Prospects for Expansion of Universal Newborn Screening in Bulgaria: A Survey among Medical Professionals
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Sample
2.2. Survey Items
2.3. Electronic Survey Properties
2.4. Data Analysis
3. Results
3.1. Socio-Demographic and Career Profile of the Respondents
3.2. Knowledge and Attitudes towards the Current NBS Program in Bulgaria
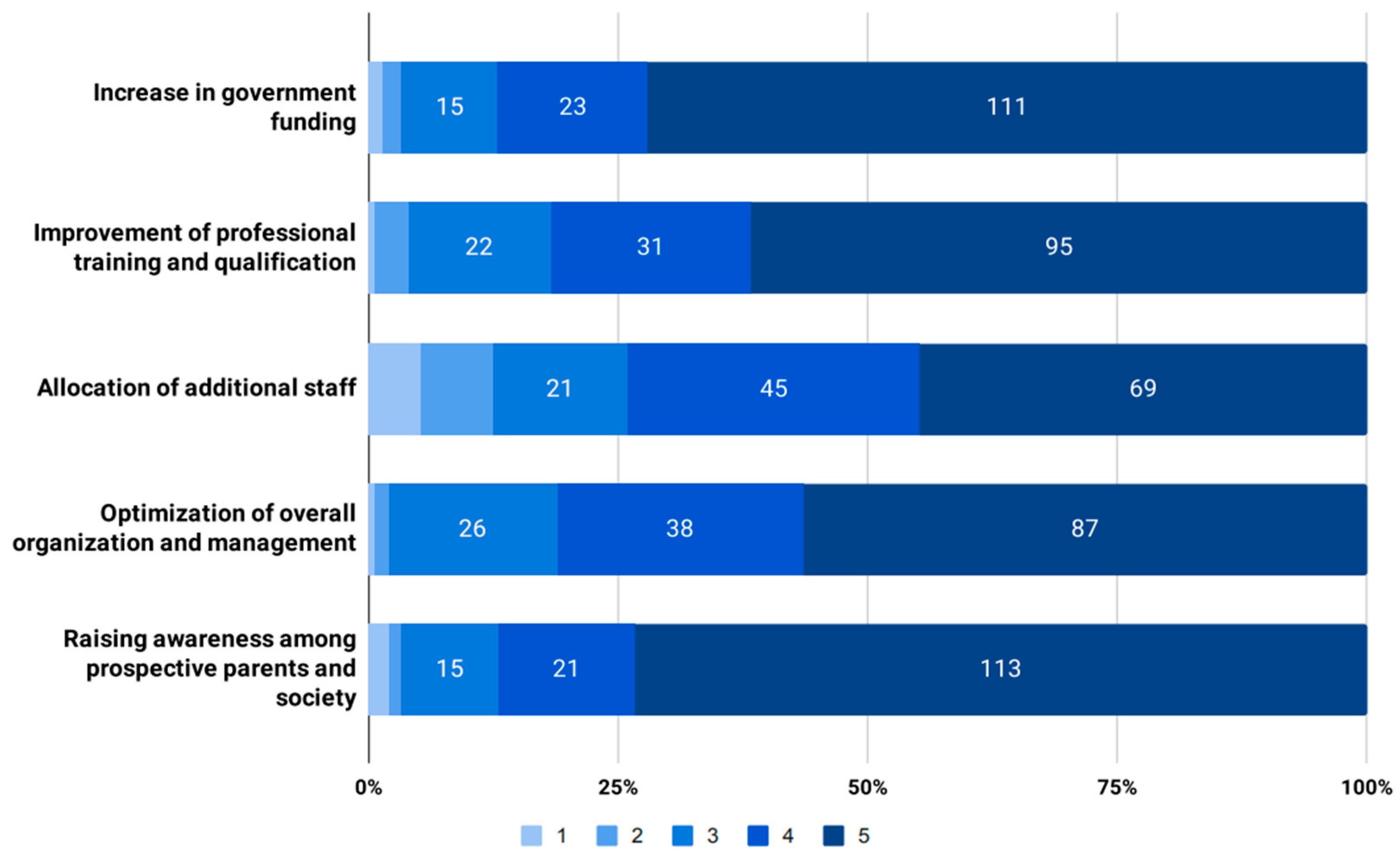
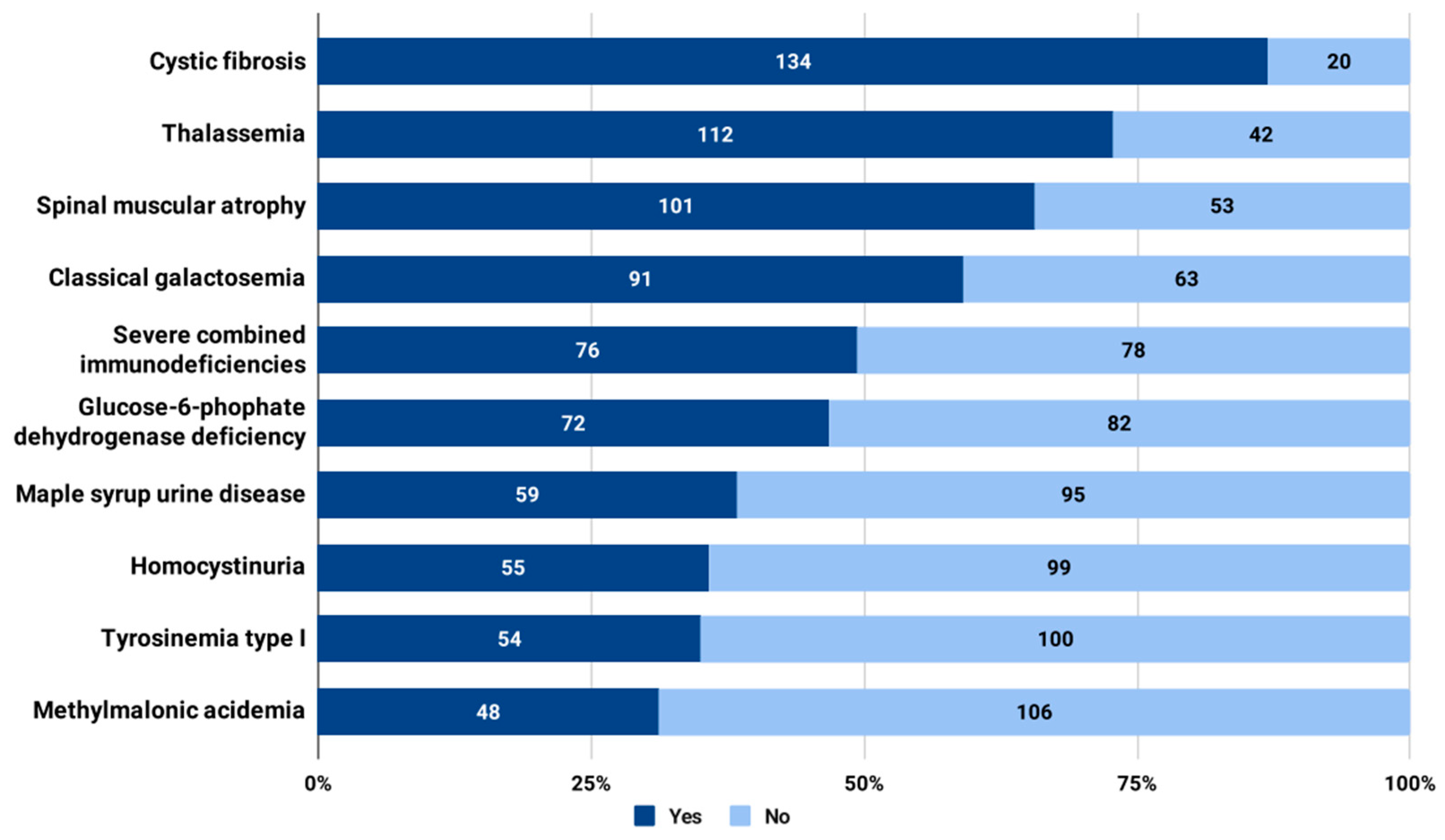
3.3. Attitudes towards the Expansion of Universal NBS in Bulgaria with Additional Disorders to Screen
4. Discussion
4.1. Prospective Conditions to Be Added to the Universal NBS Panel
4.2. Measures to Improve the Outcomes of Universal NBS Programs
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikonja, J.; Groselj, U.; Scarpa, M.; la Marca, G.; Cheillan, D.; Kölker, S.; Zetterström, R.H.; Kožich, V.; Le Cam, Y.; Gumus, G.; et al. Towards Achieving Equity and Innovation in Newborn Screening across Europe. Int. J. Neonatal Screen. 