Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency
Abstract
:1. Introduction
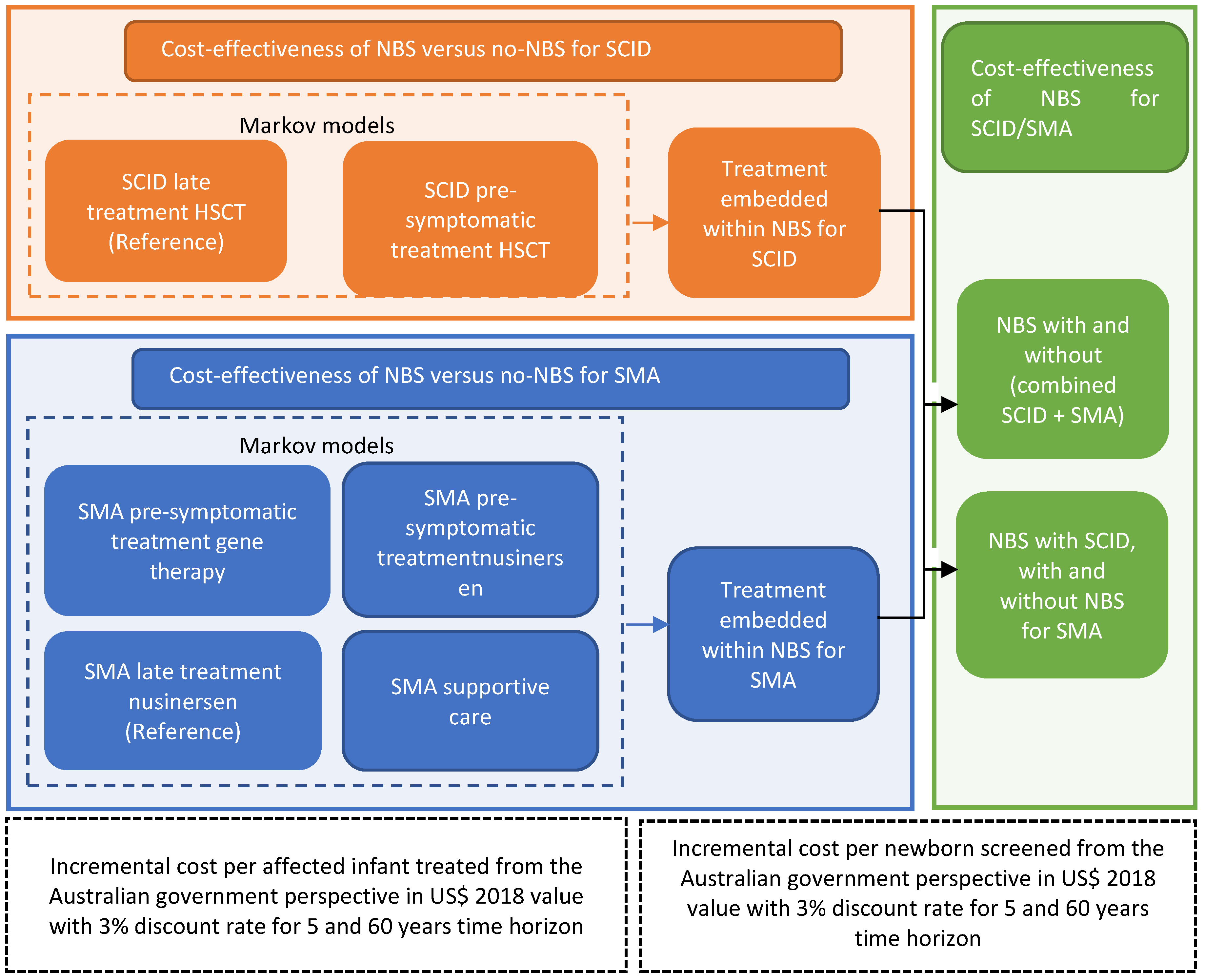
2. Materials and Methods
3. Results
3.1. Cost-Effectiveness Analysis of NBS for SMA
3.2. Cost-Effectiveness Analysis of NBS for SCID
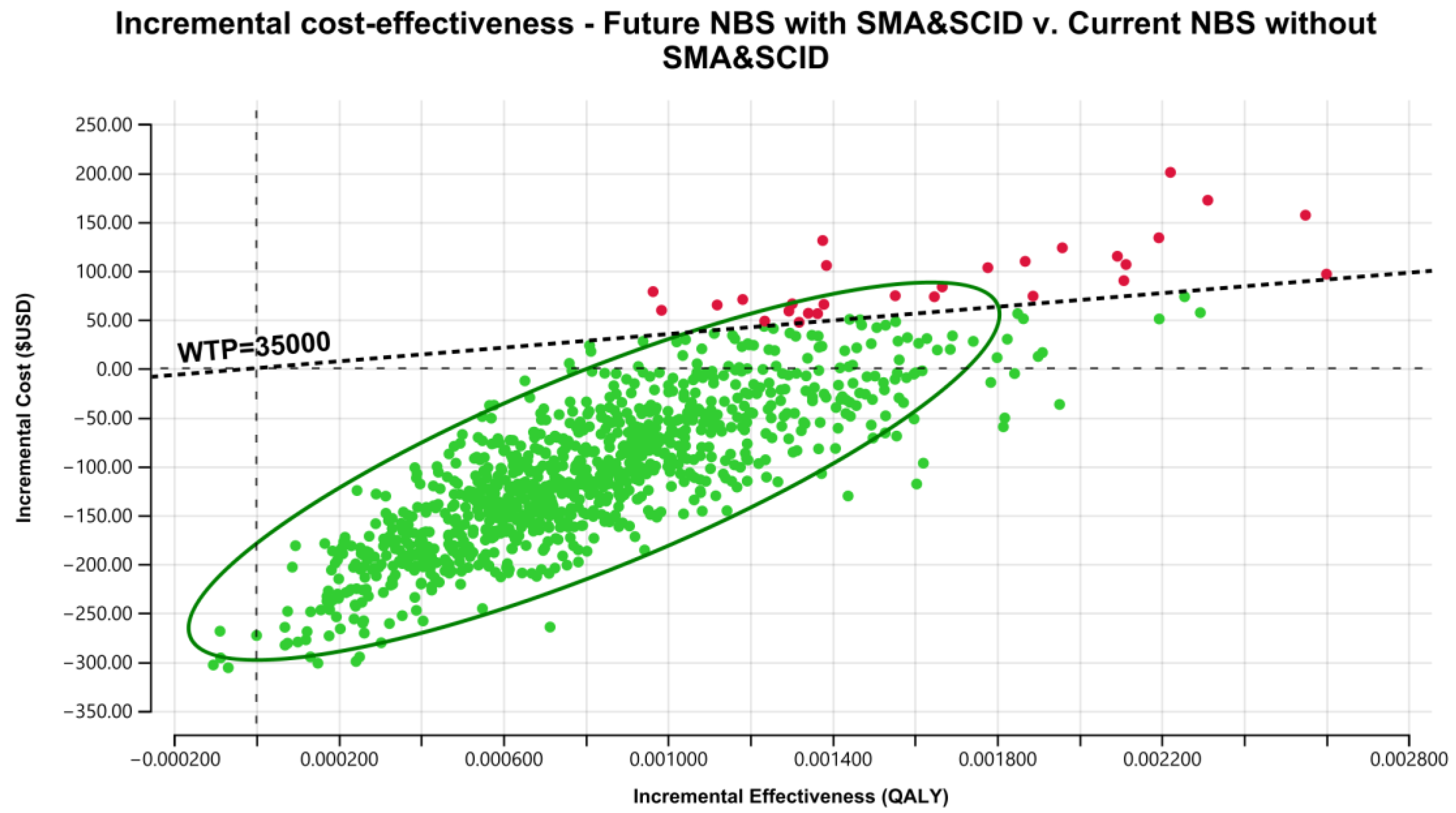
3.3. Cost-Effectiveness of the Addition of SCID and SMA Together into NBS Programs
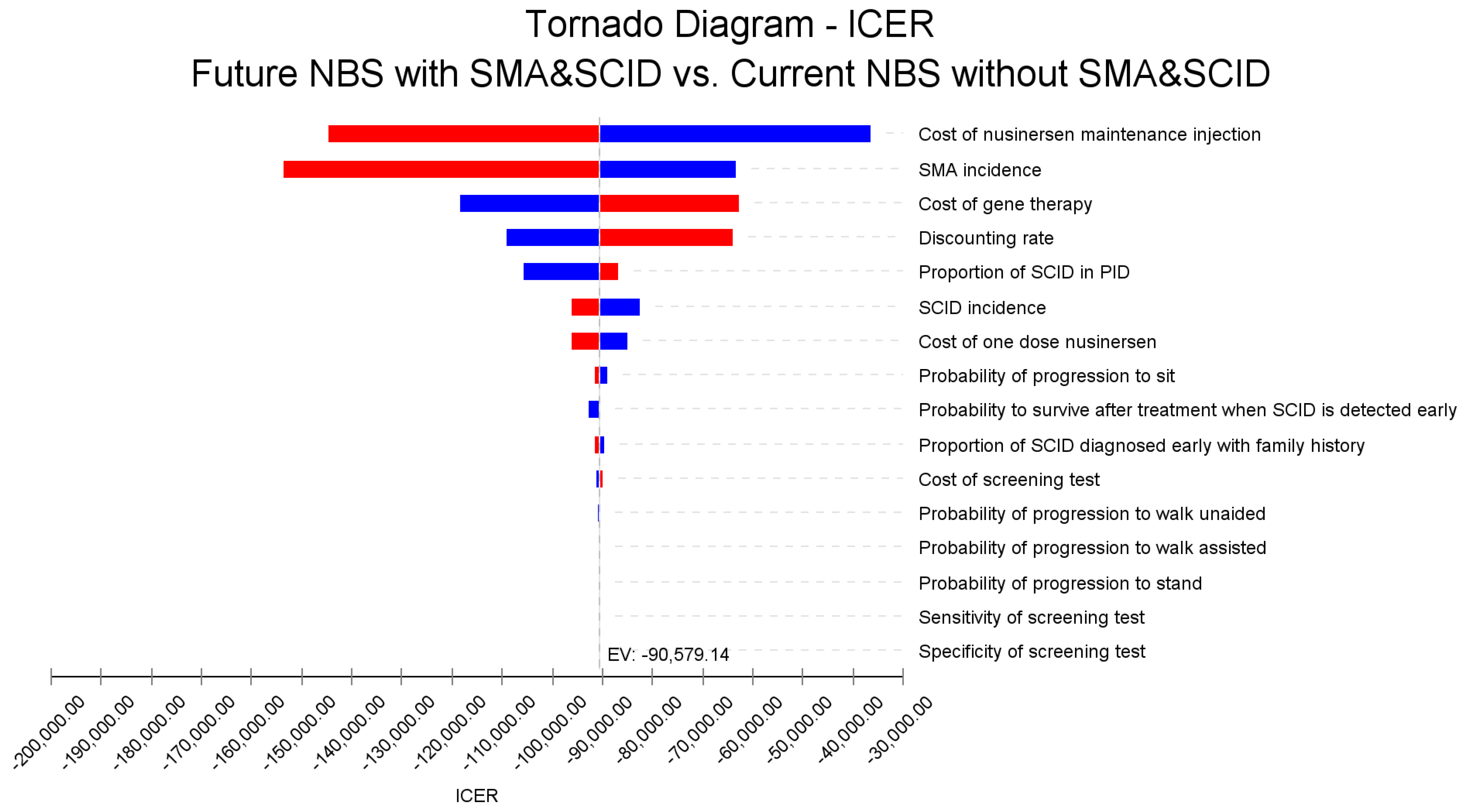
3.4. Sensitivity Analyses
3.5. Budget Impact on Government Accounts of a Combined Addition of SCID + SMA into a NBS Program
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrar, M.A.; Park, S.B.; Vucic, S.; Carey, K.; Turner, B.; Gillingwater, T.; Swoboda, K.; Kiernan, M.C. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 2017, 81, 355–368. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonilla, F.A.; et al. Newborn Screening for Severe Combined Immunodeficiency in 11 Screening Programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A. Severe combined immunodeficiencies (SCID). Clin. Exp. Immunol. 2000, 122, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, I.S.; Englund, J.A.; Burroughs, L.M.; Torgerson, T.R.; Skoda-Smith, S. Outcomes and Duration of Pneumocystis jiroveci Pneumonia Therapy in Infants with Severe Combined Immunodeficiency. Pediatr. Infect. Dis. J. 2012, 31, 95–97. [Google Scholar] [CrossRef]
- Kim, K.R.; Kim, J.M.; Kang, J.-M.; Kim, Y.-J. Pneumocystis jirovecii pneumonia in pediatric patients: An analysis of 15 confirmed consecutive cases during 14 years. Korean J. Pediatr. 2016, 59, 252–255. [Google Scholar] [CrossRef] [Green Version]
