A Delphi Survey Study to Formulate Statements on the Treatability of Inherited Metabolic Disorders to Decide on Eligibility for Newborn Screening
Abstract
:1. Introduction
2. Materials and Methods
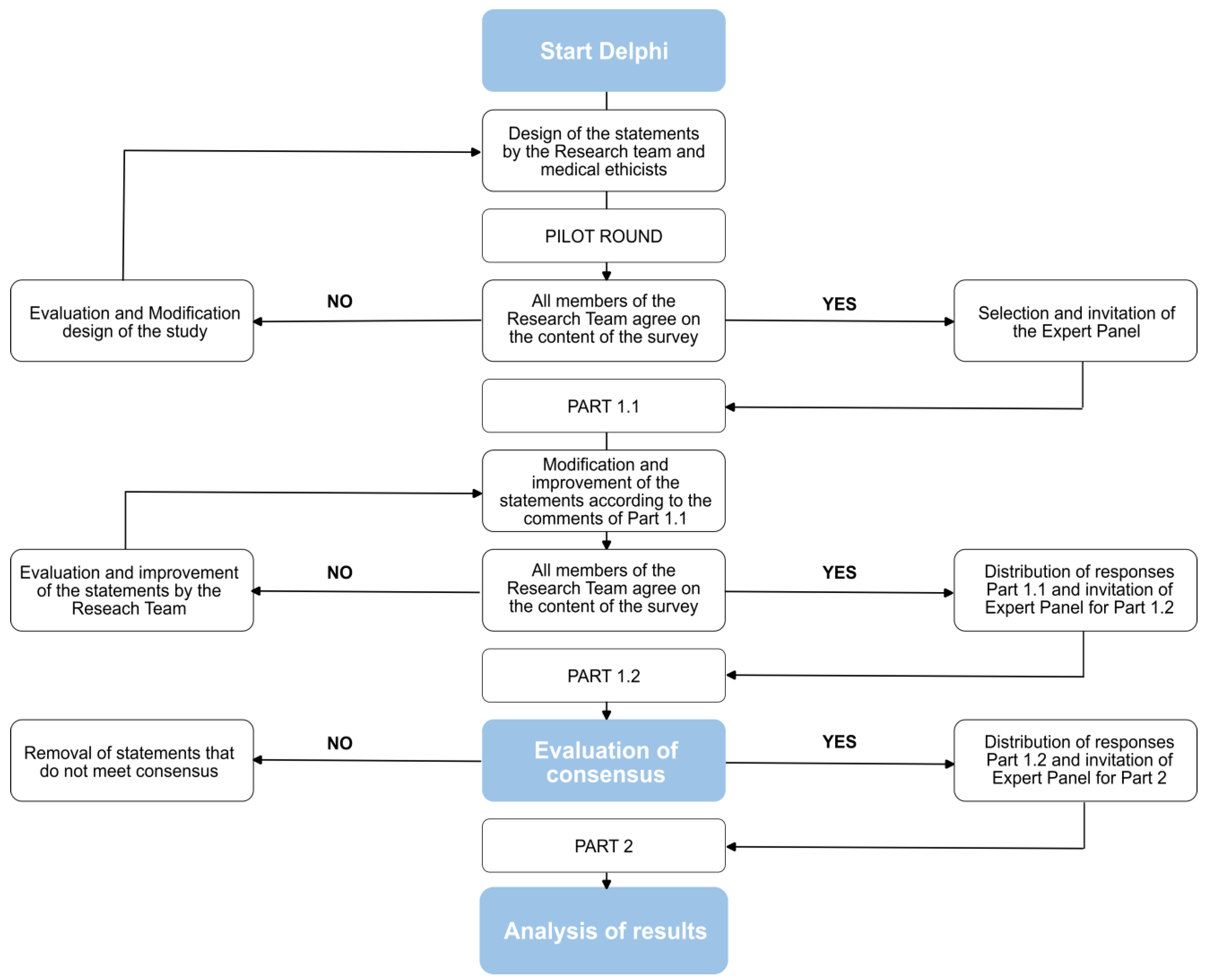
2.1. The General Design of the Delphi Study
2.1.1. Part 1
2.1.2. Part 2
2.2. Informational Input and Piloting of Materials
2.3. Strategy for Interpretation and Processing of Results
3. Results
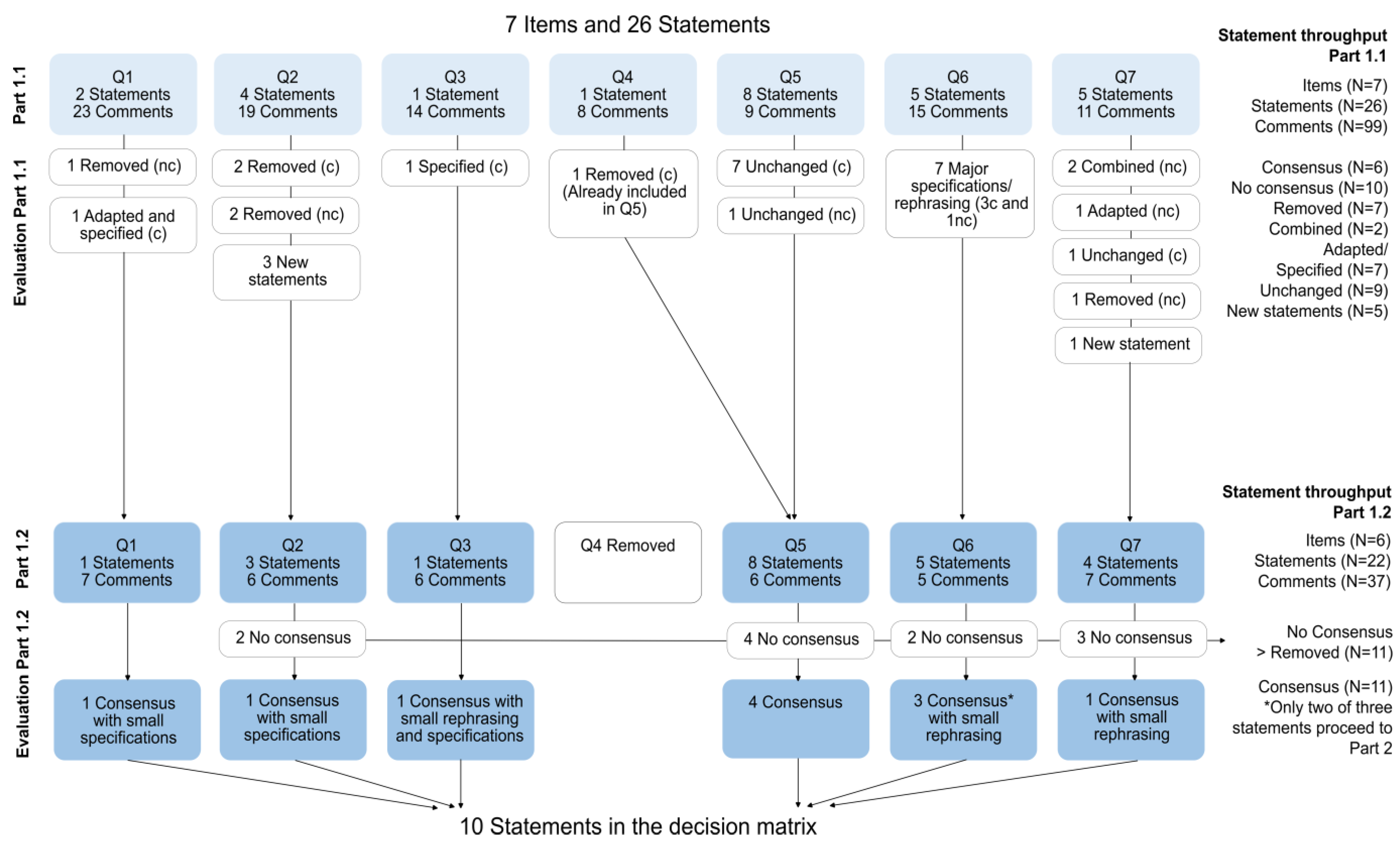
3.1. Results Part 1.1 and Part 1.2
3.1.1. Panel Participation
3.1.2. Statements and Item Throughput
3.2. Results Part 2
3.2.1. Panel Participation
3.2.2. Contribution of Statements
3.2.3. Inclusion in NBS
3.2.4. The Scoring of Statements per IMD
3.2.5. Comments and Evaluation of the EP on the Decision Matrix
- Opinion 1:
- It can be a useful tool if all data are available and experts are involved in decision-making.
- Opinion 2:
- It can be a useful tool as a starting point for a discussion for inclusion in NBS.
- Opinion 3:
- Concerns about how to proceed with this tool if disorders are not eligible for NBS.
- Opinion 4:
- Concerns about how to proceed with this tool for disorders with a broad phenotypic variability.
- Opinion 5:
- The decision matrix is not useful (yet) to quantify treatability for IMDs in NBS.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. First Draft of the Statements by the Research Team and Medical Ethicists
- (a)
- Approved by the EMA
- (b)
- Covered by insurance companies (as set by the Dutch government)
- (a)
- Little or no impact or burden with yearly blood tests, noninvasive imaging screening tests, oral medication with a low side-effect profile
- (b)
- Modest impact or burden with invasive screening tests, daily lifestyle/diet modification, and medication with a substantial side-effect profile
- (c)
- Significant impact or burden including intravenous treatment, removal, or transplantation of a non-vital organ (system) with frequent complications