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Koracin, V.; Mlinaric, M.; Baric, I.; Brincat, I.; Djordjevic, M.; Torkar, A.D.; Fumic, K.; Kocova, M.; Milenkovic, T.; Moldovanu, F.; et al. Current Status of Newborn Screening in Southeastern Europe. Front. Pediatr. 2021, 9, 648939. [Google Scholar] [CrossRef] [PubMed]
- Loeber, J.G. European Union Should Actively Stimulate and Harmonise Neonatal Screening Initiatives. Int. J. Neonatal Screen. 2018, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; WHO: Geneva, Switzerland, 1968; Available online: https://apps.who.int/iris/handle/10665/37650 (accessed on 22 August 2023).
- Sturdy, S.; Miller, F.; Hogarth, S.; Armstrong, N.; Chakraborty, P.; Cressman, C.; Dobrow, M.; Flitcroft, K.; Grossman, D.; Harris, R.; et al. Half a Century of Wilson & Jungner: Reflections on the Governance of Population Screening. Wellcome Open Res. 2020, 5, 158. [Google Scholar] [CrossRef]
- Dobrow, M.J.; Hagens, V.; Chafe, R.; Sullivan, T.; Rabeneck, L. Consolidated principles for screening based on a systematic review and consensus process. CMAJ 2018, 190, E422–E429. [Google Scholar] [CrossRef]