- Heimall, J.; Logan, B.R.; Cowan, M.J.; Notarangelo, L.D.; Griffith, L.M.; Puck, J.M.; Kohn, D.B.; Pulsipher, M.A.; Parikh, S.; Martinez, C.; et al. Immune reconstitution and survival of 100 SCID patients post–hematopoietic cell transplant: A PIDTC natural history study. Blood 2017, 130, 2718–2727. [Google Scholar] [CrossRef] [Green Version]
- Bessey, A.; Chilcott, J.; Leaviss, J.; de la Cruz, C.; Wong, R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. Int. J. Neonatal Screen. 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Davis, J.; Pai, S.-Y.; Bonilla, F.A.; Puck, J.M.; Apkon, M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol. Genet. Metab. 2011, 104, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Thompson, J.D.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S.D. Cost-Effectiveness/Cost-Benefit Analysis of Newborn Screening for Severe Combined Immune Deficiency in Washington State. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Grosse, S.D.; Thompson, J.D.; Ding, Y.; Glass, M. The Use of Economic Evaluation to Inform Newborn Screening Policy Decisions: The Washington State Experience. Milbank Q. 2016, 94, 366–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marle, M.E.V.D.A.-V.; Blom, M.; van der Burg, M.; Bredius, R.G.M.; Van der Ploeg, C.P.B. Economic Evaluation of Different Screening Strategies for Severe Combined Immunodeficiency Based on Real-Life Data. Int. J. Neonatal Screen. 