- (d)
- Extreme impact or burden with transplantation of a vital organ (system)
- (a)
- Prevent (sudden) death
- (b)
- Prevent complications of all possible involved organs
- (c)
- Reverse all manifestations of the disorder
- (d)
- Prevent symptoms clearly related to one or two primary organ(s), while it is acceptable that treatment cannot prevent all clinical problems
- (e)
- Prevent developmental delay
- (f)
- Prevent recurrent admissions to the hospital
- (g)
- Improve the quality of life
- (h)
- Improve the quality-adjusted life-years (QALYs)
- (a)
- 1 Publication of 1 institute
- (b)
- At least 2 publications of 1 institute
- (c)
- 1 Publication of at least 2 institutes each
- (d)
- At least 2 Publication of at least 2 institutes each
- (e)
- At least…
- (a)
- In <25% of patients
- (b)
- In 25–50% of patients
- (c)
- In 50–75% of patients
- (d)
- In >75% of patients
- (e)
- At least in…
Appendix B. Treatability Statements after Part 1.1
- (a)
- As long as the expected benefit/burden ratio of early treatment is positive for patients, any available treatment enabling NBS is acceptable
- (b)
- The burden of treatment is not important in determining treatability for NBS
- (c)
- In the decision to include a disorder in NBS the risk of overtreatment is less important than the risk of undertreatment
- (a)
- Prevent (sudden) death
- (b)
- Prevent complications of all possible involved organs
- (c)
- Reverse all manifestations of the disorder
- (d)
- Prevent symptoms clearly related to one or two primary organ(s), while it is acceptable that treatment cannot prevent all clinical problems
- (e)
- Prevent developmental delay
- (f)
- Prevent recurrent admissions to the hospital
- (g)
- Improve the quality of life
- (h)
- Improve the quality-adjusted life-years (QALYs)
- (a)
- In very rare disorders at least one patient should be treated with any measurable effect with quality data about a plausible mechanism and/or animal models.