- Sagan, A.; McDaid, D.; Rajan, S.; Farrington, J.; McKee, M. Screening: When Is It Appropriate and How Can We Get It Right? European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2020; Available online: https://apps.who.int/iris/handle/10665/330810 (accessed on 22 August 2023).
- Spiekerkoetter, U.; Bick, D.; Scott, R.; Hopkins, H.; Krones, T.; Gross, E.S.; Bonham, J.R. Genomic newborn screening: Are we entering a new era of screening? J. Inherit. Metab. Dis. 2023, 46, 778–795. [Google Scholar] [CrossRef]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- la Marca, G.; Carling, R.S.; Moat, S.J.; Yahyaoui, R.; Ranieri, E.; Bonham, J.R.; Schielen, P.C.J.I. Current State and Innovations in Newborn Screening: Continuing to Do Good and Avoid Harm. Int. J. Neonatal Screen. 2023, 9, 15. [Google Scholar] [CrossRef]
- Ministry of Health. Minister of Health’s Ordinance No. 26 of June 14, 2007 on Providing Obstetric Care to Women Without Health Insurance and on Conducting Examinations and Tests Outside the Scope of Compulsory Health Insurance for Children and Pregnant Women. Available online: https://www.mh.government.bg/media/filer_public/2022/08/31/naredba-izmdop-naredba26-2007-akusherska-pomosht.pdf (accessed on 22 August 2023).
