. [Google Scholar] [CrossRef] [PubMed]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chida, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of advanced glycation end products with coronary artery calcification in japanese subjects with type 2 diabetes as assessed by skin autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Wang, Y.-C.; Wang, G.-J.; Shen, M.-Y.; Chang, Y.-L.; Liou, S.-Y.; Chen, H.-C.; Chang, C.-T. Skin autofluorescence is associated with endothelial dysfunction in uremic subjects on hemodialysis. PLoS ONE 2016, 11, e0147771. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, B.; Jacobs, K.; Santos, A.N.; Wienke, A.; Silber, R.; Simm, A. Relationship between cardiac tissue glycation and skin autofluorescence in patients with coronary artery disease. Diabetes Metab. 2015, 41, 410–415. [Google Scholar] [CrossRef]
- McIntyre, N.J.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin. J. Am. Soc. Nephrol. 2011, 6, 2356–2363. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Tanaka, K.; Kanno, M.; Watanabe, K.; Hayashi, Y.; Asahi, K.; Suzuki, H.; Sato, K.; Sakaue, M.; Terawaki, H.; et al. Skin autofluorescence predicts cardiovascular mortality in patients on chronic hemodialysis. Ther. Apher. Dial. 2014, 18, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerwaldt, R.; Hartog, J.W.; Graaff, R.; Huisman, R.J.; Links, T.P.; Hollander, N.C.D.; Thorpe, S.R.; Baynes, J.W.; Navis, G.; Gans, R.O.; et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Coli, L.; De Sanctis, L.B.; Feliciangeli, G.; Iannelli, S.; Scolari, M.P.; Todeschini, P.; Tumietto, F.; Costigliola, P.; Chiodo, F. Dialysis membrane biocompatibility: Effects on cellular elements. Nephrol. Dial. Transplant. 1995, 10, 27–32. [Google Scholar] [PubMed]