- (b)
- At least one paper on a new treatment by one institute with good quality data about a plausible mechanism with an “adequate number of patients” with clear effect size and suggesting the efficacy of early treatment is sufficient to accept that treatment for NBS.
- (c)
- Papers on a new treatment by at least two institutes with good quality data about a plausible mechanism with an “adequate number of patients” with clear effect size and suggesting the efficacy of early treatment is sufficient to accept that treatment for NBS.
- (d)
- Papers on a new treatment by at least three institutes with good quality data about a plausible mechanism with an “adequate number of patients” with clear effect size and suggesting the efficacy of early treatment is sufficient to accept that treatment for NBS.
- (e)
- There must be consensus on the positive outcome by an (international) expert meeting
- (a)
- In <50% of patients
- (b)
- In >50% of patients
- (c)
- In >75% of patients
- (d)
- Basically all patients
Appendix C. Comments from the Expert Panel on Part 2
“Proven treatment efficacy. Readily available treatment”.
“In PDE you want to treat seizures but also PMR, for the last there is evidence but may be less clear and spectacular than for seizure control. It would help to answer these questions if the latest papers are provided (if you do not know the last up-to-date info for this disease)”.
“We test consistently for PDE (and treat accordingly) in infants with unexplained epilepsy, not sure if implementation in NBS would lead to earlier detection/treatment”.
“Not financially covered or reimbursed for adults (yet) and almost no one will be capable of living a normal independent life. Mortality prevention is not reason enough to include a disorder in NBS. The treatment of epilepsy could also be done by changing guidelines, that make sure that every child with epilepsy will be screened directly for this disorder”.
“I missed questions on the appropriateness of biomarkers in body fluids”.
“No doubt”.
“Yet unknown, insufficient data with a possible positive response but in only nine patients, future studies are necessary as well as a valid screening parameter”.
“Yes, but is proven effective screening is still lacking”!
“Relevance of NBS for CG is undisputed in my opinion”.
“Questionable what ‘a patient’ is, Duarte variants included or not”?
“I actually do not know if there is a treatment effect in > 75% of patients, I assume there is but I don’t know if this is studied that way”.
“Although the appearance of long-term complications are not affected by current intervention after NBS it can be lifesaving for the child with neonatal illness to know that it is due to CG because NBS is positive (avoids the chance that this diagnosis is overlooked with fatal consequences)”.
“The issue is that death is prevented and liver disease as well but mental retardation is not influenced really”.
“No doubt”.
“For reasons of the life-threatening newborn crisis, it does not prevent developmental delay and other long-term effects”.
“A big problem is that early disease and possible death, as well as cataract, are prevented BUT the treatment does not prevent significant developmental delay in many patients”.
“More studies needed to confirm NBS efficacy on lowering disease-related mortality, but in principle a good candidate for NBS”.
“Exceedingly rare, but treatable, should be included don’t know about how many patients have been treated, and how many mild patients”.
“I don’t know how many centers have thought about this treatment or whether this builds on knowledge for other fatty acid oxidation disorders”.
“Yes, if one can differentiate between myopathic and the other forms”.
“One of the issues will be that not all CPT2 patients need treatment from birth onwards and that you will treat persons unnecessarily with a mild disease as patients”.
“Research is necessary to investigate this. There is a discrepancy between severe phenotypes and late presentation. More research is needed to look e.g., how to discriminate between the severity of phenotypes”.
“Most cases are milder cases”.
“Easily treatable, for the ones at risk for hypoglycemia”.
“More study is needed to make sure if CPT2 should be added to NBS. This will at least consist of a cohort study including all Dutch patients, and if numbers are too low, an international study”.
“Starting shortly after birth in classic infantile-onset Pompe disease will positively affect treatment outcome. Effect of NBS on development of long term complications likely limited”.
“Don’t know about consensus meetings. don’t know about diagnostic assays”.
“I think early treatment helps but does not prevent all disease manifestations. I think it might also help in the diagnostic delay and uncertainty in parents”.