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, C.P.B.; Blom, M.; Bredius, R.G.B.; van der Burg, M.; Schielen, P.J.I.; Verkerk, P.H.; Akker-van, M.E. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur. J. Pediatr. 2019, 178, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH Common Drug Review Pharmacoeconomic Review Report for Nusinersen; Canadian Agency for Drugs and Technologies in Health (CADTH): Ottawa, ON, Canada, 2018. [Google Scholar]
- Ellis, A.; Mickle, K.; Herron-Smith, S.; Kumar, V.M.; Cianciolo, L.; Seidner, M.; Rind, D.; Pearson, S.D.; Thokala, P.; Stevenson, M. Spinraza® and Zolgensma® for Spinal Muscular Atrophy: Effectiveness and Value—Final Evidence Report; Institute for Clinical and Economic Review: Boston, MA, USA, 2019. [Google Scholar]
- Malone, D.C.; Dean, R.; Arjunji, R.; Jensen, I.; Cyr, P.; Miller, B.; Maru, B.; Sproule, D.M.; Feltner, D.E.; Dabbous, O. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J. Mark. Access Health Policy 2019, 7, 1601484. [Google Scholar] [CrossRef] [PubMed]
- National Centre for Pharmacoeconomics. Cost-Effectiveness of Nusinersen (Spinraza) for the Treatment of 5q Spinal Muscular Atrophy (SMA); National Centre for Pharmacoeconomics: Dublin, Ireland, 2017. [Google Scholar]
- Pharmaceutical Benefits Scheme (PBS). Public Summary Document—Nusinersen; Pharmaceutical Benefits Advisory Committee (PBAC): Canberra, Australia, 2018.
- Thokala, P.; Stevenson, M.; Kumar, V.M.; Ren, S.; Ellis, A.G.; Chapman, R.H. Cost effectiveness of nusinersen for patients with infantile-onset spinal muscular atrophy in US. Cost Eff. Resour. Alloc. 2020, 18, 41. [Google Scholar] [CrossRef]