- Eysenbach, G. Improving the quality of Web surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34, Erratum in J. Med. Internet Res. 2012, 14, e8. https://doi.org/10.2196/jmir.2042. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef]
- Munck, A.; Berger, O.D.; Southern, K.W.; Carducci, C.; Groot, K.M.d.W.-D.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Proesmans, M.; Sands, D.; et al. European survey of newborn bloodspot screening for CF: Opportunity to address challenges and improve performance. J. Cyst. Fibros. 2023, 22, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Iskrov, G.G.; Stefanov, R.S.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; BURQOL-RD Research Network. Economic Burden and Health-Related Quality of Life of Patients with Cystic Fibrosis in Bulgaria. Folia Med. 2015, 57, 56–64. [Google Scholar] [CrossRef][Green Version]
- Petrova, G.; Yaneva, N.; Hrbková, J.; Libik, M.; Savov, A.; Macek, M., Jr. Identification of 99% of CFTR gene mutations in Bulgarian-, Bulgarian Turk-, and Roma cystic fibrosis patients. Mol. Genet. Genom. Med. 2019, 7, e696. [Google Scholar] [CrossRef]
- Rolla, R.; Castagno, M.; Zaffaroni, M.; Grigollo, B.; Colombo, S.; Piccotti, S.; Dellora, C.; Bona, G.; Bellomo, G. Neonatal screening for sickle cell disease and other hemoglobinopathies in “the changing Europe”. Clin. Lab. 2014, 60, 2089–2093. [Google Scholar] [CrossRef]
- Kalaydjieva, L.; Eigel, A.; Horst, J. The molecular basis of beta thalassaemia in Bulgaria. J. Med. Genet. 1989, 26, 614–618. [Google Scholar] [CrossRef]
- Petkov, G.H.; Efremov, G.D. Molecular basis of beta-thalassemia and other hemoglobinopathies in Bulgaria: An update. Hemoglobin 2007, 31, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J.; Daniel, Y.; Henthorn, J.; de la Salle, B.; Hogan, A.; Roy, N.B.A.; Mooney, C.; Langabeer, L.; Rees, D.C.; the BSH Committee. Significant haemoglobinopathies: A guideline for screening and diagnosis: A British Society for Haematology Guideline: A British Society for Haematology Guideline. Br. J. Haematol. 2023, 201, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Dick, M.C. Antenatal screening for haemoglobinopathies: Current status, barriers and ethics. Br. J. Haematol. 2019, 187, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.C. Newborn screening for hemoglobin disorders. Hemoglobin 2011, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Aramayo-Singelmann, C.; Halimeh, S.; Proske, P.; Vignalingarajah, A.; Cario, H.; Christensen, M.O.; Yamamoto, R.; Röth, A.; Reinhardt, D.; Reinhardt, H.C.; et al. Screening and diagnosis of hemoglobinopathies in Germany: Current state and future perspectives. Sci. Rep. 2022, 12, 9762. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Deng, S.; Chiriboga, C.A.; Kay, D.M.; Irumudomon, O.; Laureta, E.; Delfiner, L.; Treidler, S.O.; Anziska, Y.; Sakonju, A.; et al. Newborn Screening for Spinal Muscular Atrophy in New York State: Clinical Outcomes from the First 3 Years. Neurology 2022, 99, e1527–e1537. [Google Scholar] [CrossRef] [PubMed]
- Kimizu, T.; Ida, S.; Okamoto, K.; Awano, H.; Niba, E.T.E.; Wijaya, Y.O.S.; Okazaki, S.; Shimomura, H.; Lee, T.; Tominaga, K.; et al. Spinal Muscular Atrophy: Diagnosis, Incidence, and Newborn Screening in Japan. Int. J. Neonatal Screen. 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, D.S.T.; Russell, J.S.; Wiley, V.; Alexander, I.E.; Farrar, M.A. The implementation of newborn screening for spinal muscular atrophy: The Australian experience. Genet. Med. 2020, 22, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Vrščaj, E.; Servais, L.; Osredkar, D.; SMA NBS World Study Group. Newborn screening programs for spinal muscular atrophy worldwide: Where we stand and where to go. Neuromuscul. Disord. 2021, 31, 574–582. [Google Scholar] [CrossRef]
- Vill, K.; Schwartz, O.; Blaschek, A.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Czibere, L.; et al. Newborn screening for spinal muscular atrophy in Germany: Clinical results after 2 years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- National Health Insurance Fund (NHIF). Database of Medicinal Products That Are Paid by NHIF. Available online: https://services.nhif.bg/references/lists/medicine.xhtml (accessed on 22 August 2023).