“Questions about inclusion will not be related to treatability”.
“No because we cannot filter out the late-onset forms in an efficient way in a neonatal blood spot; not even with DNA techniques”.
“If detection of mild (late or adult-onset) variants can be prevented”.
“Detection of phenylalanine in NBS unsuitable test as AR form can be missed. Most symptoms can be reversed with treatment after diagnosis, even after a large diagnostic delay”.
“Don’t know about variants, don’t know about literature”.
“Also for this disease, I don’t have all the UpToDate knowledge but I guess there has not been an international consensus meeting”.
“Based on treatability this could fly in but we have to accept that MR cannot be easily prevented as in PKU”.
“I have no experience with this disorder. At large risk at over-diagnosing in my opinion”.
“For me, that would depend on the number of patients with biallelic mutations versus cases with dominant inheritance. That complicates the decision for defects in this gene. For this disease, I do have not enough overview of the current literature to decide on this question”.
“Insufficient data yet”.
“Insufficient knowledge about this disorder”.
“Patients still exhibit many metabolic decompensations despite early detection. If other treatment modalities become standard of care (liver Tx, gene therapy), the efficacy of NBS for OTC deficiency may become more apparent”.
“I am afraid that the most severe type will already give symptoms before the neonatal screening result and therefore you won’t fully prevent death or developmental delay, also diet and scavengers will not fully prevent this”.
“Not taken into account that it is X-linked affected girls: risk/benefit longitudinal data needed the question will be how you aim to tackle the difference between the very severe presentation of male newborns with usually later presenting female children”.
“For severe cases, NBS results may come too late”.
“There are insufficient good treatment options but harm can definitely be avoided and parents could be counseled for subsequent children. But that is not the aim of NBS”.
“In general X-linked diseases are more difficult for NBS. I see too many drawbacks”.
“Not the severe OTC in boys milder forms may benefit”.
“As we are discussing only the severe end of the phenotypic spectrum, I think screening will be too late to prevent the first clinical metabolic decompensation”. “It is the first decompensation that often determines the outcome of cognitive and motor impairment”.
“Early detection and treatment will prevent complications of WD, but optimization of treatment still needed”.
“I am insufficiently aware of this condition and screening & treatment to comment”.
“Which patients are the true patients in need of treatment? If that can be answered then NBS is justified”.
“Looking back to Pompe disease, I guess there was an extra question on Qualys not included for other diseases, so should be left out in Pompe”.
“I lack experience in the various forms (liver/neurologic) of Wilson’s disease and effects of therapy”.
“No doubt”.
“Still significant disease burden despite early detection, the effect of long term complications (i.e., kidney failure) unclear”.
“Already part of NBS”.
“The available treatment helps but does not fully prevents longterm complications in MMA in addition when the NBS results are available the most severe MMA patients are often already symptomatic so yes I think NBS helps but does not prevent all MMA complications”.
“We do not -by the method now at the time performed now- achieve that we will prevent death etc. in severe forms, but if it will be early enough it should prevent much of the disease. So, the answer depends on the interpretation of the question”.
“Unfortunately, the effects of early treatment (when comparing early identified patients by screening with their clinically identified sib) are most effective in preventing the initial presentation and consequences thereof”.
“I am not fully convinced”.
“Not for the severe MMA mut 0 or mut—as they will have presented already the milder CBLA CBLB will have a major benefit”.
“Severe MCM is not fit for inclusion in NBS. However, the milder causes of MMA are”!
“Currently unclear how NBS for TH would affect the clinical outcome. Variable response to treatment”.
“Yes, but not all types will have full treatment effect (for the severe type this effect can be limited) in addition I don’t know all the evidence for this disease”.
“Limited experience”.
“Difficult to diagnose, a long delay and treatment has a very good effect, that is why I have chosen this”
“No doubt”.
IMD 10—PKU in NBS
“Proven benefit. No discussion on NBS”.
“Included as positive control? I indeed scored high in all aspects of treatability”
“No doubt”.
Appendix D. Comments from the Expert Panel on the Final Treatability Statements
“Yes I think so but to fill in you have to know the up-to-date knowledge and I think you can better fill this in if you have read the relevant literature beforehand”.
“Yes, but must be performed by (international) experts in treatment of that particular disease”.
“It can be”.
“Yes if all the data (evidence for consensus meetings etc.) is easily available”.
“Overall this is a difficult subject. On a few of these diseases, I am not an expert, and in my opinion, experts should describe the effect of early treatment and then together with these experts we can make a decision”.
“It would be a useful tool to start the discussion”.
“I guess so, counting the produced marks together. Would think it can be used as a pyramid. If you have gone through this level the matrix using the other Wilson & Jungner criteria can be put in a matrix receiving marks/points to pass the criteria to be included”.
“Yes, this may work. I had some troubles with the ‘dubbele ontkenning’ (double denial?), which is when you decide an IMD is NOT fit for inclusion, and then you have to state how important ‘the presence of an effective treatment’ is, as for some there is no effective treatment, so you tend to say that it is not important, while it actually is (though there is not a treatment). Rather difficult to write this down, but I hope you get my point.