- Angeletti, A.; Zappulo, F.; Donadei, C.; Cappuccilli, M.; Di Certo, G.; Conte, D.; Comai, G.; Donati, G.; La Manna, G. Immunological effects of a single hemodialysis treatment. Medicina 2020, 56, 71. [Google Scholar] [CrossRef] [Green Version]
- Pupim, L.B.; Himmelfarb, J.; McMonagle, E.; Shyr, Y.; Ikizler, T.A. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004, 65, 2371–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memoli, B.; Postiglione, L.; Cianciaruso, B.; Bisesti, V.; Cimmaruta, C.; Marzano, L.; Minutolo, R.; Cuomo, V.; Guida, B.; Andreucci, M.; et al. Role of different dialysis membranes in the release of interleukin-6-soluble receptor in uremic patients. Kidney Int. 2000, 58, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Menegatti, E.; Rossi, D.; Chiara, M.; Alpa, M.; Sena, L.M.; Roccatello, D. Cytokine release pathway in mononuclear cells stimulated in vitro by dialysis membranes. Am. J. Nephrol. 2002, 22, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Sutherland, W.; De Jong, S. Effect of changing from a cellulose acetate to a polysulphone dialysis membrane on protein oxidation and inflammation markers. Clin. Nephrol. 2004, 61, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Kuragano, T.; Kida, A.; Kitamura, R.; Nanami, M.; Otaki, Y.; Nonoguchi, H.; Matsumoto, A.; Nakanishi, T. A crossover study of the acrylonitrile-co-methallyl sulfonate and polysulfone membranes for elderly hemodialysis patients: The effect on hemodynamic, nutritional, and inflammatory conditions. ASAIO J. 2011, 57, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, R.; Arias-Guillén, M.; Gómez, M.; Vera, M.; Fontseré, N.; Rodas, L.; Filella, X.; Reverter, J.C.; Lozano, F.; Villamor, N.; et al. Study of biocompatibility of membranes in online hemodiafiltration. Blood Purif. 2020, 49, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobayashi, K.; Suzuki, H. Pulse wave velocity as an indicator of arteriosclerosis in hemodialysis patients. Atherosclerosis 2004, 176, 405–409. [Google Scholar] [CrossRef] [PubMed]
