Towards On-Demand E. coli-Based Cell-Free Protein Synthesis of Tissue Plasminogen Activator
Abstract
:1. Introduction
2. Materials and Methods
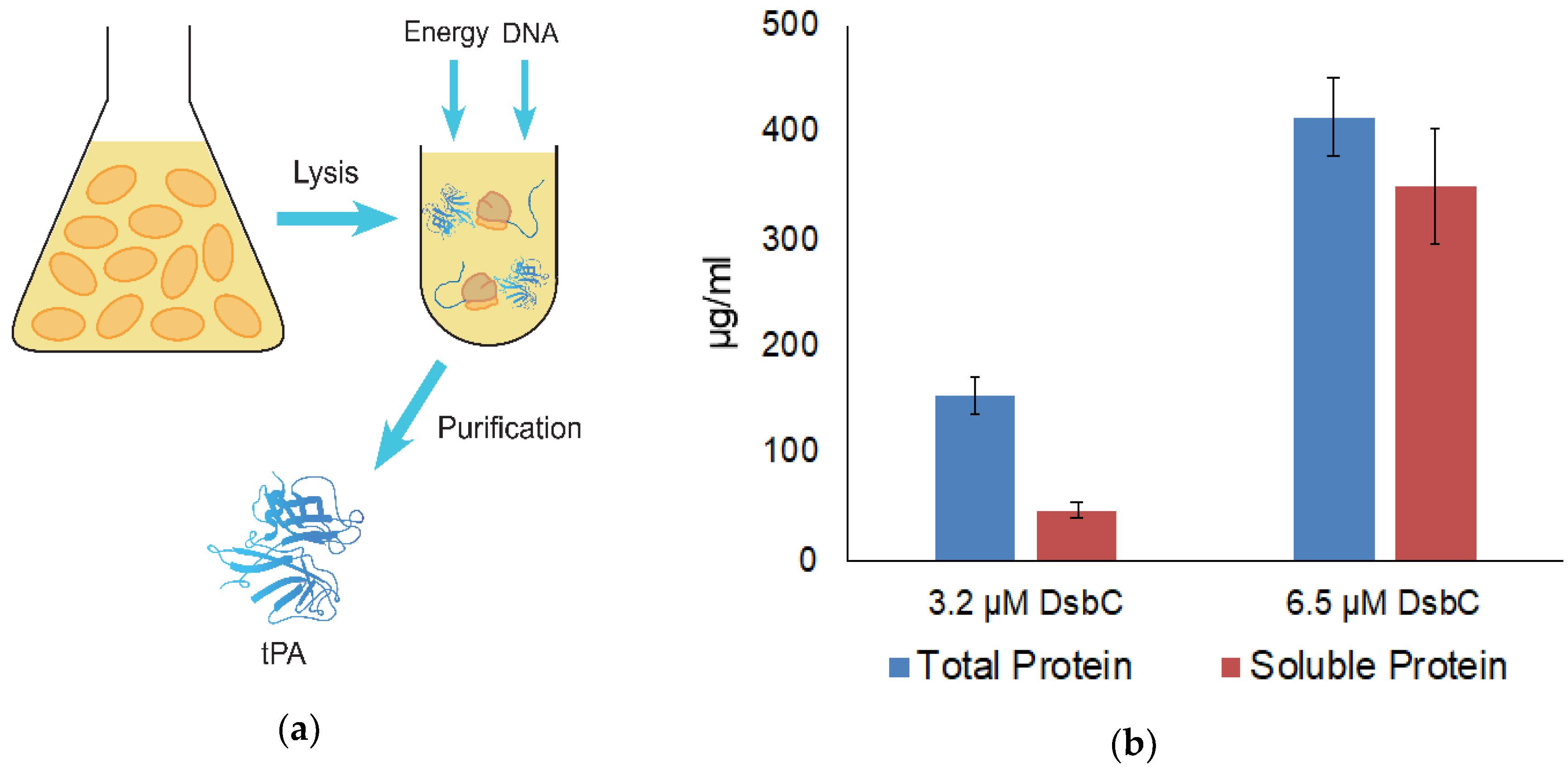
2.1. Extract Preparation
2.2. Cell-Free Protein Synthesis
2.3. Measuring Protein Concentration
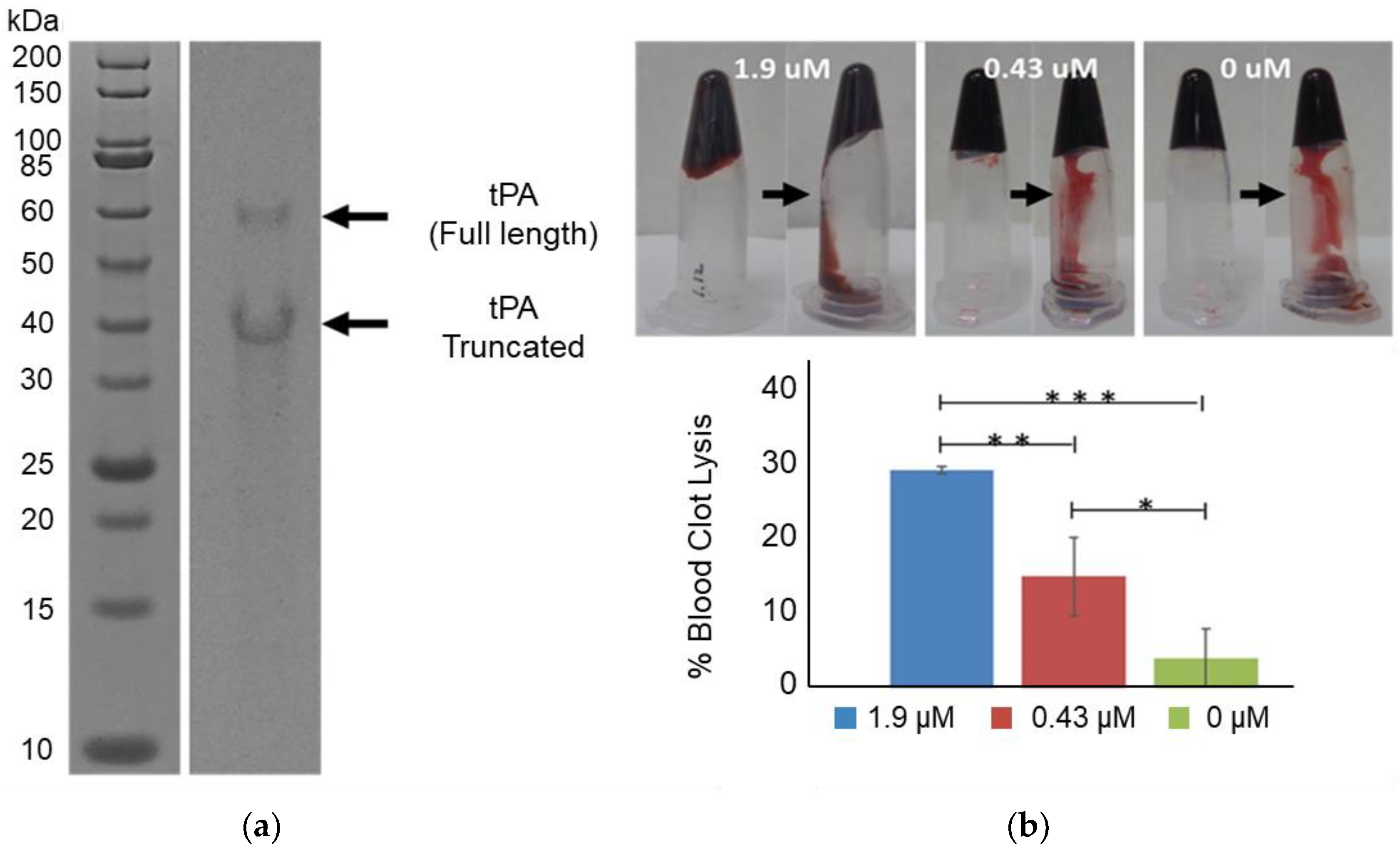
2.4. tPa Purification
2.5. tPa Activity Assay
2.6. Blood Clot Lysis Assay
3. Results and Discussion
3.1. tPA Expression and Specific Activity
3.2. Blood Clot Lysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gravanis, I.; Tsirka, S.E. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin. Ther. Targets 2008, 12, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, A.S.M.; Earl, C.C.; Muhlestein, C.; Bundy, B.C. Escherichia coli-based cell-free extract development for protein-based cancer therapeutic production. Int. J. Dev. Biol. 2016, 60, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Senis, Y.A.; Barr, A.J. Targeting Receptor-Type Protein Tyrosine Phosphatases with Biotherapeutics: Is Outside-in Better than Inside-Out? Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Guzauskas, G.; Villa, K.F.; Fagan, S.C.; Veenstra, D.L. A model of cost-effectiveness of tissue plasminogen activator in patient subgroups 3 to 4.5 hours after onset of acute ischemic stroke. Ann. Emerg. Med. 2013, 61, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [PubMed]
- Mehr, S.R.; Brook, R.A. Factors influencing the economics of biosimilars in the US. J. Med. Econ. 2017, 20, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Cornes, P. The economic pressures for biosimilar drug use in cancer medicine. Target. Oncol. 2012, 7 (Suppl. 1), S57–S67. [Google Scholar] [CrossRef]
- Wilding, K.M.; Smith, A.K.; Wilkerson, J.W.; Bush, D.B.; Knotts, T.A.t.; Bundy, B.C. The Locational Impact of Site-Specific PEGylation: Streamlined Screening with Cell-Free Protein Expression and Coarse-Grain Simulation. ACS Synth. Biol. 2018, 7, 510–521. [Google Scholar] [CrossRef]
- Shrestha, P.; Smith, M.T.; Bundy, B.C. Cell-free unnatural amino acid incorporation with alternative energy systems and linear expression templates. New Biotechnol. 2014, 31, 28–34. [Google Scholar] [CrossRef]
- Salehi, A.S.; Smith, M.T.; Bennett, A.M.; Williams, J.B.; Pitt, W.G.; Bundy, B.C. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol. J. 2016, 11, 274–281. [Google Scholar] [CrossRef]