“In particular the questions are not suitable to help to support the advice for not selecting the disease for NBS”.
“In addition, it is kind of difficult not to think about severe vs. less severe or even adult-onset disease. If I am correct it was stated in the introduction that we should think about the severe phenotypes when doing this survey, but at the end of the matrix, it suddenly states ‘severe and less severe’ (or something like that). Finally, quality of life is a very complex issue here. How to measure this? How reliable? Disease-specific scales, etc., etc”.
“It seems useful for diseases with limited phenotypic variability. For diseases with a wider phenotypic variability, it seems more difficult because the two ends of the spectrum cannot be compared and usually not enough data are available on late-onset disease. Example: Pompe disease…………”.
“No, too oversimplified, without profound knowledge of every disorder”.
“Sometimes questions are composed of more statements and therefore are difficult to answer clearly. Some questions are still difficult to understand, e.g., the question: ‘The treatment/diet results… disease-related mortality.’ This matrix is rather global and not all important issues per disease are addressed. In my opinion, the clarity/interpretability of this final decision-matrix is not yet sufficient to be useful to quantify treatability for IMDs in NBS”.
“In general, yes, but:—not all questions applicable to all diseases (e.g., preventing (acute) death, developmental delay). ‘Not applicable’ could be added as an option.—due to limited knowledge on some diseases several questions hard to answer.—question should be added about the level of expertise of the respondent with the specific disorder which is assayed”.
References
- Wilson, J.M.G.; Jungner, G.; World Health Organization. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisting wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Costea, I. Guiding policy decisions for genetic screening: Developing a systematic and transparent approach. Public Health Genom. 2011, 14, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sturdy, S.; Miller, F.; Hogarth, S.; Armstrong, N.; Chakraborty, P.; Cressman, C.; Dobrow, M.; Flitcroft, K.; Grossman, D.; Harris, R.; et al. Half a Century of Wilson & Jungner: Reflections on the Governance of Population Screening. Wellcome Open Res. 2020, 5, 158. [Google Scholar] [PubMed]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R. Newborn Screening: Toward a Uniform Screening Panel and System—Executive Summary. Pediatrics 2006, 117 (Suppl. S3), S296–S307. [Google Scholar] [CrossRef]
- Kemper, A.R.; Green, N.S.; Calonge, N.; Lam, W.K.; Comeau, A.M.; Goldenberg, A.J.; Ojodu, J.; Prosser, L.A.; Tanksley, S.; Bocchini, J.A., Jr. Decision-making process for conditions nominated to the Recommended Uniform Screening Panel: Statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2014, 16, 183–187. [Google Scholar] [CrossRef]
- Children ACoHDiNa. Recommended Uniform Screening Panel; Health Resources & Services Administration: Rockville, MD, USA, 2018. [Google Scholar]
- Lund, A.M.; Wibrand, F.; Skogstrand, K.; Bækvad-Hansen, M.; Gregersen, N.; Andresen, B.S.; Hougaard, D.M.; Dunø, M.; Olsen, R.K.J. Use of Molecular Genetic Analyses in Danish Routine Newborn Screening. Int. J. Neonatal Screen. 2021, 7, 50. [Google Scholar] [CrossRef]
- Australian Health Ministers’ Advisory Council. Newborn Bloodspot Screening National Policy Framework; Australian Health Ministers’ Advisory Council: Canberra, Australia, 2018. [Google Scholar]
- Committee CP; Screening SCO. Population-Based Screening Framework; Australian Government Department of Health and Aged Care: Canberra, Australia, 2018. [Google Scholar]
- Australian Goverment. NBS Conditions Screened in Australia, September 2022; Australian Government Department of Health and Aged Care: Canberra, Australia, 2022. [Google Scholar]
- Pollitt, R.J. Introducing new screens: Why are we all doing different things? J. Inherit. Metab. Dis. 2007, 30, 423–429. [Google Scholar] [CrossRef]
- Furnier, S.M.; Durkin, M.S.; Baker, M.W. Translating Molecular Technologies into Routine Newborn Screening Practice. Int. J. Neonatal Screen. 2020, 6, 80. [Google Scholar] [CrossRef]
- Adhikari, A.N.; Gallagher, R.C.; Wang, Y.; Currier, R.J.; Amatuni, G.; Bassaganyas, L.; Chen, F.; Kundu, K.; Kvale, M.; Mooney, S.D.; et al. The role of exome sequencing in newborn screening for inborn errors of metabolism. Nat. Med. 2020, 26, 1392–1397. [Google Scholar] [CrossRef]