- Stoeva, I. Current status of endocrine screening programs in Bulgaria. In Proceedings of the 2nd National Conference for Rare Diseases and Orphan Drugs, Plovdiv, Bulgaria, 9–11 September 2011; pp. 16–21. [Google Scholar]
- Kremensky, I. Genetic Screening and Diagnostics. DSc Thesis, Medical University of Sofia, Sofia, Bulgaria, 2006. [Google Scholar]
- Franková, V.; Dohnalová, A.; Pešková, K.; Hermánková, R.; O’driscoll, R.; Ješina, P.; Kožich, V. Factors Influencing Parental Awareness about Newborn Screening. Int. J. Neonatal Screen. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Wilaiwongsathien, K.; Wattanasirichaigoon, D.; Rattanasiri, S.; Aonnuam, C.; Tangshewinsirikul, C.; Tim-Aroon, T. Parental Awareness, Knowledge, and Attitudes Regarding Current and Future Newborn Bloodspot Screening: The First Report from Thailand. Int. J. Neonatal Screen. 2023, 9, 25. [Google Scholar] [CrossRef]
- Kerruish, N.J.; Webster, D.; Dickson, N. Information and consent for newborn screening: Practices and attitudes of service providers. J. Med. Ethics 2008, 34, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Franková, V.; Driscoll, R.O.; Jansen, M.E.; Loeber, J.G.; Kožich, V.; Bonham, J.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Regulatory landscape of providing information on newborn screening to parents across Europe. Eur. J. Hum. Genet. 2021, 29, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ijzebrink, A.; van Dijk, T.; Franková, V.; Loeber, G.; Kožich, V.; Henneman, L.; Jansen, M. Informing Parents about Newborn Screening: A European Comparison Study. Int. J. Neonatal Screen. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Hiller, E.H.; Landenburger, G.; Natowicz, M.R. Public participation in medical policy-making and the status of consumer autonomy: The example of newborn-screening programs in the United States. Am. J. Public Health 1997, 87, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.E.; Lister, K.J.; van Kranen, H.J.; Cornel, M.C. Policy Making in Newborn Screening Needs a Structured and Transparent Approach. Front. Public Health 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.E.; Klein, A.W.; Buitenhuis, E.C.; Rodenburg, W.; Cornel, M.C. Expanded Neonatal Bloodspot Screening Programmes: An Evaluation Framework to Discuss New Conditions with Stakeholders. Front. Pediatr. 2021, 9, 635353. [Google Scholar] [CrossRef] [PubMed]
- Octavius, G.S.; Daleni, V.A.; Sagala, Y.D.S. An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications. Children 2023, 10, 1216. [Google Scholar] [CrossRef]
- Png, M.E.; Yang, M.; Roberts, N.; Taylor-Phillips, S.; Rivero-Arias, O.; Petrou, S. Methods for evaluating the benefits and harms of antenatal and newborn screening programmes adopted by health economic assessments: Protocol for a systematic review. BMJ Open 2021, 11, e048031. [Google Scholar] [CrossRef] [PubMed]
- Png, M.E.; Yang, M.; Taylor-Phillips, S.; Ratushnyak, S.; Roberts, N.; White, A.; Hinton, L.; Boardman, F.; McNiven, A.; Fisher, J.; et al. Benefits and harms adopted by health economic assessments evaluating antenatal and newborn screening programmes in OECD countries: A systematic review of 336 articles and reports. Soc. Sci. Med. 2022, 314, 115428. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, V.; Friedman, J.M.; de Wert, G.; Knoppers, B.M. Exome/Genome-Wide Testing in Newborn Screening: A Proportionate Path Forward. Front. Genet. 2022, 13, 865400. [Google Scholar] [CrossRef] [PubMed]