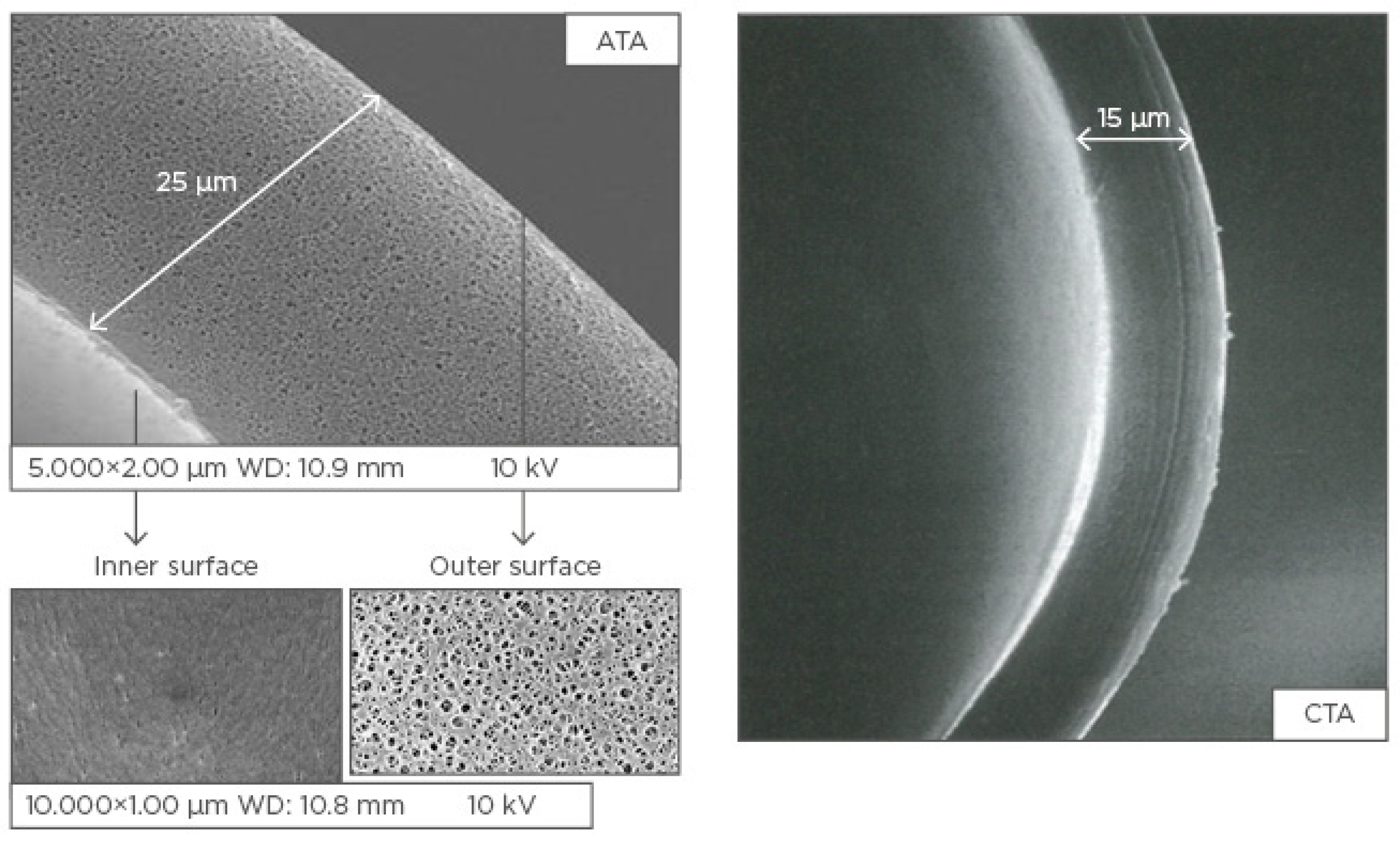
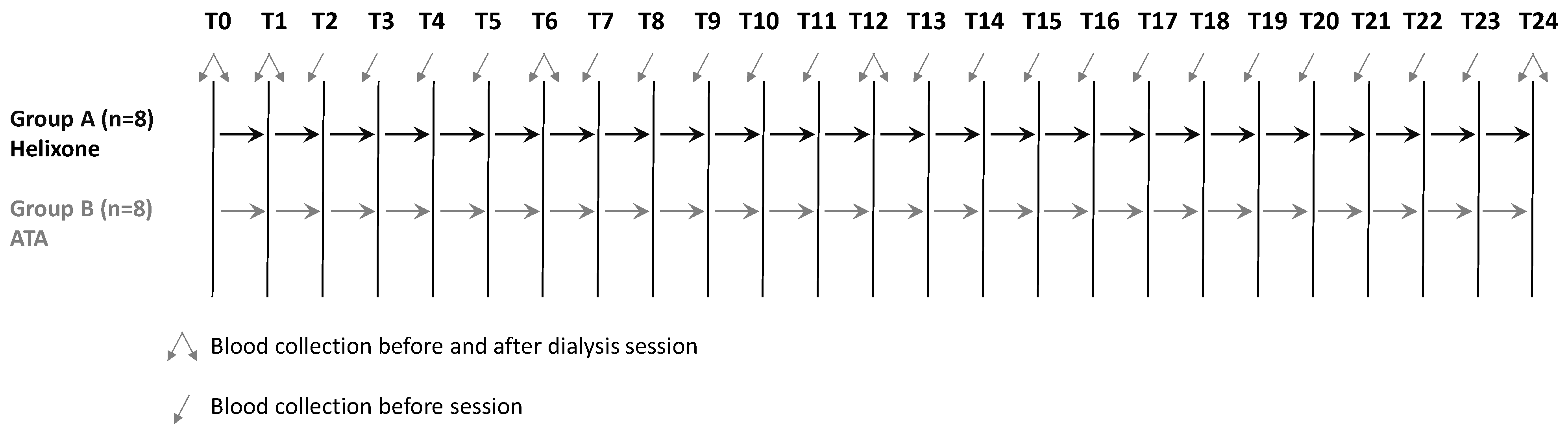
| Visit Timing | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | T14 | T15 | T16 | T17 | T18 | T19 | T20 | T21 | T22 | T23 | T24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment | X | ||||||||||||||||||||||||
| Informed consent | X | ||||||||||||||||||||||||
| Clinical history | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Therapies | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| General assessment | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Lab tests | X | X | X | X | X | X | |||||||||||||||||||
| AGEs measurements | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| BIA | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| PWV | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Adverse events | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, G.; Cappuccilli, M.; Donadei, C.; Righini, M.; Scrivo, A.; Gasperoni, L.; Zappulo, F.; La Manna, G. Toxin Removal and Inflammatory State Modulation during Online Hemodiafiltration Using Two Different Dialyzers (TRIAD2 Study). Methods Protoc. 2021, 4, 26. https://doi.org/10.3390/mps4020026
Donati G, Cappuccilli M, Donadei C, Righini M, Scrivo A, Gasperoni L, Zappulo F, La Manna G. Toxin Removal and Inflammatory State Modulation during Online Hemodiafiltration Using Two Different Dialyzers (TRIAD2 Study). Methods and Protocols. 2021; 4(2):26. https://doi.org/10.3390/mps4020026
Chicago/Turabian StyleDonati, Gabriele, Maria Cappuccilli, Chiara Donadei, Matteo Righini, Anna Scrivo, Lorenzo Gasperoni, Fulvia Zappulo, and Gaetano La Manna. 2021. "Toxin Removal and Inflammatory State Modulation during Online Hemodiafiltration Using Two Different Dialyzers (TRIAD2 Study)" Methods and Protocols 4, no. 2: 26. https://doi.org/10.3390/mps4020026