- Holm, I.A.; Agrawal, P.B.; Ceyhan-Birsoy, O.; Christensen, K.D.; Fayer, S.; Frankel, L.A.; Genetti, C.A.; Krier, J.B.; LaMay, R.C.; Levy, H.L.; et al. The BabySeq project: Implementing genomic sequencing in newborns. BMC Pediatr. 2018, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Milko, L.V.; O’Daniel, J.M.; DeCristo, D.M.; Crowley, S.B.; Foreman, A.K.M.; Wallace, K.E.; Mollison, L.F.; Strande, N.T.; Girnary, Z.S.; Boshe, L.J.; et al. An Age-Based Framework for Evaluating Genome-Scale Sequencing Results in Newborn Screening. J. Pediatr. 2019, 209, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Veldman, A.; Kiewiet, M.B.G.; Heiner-Fokkema, M.R.; Nelen, M.R.; Sinke, R.J.; Sikkema-Raddatz, B.; Voorhoeve, E.; Westra, D.; Dollé, M.E.T.; Schielen, P.C.J.I.; et al. Towards Next-Generation Sequencing (NGS)-Based Newborn Screening: A Technical Study to Prepare for the Challenges Ahead. Int. J. Neonatal Screen. 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F.; Smith, L.D.; Kunard, C.M.; Bainbridge, M.; Batalov, S.; Benson, W.; Blincow, E.; Caylor, S.; Chambers, C.; Del Angel, G.; et al. A genome sequencing system for universal newborn screening, diagnosis, and precision medicine for severe genetic diseases. Am. J. Hum. Genet. 2022, 109, 1605. [Google Scholar] [CrossRef] [PubMed]
- Van Karnebeek, C.D.M.; Shevell, M.; Zschocke, J.; Moeschler, J.B.; Stockler, S. The metabolic evaluation of the child with an intellectual developmental disorder: Diagnostic algorithm for identification of treatable causes and new digital resource. Mol. Genet. Metab. 2014, 111, 428–438. [Google Scholar] [CrossRef]
- Van Konijnenburg, E.M.M.H.; Wortmann, S.B.; Koelewijn, M.J.; Tseng, L.A.; Houben, R.; Stöckler-Ipsiroglu, S.; Ferreira, C.R.; van Karnebeek, C.D.M. Treatable inherited metabolic disorders causing intellectual disability: 2021 review and digital app. Orphanet J. Rare Dis. 2021, 16, 170. [Google Scholar] [CrossRef]
- Jünger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef]
- Veugelers, R.; Gaakeer, M.I.; Patka, P.; Huijsman, R. Improving design choices in Delphi studies in medicine: The case of an exemplary physician multi-round panel study with 100% response. BMC Med. Res. Methodol. 2020, 20, 156. [Google Scholar] [CrossRef]
- Wainwright, P.; Gallagher, A.; Tompsett, H.; Atkins, C. The use of vignettes within a Delphi exercise: A useful approach in empirical ethics? J. Med. Ethics 2010, 36, 656–660. [Google Scholar] [CrossRef]
- Vogel, C.; Zwolinsky, S.; Griffiths, C.; Hobbs, M.; Henderson, E.; Wilkins, E. A Delphi study to build consensus on the definition and use of big data in obesity research. Int. J. Obes. 2019, 43, 2573–2586. [Google Scholar] [CrossRef]
- Beiderbeck, D.; Frevel, N.; Von Der Gracht, H.A.; Schmidt, S.L.; Schweitzer, V.M. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX 2021, 8, 101401. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Hasson, F.; McKenna, H.P. The Delphi Technique in Nursing and Health Research; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, T. Reliability and validity of the Delphi method in guideline development for family physicians. Qual. Prim. Care 2010, 18, 317–326. [Google Scholar] [PubMed]
- Ceyhan-Birsoy, O.; Murry, J.B.; Machini, K.; Lebo, M.S.; Yu, T.W.; Fayer, S.; Genetti, C.A.; Schwartz, T.S.; Agrawal, P.B.; Parad, R.B.; et al. Interpretation of Genomic Sequencing Results in Healthy and Ill Newborns: Results from the BabySeq Project. Am. J. Hum. Genet. 2019, 104, 76–93. [Google Scholar] [CrossRef]
- Wojcik, M.H.; Zhang, T.; Ceyhan-Birsoy, O.; Genetti, C.A.; Lebo, M.S.; Yu, T.W.; Parad, R.B.; Holm, I.A.; Rehm, H.L.; Beggs, A.H.; et al. Discordant results between conventional newborn screening and genomic sequencing in the BabySeq Project. Genet. Med. 2021, 23, 1372–1375. [Google Scholar] [CrossRef]
- Keeney, S.; Hasson, F.; McKenna, H. Consulting the oracle: Ten lessons from using the Delphi technique in nursing research. J. Adv. Nurs. 2006, 53, 205–212. [Google Scholar] [CrossRef]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar] [CrossRef]