- Appelberg, K.; Sörensen, L.; Zetterström, R.H.; Henriksson, M.; Wedell, A.; Levin, L.Å. Cost-Effectiveness of Newborn Screening for Phenylketonuria and Congenital Hypothyroidism. J. Pediatr. 2023, 256, 38–43.e3. [Google Scholar] [CrossRef] [PubMed]
- Stark, Z.; Scott, R.H. Genomic newborn screening for rare diseases. Nat. Rev. Genet. 2023, 24, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Vears, D.F.; Savulescu, J.; Christodoulou, J.; Wall, M.; Newson, A.J. Are We Ready for Whole Population Genomic Sequencing of Asymptomatic Newborns? Pharmacogenom. Pers. Med. 2023, 16, 681–691. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, F.M.; Chowdhury, S.; Moore, B.; Frise, E.; McCarthy, J.; Hernandez, E.J.; Wong, T.; James, K.; Guidugli, L.; Agrawal, P.B.; et al. Artificial intelligence enables comprehensive genome interpretation and nomination of candidate diagnoses for rare genetic diseases. Genome Med. 2021, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Bick, D.; Ahmed, A.; Deen, D.; Ferlini, A.; Garnier, N.; Kasperaviciute, D.; Leblond, M.; Pichini, A.; Rendon, A.; Satija, A.; et al. Newborn Screening by Genomic Sequencing: Opportunities and Challenges. Int. J. Neonatal Screen. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 124 (80.5) |
| Male | 30 (19.5) |
| Mean age in years (±SD) | 49.7 ± 12.4 |
| Highest educational degree | |
| M.Sc. | 52 (33.8) |
| Ph.D. | 83 (53.9) |
| D.Sc. | 19 (12.3) |
| Medical specialty (multiple responses allowed) | |
| Medical genetics | 22 (14.3) |
| Pediatrics | 105 (68.2) |
| Pediatric gastroenterology | 10 (6.5) |
| Pediatric endocrinology and metabolic diseases | 23 (14.9) |
| Pediatric cardiology | 4 (2.6) |
| Pediatric clinical hematology and oncology | 8 (5.2) |
| Pediatric neurology | 10 (6.5) |
| Pediatric nephrology and hemodialysis | 4 (2.6) |
| Pediatric pneumology and phthisiology | 15 (9.7) |
| Pediatric rheumatology | 4 (2.6) |
| Neonatology | 12 (7.8) |
| Biochemistry | 4 (2.6) |
| Other | 21 (13.6) |
| Mean professional experience in years (±SD) | 21.5 ± 13.2 |
| Main professional role (>50% of the time) | |
| Diagnosis and treatment | 129 (83.8) |
| Teaching | 15 (9.7) |
| Research | 6 (3.4) |
| Administration | 2 (1.3) |
| Other | 2 (1.3) |
| Topic | n (%) |
|---|---|
| Self-rated knowledge about the current universal NBS program on a 1–5 scale (1 being the lowest and 5 being the highest) | |
| 1 | 2 (1.3) |
| 2 | 5 (3.2) |
| 3 | 30 (19.5) |
| 4 | 70 (45.5) |
| 5 | 47 (30.5) |
| Participation in activities that are related to the current NBS program (multiple responses allowed) | |
| Collection of samples from newborns | 13 (8.4) |
| Primary processing and analysis of the collected samples | 5 (3.2) |
| Confirmation of diagnosis | 28 (18.2) |
| Treatment and follow-up of patients | 48 (31.2) |
| Maintenance of epidemiological registers | 2 (1.3) |