- Smith, M.T.; Berkheimer, S.D.; Werner, C.J.; Bundy, B.C. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques 2014, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Wilding, K.M.; Hunt, J.M.; Bennett, A.M.; Bundy, B.C. The emerging age of cell-free synthetic biology. FEBS Lett. 2014, 588, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Earl, C.C.; Smith, M.T.; Lease, R.A.; Bundy, B. Polyvinylsulfonic Acid: A Low-cost RNase Inhibitor for Enhanced RNA Preservation and Cell-free Protein Translation. Bioengineered 2018, 9, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wilding, K.M.; Schinn, S.M.; Long, E.A.; Bundy, B.C. The emerging impact of cell-free chemical biosynthesis. Curr. Opin. Biotechnol. 2018, 53, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bundy, B.C.; Hunt, J.P.; Jewett, M.C.; Swartz, J.R.; Wood, D.W.; Frey, D.D.; Rao, G. Cell-free biomanufacturing. Curr. Opin. Chem. Eng. 2018, 22, 177–183. [Google Scholar] [CrossRef]
- Hunt, J.P.; Yang, S.O.; Wilding, K.M.; Bundy, B.C. The growing impact of lyophilized cell-free protein expression systems. Bioengineered 2017, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Schinn, S.M.; Bradley, W.; Groesbeck, A.; Wu, J.C.; Broadbent, A.; Bundy, B.C. Rapid in vitro screening for the location-dependent effects of unnatural amino acids on protein expression and activity. Biotechnol. Bioeng. 2017, 114, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.S.; Shakalli Tang, M.J.; Smith, M.T.; Hunt, J.M.; Law, R.A.; Wood, D.W.; Bundy, B.C. Cell-Free Protein Synthesis Approach to Biosensing hTRbeta-Specific Endocrine Disruptors. Anal. Chem. 2017, 89, 3395–3401. [Google Scholar] [CrossRef]
- Yang, J.H.; Kanter, G.; Voloshin, A.; Levy, R.; Swartz, J.R. Expression of active murine granulocyte-macrophage colony-stimulating factor in an Escherichia coli cell-free system. Biotechnol. Progr. 2004, 20, 1689–1696. [Google Scholar] [CrossRef]
- Zimmerman, E.S.; Heibeck, T.H.; Gill, A.; Li, X.; Murray, C.J.; Madlansacay, M.R.; Tran, C.; Uter, N.T.; Yin, G.; Rivers, P.J.; et al. Production of Site-Specific Antibody–Drug Conjugates Using Optimized Non-Natural Amino Acids in a Cell-Free Expression System. Bioconjugate Chem. 2014, 25, 351–361. [Google Scholar] [CrossRef]
- Wilding, K.M.; Hunt, J.P.; Wilkerson, J.W.; Funk, P.J.; Swensen, R.L.; Carver, W.C.; Christian, L.M. Endotoxin-free E. coli-based cell-free protein synthesis: Pre-expression endotoxin removal approaches for on-demand cancer therapeutic production. Biotechnol. J. 2019, 14, 1800271. [Google Scholar] [CrossRef] [PubMed]