- Hohmann, E.; Brand, J.C.; Rossi, M.J.; Lubowitz, J.H. Expert Opinion Is Necessary: Delphi Panel Methodology Facilitates a Scientific Approach to Consensus. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 349–351. [Google Scholar] [CrossRef]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ Br. Med. J. 2001, 323, 334. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924. [Google Scholar] [CrossRef]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Markmann, C.; Spickermann, A.; Von Der Gracht, H.A.; Brem, A. Improving the question formulation in Delphi-like surveys: Analysis of the effects of abstract language and amount of information on response behavior. Futures Foresight Sci. 2021, 3, 56. [Google Scholar] [CrossRef]
- Yaniv, I. Group diversity and decision quality: Amplification and attenuation of the framing effect. Int. J. Forecast. 2011, 27, 41–49. [Google Scholar] [CrossRef]
- Winkler, J.; Moser, R. Biases in future-oriented Delphi studies: A cognitive perspective. Technol. Forecast. Soc. Change 2016, 105, 63–76. [Google Scholar] [CrossRef]
- Van Dijk, T.; Kater, A.; Jansen, M.; Dondorp, W.J.; Blom, M.; Kemp, S.; Langeveld, M.; Cornel, M.C.; van der Pal, S.M.; Henneman, L. Expanding Neonatal Bloodspot Screening: A Multi-Stakeholder Perspective. Front. Pediatr. 2021, 9, 706394. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Christensen, K.D.; Genetti, C.A.; Parad, R.B.; Robinson, J.O.; Zawatsky, C.L.B.; Zettler, B.; Beggs, A.H.; Holm, I.A.; Green, R.C.; et al. Parental Attitudes Toward Standard Newborn Screening and Newborn Genomic Sequencing: Findings from the BabySeq Study. Front. Genet. 2022, 13, 867371. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Bredius, R.G.M.; Jansen, M.E.; Weijman, G.; Kemper, E.A.; Vermont, C.L.; Hollink, I.H.I.M.; Dik, W.A.; van Montfrans, J.M.; van Gijn, M.E.; et al. Parents’ Perspectives and Societal Acceptance of Implementation of Newborn Screening for SCID in the Netherlands. J. Clin. Immunol. 2021, 41, 99–108. [Google Scholar] [CrossRef]
| Part 1.1/Round 1 | Part 1.2/Round 2 | Part 2/Round 3 |
|---|---|---|
| Consensus was declared if a statement scored a mean of ≥7.0, a median of ≥7.0, and a mode of ≥7.0. Regardless of whether consensus was reached, statements proceeded to Part 1.2 to be discussed in an altered form. | Consensus was declared if a statement scored a mean of ≥7.0, a median of ≥7.0, and a mode of ≥7.0. Only statements reaching consensus proceeded to Part 2 1. | A ≥ 75% majority agreement by the EP had to be reached to include a disorder in the NBS based only on treatability. |
| The RT was free to add small grammatical or textual changes to clarify the statement (also based on individual comments from the EP), provided that it did not compromise the meaning or implication of the statement. | The RT was free to add small grammatical or textual changes to clarify the statement (also based on individual comments from the EP), provided that it did not compromise the meaning or implication of the statement. | The RT was free to add small grammatical or textual changes to clarify the statement (also based on individual comments from the EP), provided that it did not compromise the meaning or implication of the statement. |
| If 3 or more EPs requested the same alteration, the statement was adapted accordingly. | No new statements were added after Part 1.2. | |
| If 3 or more EPs found the statement irrelevant, it could be removed, adapted, specified, or combined with another statement. | ||
| The RT was free to add statements, based on the suggestions of individual panelists. |
| Statement (S)/Question (Q) | |
|---|---|
| Q1./S1 | There is a treatment available that is fully financially covered or reimbursed by standard health care (also when a patient reaches adulthood). |
| Q2./S2 | The expected benefit/burden ratio of early treatment is positive and results in significant health benefits. |
| Q3./S3 | The treatment/diet results in enough significant health benefits (compared to no treatment) to accept a small risk for disease-related mortality. |
| Q5.1./S4 | Early detection with subsequent treatment, when compared to clinical presentation with subsequent treatment, prevents (sudden) death. |
| Q5.4./S5 | Early detection with subsequent treatment, when compared to clinical presentation with subsequent treatment, prevents symptoms clearly related to one or two primary organ(s). |
| Q5.5./S6 | Early detection with subsequent treatment, when compared to clinical presentation with subsequent treatment, prevents developmental delay. |
| Q5.7./S7 | Early detection with subsequent treatment, when compared to clinical presentation with subsequent treatment, improves quality of life. |