| Administration and control | 7 (4.5) |
| None | 85 (55.2) |
| Assessment of the outcomes (coverage of all newborns and early diagnosis) of the current universal NBS program on a 1–5 scale (1 being the lowest and 5 being the highest) | |
| 1 | 1 (0.6) |
| 2 | 9 (5.8) |
| 3 | 30 (19.5) |
| 4 | 75 (48.7) |
| 5 | 39 (25.3) |
| Expansion of the current NBS program by including additional disorders to screen | |
| Yes | 150 (97.4) |
| No | 4 (2.6) |
| Wilson–Jungner Principle | Cystic Fibrosis (n = 134) | Thalassemia (n = 112) | Spinal Muscular Atrophy (n = 101) |
|---|---|---|---|
| The condition sought should be an important health problem | 117 (87.3%) | 94 (83.9%) | 91 (90.1%) |
| There should be an accepted treatment for patients with recognized disease | 82 (61.2%) | 74 (66.1%) | 50 (49.5%) |
| Facilities for diagnosis and treatment should be available | 58 (43.3%) | 61 (54.5%) | 43 (42.6%) |
| There should be a recognizable latent or early symptomatic stage | 67 (50.0%) | 57 (50.9%) | 60 (59.4%) |
| There should be a suitable test or examination | 92 (68.7%) | 71 (63.4%) | 45 (44.6%) |
| The test should be acceptable to the population | 55 (41.0%) | 51 (45.5%) | 24 (23.8%) |
| The natural history of the condition, including development from latent to declared disease, should be adequately understood | 46 (34.3%) | 51 (45.5%) | 44 (43.6%) |
| There should be an agreed policy on whom to treat as patients | 64 (47.8%) | 62 (55.4%) | 31 (30.7%) |
| The cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole | 40 (29.9%) | 37 (33.0%) | 17 (16.8%) |
| Case-finding should be a continuing process and not a “once and for all” project | 71 (53.0%) | 51 (45.5%) | 47 (46.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskrov, G.; Angelova, V.; Bochev, B.; Valchinova, V.; Gencheva, T.; Dzhuleva, D.; Dichev, J.; Nedkova, T.; Palkova, M.; Tyutyukova, A.; et al. Prospects for Expansion of Universal Newborn Screening in Bulgaria: A Survey among Medical Professionals. Int. J. Neonatal Screen. 2023, 9, 57. https://doi.org/10.3390/ijns9040057
Iskrov G, Angelova V, Bochev B, Valchinova V, Gencheva T, Dzhuleva D, Dichev J, Nedkova T, Palkova M, Tyutyukova A, et al. Prospects for Expansion of Universal Newborn Screening in Bulgaria: A Survey among Medical Professionals. International Journal of Neonatal Screening. 2023; 9(4):57. https://doi.org/10.3390/ijns9040057
Chicago/Turabian StyleIskrov, Georgi, Vyara Angelova, Boyan Bochev, Vaska Valchinova, Teodora Gencheva, Desislava Dzhuleva, Julian Dichev, Tanya Nedkova, Mariya Palkova, Anelia Tyutyukova, and et al. 2023. "Prospects for Expansion of Universal Newborn Screening in Bulgaria: A Survey among Medical Professionals" International Journal of Neonatal Screening 9, no. 4: 57. https://doi.org/10.3390/ijns9040057
APA StyleIskrov, G., Angelova, V., Bochev, B., Valchinova, V., Gencheva, T., Dzhuleva, D., Dichev, J., Nedkova, T., Palkova, M., Tyutyukova, A., Hristova, M., Hristova-Atanasova, E., & Stefanov, R. (2023). Prospects for Expansion of Universal Newborn Screening in Bulgaria: A Survey among Medical Professionals. International Journal of Neonatal Screening, 9(4), 57. https://doi.org/10.3390/ijns9040057