- Majidzadeh, A.K.; Mahboudi, F.; Hemayatkar, M.; Davami, F.; Barkhordary, F.; Adeli, A.; Soleimani, M.; Davoudi, N.; Khalaj, V. Human Tissue Plasminogen Activator Expression in Escherichia coli using Cytoplasmic and Periplasmic Cumulative Power. Avicenna J. Med. Biotechnol. 2010, 2, 131–136. [Google Scholar]
- Qiu, J.; Swartz, J.R.; Georgiou, G. Expression of active human tissue-type plasminogen activator in Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 4891–4896. [Google Scholar] [PubMed]
- Lee, H.J.; Im, H. Soluble Expression and Purification of Human Tissue-type Plasminogen Activator Protease Domain. Bull. Korean Chem. Soc. 2010, 31, 2607–2612. [Google Scholar] [CrossRef] [Green Version]
- Fathi-Roudsari, M.; Akhavian-Tehrani, A.; Maghsoudi, N. Comparison of Three Escherichia coli Strains in Recombinant Production of Reteplase. Avicenna J. Med. Biotechnol. 2016, 8, 16–22. [Google Scholar] [PubMed]
- Pennica, D.; Holmes, W.E.; Kohr, W.J.; Harkins, R.N.; Vehar, G.A.; Ward, C.A.; Bennett, W.F.; Yelverton, E.; Seeburg, P.H.; Heyneker, H.L.; et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 1983, 301, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Furlong, A.M.; Thomsen, D.R.; Marotti, K.R.; Post, L.E.; Sharma, S.K. Active human tissue plasminogen activator secreted from insect cells using a baculovirus vector. Biotechnol. Appl. Biochem. 1988, 10, 454–464. [Google Scholar]
- Martegani, E.; Forlani, N.; Mauri, I.; Porro, D.; Schleuning, W.D.; Alberghina, L. Expression of high levels of human tissue plasminogen activator in yeast under the control of an inducible GAL promoter. Appl. Microbiol. Biotechnol. 1992, 37, 604–608. [Google Scholar] [CrossRef]
- Steiner, H.; Pohl, G.; Gunne, H.; Hellers, M.; Elhammer, A.; Hansson, L. Human tissue-type plasminogen activator synthesized by using a baculovirus vector in insect cells compared with human plasminogen activator produced in mouse cells. Gene 1988, 73, 449–457. [Google Scholar] [CrossRef]
- Goerke, A.R.; Swartz, J.R. Development of cell-free protein synthesis platforms for disulfide bonded proteins. Biotechnol. Bioeng. 2008, 99, 351–367. [Google Scholar] [CrossRef]