| Q6.4./S8 | Papers on treatments were published by at least two institutes with good quality data about a plausible mechanism with an “adequate number of patients” with clear effect size and suggesting the efficacy of early treatment is sufficient to accept that treatment for newborn screening. |
| Q6.5./S9 | There is consensus on positive outcome of early detection by an (international) expert meeting. |
| Q7.3./S10 | The expected effect of treatment was demonstrated in more than 75% of patients (both mild and severe variants). |
| Assessment of Genes in Studies (Yes/No) * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name of Disorder | Associated Gene | OMIM 2 | NGSf4NBS Project [18] | RUSP [7] | NEXUS [17] | Babyseq [16] Project | Treatability Delphi Study | |
| IMD 1 | PDE | ALDH7A1 | 266100 | Yes | No | No | No | Yes |
| IMD 2 | CG | GALT | 606999 | Yes | Yes | Yes | Yes | Yes |
| IMD 3 | CPT2 | CPT2 | 255110 | Yes | Yes | Yes | No | No |
| 600649 | ||||||||
| 608836 | ||||||||
| IMD 4 | GSD2 | GAA | 232300 | No | Yes | Yes | Yes | No |
| IMD 5 | GCH1 | GCH1 | 233910 | Yes | Yes | Yes | No | No |
| IMD 6 | OTC | OTC | 311250 | Yes | No | Yes | Yes | No |
| IMD 7 | WD | ATP7B | 277900 | Yes | No | No | Yes | No |
| IMD 8 | MCM | MMUT | 251000 | Yes | Yes | No | No | Yes |
| IMD 9 | TH | TH | 605407 | Yes | No | No | No | Yes |
| IMD 10 | PKU | PAH | 261600 | Yes | Yes | Yes | Yes | Yes |
| PKU | CG | MCM | PDE | TH | CPT2 | WD | GCH1 | GSD2 | OTC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Consensus on eligibility for NBS by the EP | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No | |
| (−100.00%) | (−94.70%) | (−87.60%) | (−86.40%) | (−80.00%) | (−72.20%) | (−71.20%) | (−66.70%) | (−58.80%) | (−50.00%) | ||
| Response rate | N = 16 | N = 19 | N = 16 | N = 22 | N = 15 | N = 18 | N = 14 | N = 12 | N = 17 | N = 16 | |
| S1 | 4.75 | 4.78 | 4.5 | 3.9 | 4.56 | 4.38 | 4.27 | 4.23 | 4.24 | 4.44 | |
| S2 | 4.88 | 4.42 | 3.94 | 4.05 | 4.19 | 3.88 | 3.93 | 4.08 | 4.06 | 3.63 | |
| S3 | 4.43 | 4.26 | 3.63 | 4.14 | 3.67 | 3.39 | 4.07 | 3.55 | 4.12 | 4 | |
| S4 | 2.79 | 4.22 | 3.69 | 3.4 | 2.87 | 3.56 | 2.92 | 2.33 | 3.59 | 3.94 | |
| S5 | 4.75 | 4.35 | 3.56 | 3.86 | 3.69 | 3.78 | 4.07 | 3.77 | 4.24 | 3.88 | |
| S6 | 4.75 | 3 | 3.56 | 3.43 | 3.69 | 3 | 3.83 | 3.38 | 2.88 | 3.44 | |
| S7 | 4.56 | 3.89 | 3.6 | 4.1 | 4 | 3.44 | 4.21 | 3.85 | 4.12 | 3.69 | |
| S8 | 4.88 | 4.32 | 3.75 | 4 | 3.21 | 3.22 | 3.54 | 2.67 | 4.12 | 3.75 | |
| S9 | 4.88 | 4.21 | 3.53 | 3.76 | 2.87 | 3 | 3.46 | 2.92 | 4.07 | 3.56 | |
| S10 | 4.75 | 3.95 | 3.13 | 3.9 | 3.21 | 3 | 3.21 | 3.17 | 3.56 | 3.38 | |
| Mean 2 per IMD | 4.54 | 4.14 | 3.69 | 3.85 | 3.6 | 3.46 | 3.75 | 3.39 | 3.9 | 3.77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldman, A.; Kiewiet, M.B.G.; Westra, D.; Bosch, A.M.; Brands, M.M.G.; de Coo, R.I.F.M.; Derks, T.G.J.; Fuchs, S.A.; van den Hout, J.M.P.; Huidekoper, H.H.; et al. A Delphi Survey Study to Formulate Statements on the Treatability of Inherited Metabolic Disorders to Decide on Eligibility for Newborn Screening. Int. J. Neonatal Screen. 2023, 9, 56. https://doi.org/10.3390/ijns9040056
Veldman A, Kiewiet MBG, Westra D, Bosch AM, Brands MMG, de Coo RIFM, Derks TGJ, Fuchs SA, van den Hout JMP, Huidekoper HH, et al. A Delphi Survey Study to Formulate Statements on the Treatability of Inherited Metabolic Disorders to Decide on Eligibility for Newborn Screening. International Journal of Neonatal Screening. 2023; 9(4):56. https://doi.org/10.3390/ijns9040056
Chicago/Turabian StyleVeldman, Abigail, M. B. Gea Kiewiet, Dineke Westra, Annet M. Bosch, Marion M. G. Brands, René I. F. M. de Coo, Terry G. J. Derks, Sabine A. Fuchs, Johanna. M. P. van den Hout, Hidde H. Huidekoper, and et al. 2023. "A Delphi Survey Study to Formulate Statements on the Treatability of Inherited Metabolic Disorders to Decide on Eligibility for Newborn Screening" International Journal of Neonatal Screening 9, no. 4: 56. https://doi.org/10.3390/ijns9040056
APA StyleVeldman, A., Kiewiet, M. B. G., Westra, D., Bosch, A. M., Brands, M. M. G., de Coo, R. I. F. M., Derks, T. G. J., Fuchs, S. A., van den Hout, J. M. P., Huidekoper, H. H., Kluijtmans, L. A. J., Koop, K., Lubout, C. M. A., Mulder, M. F., Panis, B., Rubio-Gozalbo, M. E., de Sain-van der Velden, M. G., Schaefers, J., Schreuder, A. B., ... van Spronsen, F. J. (2023). A Delphi Survey Study to Formulate Statements on the Treatability of Inherited Metabolic Disorders to Decide on Eligibility for Newborn Screening. International Journal of Neonatal Screening, 9(4), 56. https://doi.org/10.3390/ijns9040056