- Pohl, G.; Kallstrom, M.; Bergsdorf, N.; Wallen, P.; Jornvall, H. Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence, identify the active site serine residue, establish glycosylation sites, and localize variant differences. Biochemistry 1984, 23, 3701–3707. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Ogiwara, K.; Takano, N.; Moriyama, A.; Takahashi, T. Biochemical characterization of human kallikrein 8 and its possible involvement in the degradation of extracellular matrix proteins. FEBS Lett. 2005, 579, 6879–6884. [Google Scholar] [CrossRef] [PubMed]
- Rijken, D.C.; Hoylaerts, M.; Collen, D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J. Biol. Chem. 1982, 257, 2920–2925. [Google Scholar]
- Wallen, P.; Pohl, G.; Bergsdorf, N.; Ranby, M.; Ny, T.; Jornvall, H. Purification and characterization of a melanoma cell plasminogen activator. Eur. J. Biochem. 1983, 132, 681–686. [Google Scholar] [CrossRef]
- Strongin, A.Y.; Gorodetsky, D.I.; Stepanov, V.M. The study of Escherichia coli proteases. Intracellular serine protease of E. coli-an analogue of bacillus proteases. J. Gen. Microbiol. 1979, 110, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Rodier, M.; Prigent-Tessier, A.; Bejot, Y.; Jacquin, A.; Mossiat, C.; Marie, C.; Garnier, P. Exogenous t-PA Administration Increases Hippocampal Mature BDNF Levels. Plasmin- or NMDA-Dependent: Mechanism? PLoS ONE 2014, 9, e92416. [Google Scholar] [CrossRef] [PubMed]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-O.; Nielsen, G.H.; Wilding, K.M.; Cooper, M.A.; Wood, D.W.; Bundy, B.C. Towards On-Demand E. coli-Based Cell-Free Protein Synthesis of Tissue Plasminogen Activator. Methods Protoc. 2019, 2, 52. https://doi.org/10.3390/mps2020052
Yang S-O, Nielsen GH, Wilding KM, Cooper MA, Wood DW, Bundy BC. Towards On-Demand E. coli-Based Cell-Free Protein Synthesis of Tissue Plasminogen Activator. Methods and Protocols. 2019; 2(2):52. https://doi.org/10.3390/mps2020052
Chicago/Turabian StyleYang, Seung-Ook, Gregory H. Nielsen, Kristen M. Wilding, Merideth A. Cooper, David W. Wood, and Bradley C. Bundy. 2019. "Towards On-Demand E. coli-Based Cell-Free Protein Synthesis of Tissue Plasminogen Activator" Methods and Protocols 2, no. 2: 52. https://doi.org/10.3390/mps2020052
APA StyleYang, S.-O., Nielsen, G. H., Wilding, K. M., Cooper, M. A., Wood, D. W., & Bundy, B. C. (2019). Towards On-Demand E. coli-Based Cell-Free Protein Synthesis of Tissue Plasminogen Activator. Methods and Protocols, 2(2), 52. https://doi.org/10.3390/mps2020052