- Zuluaga-Sanchez, S.; Teynor, M.; Knight, C.; Thompson, R.; Lundqvist, T.; Ekelund, M.; Forsmark, A.; Vickers, A.D.; Lloyd, A. Cost Effectiveness of Nusinersen in the Treatment of Patients with Infantile-Onset and Later-Onset Spinal Muscular Atrophy in Sweden. PharmacoEconomics 2019, 37, 845–865. [Google Scholar] [CrossRef] [Green Version]
- Jalali, A.; Rothwell, E.; Botkin, J.R.; Anderson, R.A.; Butterfield, R.J.; Nelson, R.E. Cost-Effectiveness of Nusinersen and Universal Newborn Screening for Spinal Muscular Atrophy. J. Pediatr. 2020, 227, 274–280.e2. [Google Scholar] [CrossRef]
- Shih, S.T.; Farrar, M.A.; Wiley, V.; Chambers, G. Newborn screening for spinal muscular atrophy with disease-modifying therapies: A cost-effectiveness analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1296–1304. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Lehman, K.J.; McColly, M.; Lowes, L.P.; Alfano, L.N.; Reash, N.F.; Iammarino, M.A.; Church, K.R.; Kleyn, A.; et al. Five-Year Extension Results of the Phase 1 START Trial of Onasemnogene Abeparvovec in Spinal Muscular Atrophy. JAMA Neurol. 2021, 78, 831–834. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Purchasing Power Parities (PPP). Available online: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm (accessed on 3 July 2020).
- Kariyawasam, D.S.T.; Russell, J.S.; Wiley, V.; Alexander, I.E.; Farrar, M.A. The implementation of newborn screening for spinal muscular atrophy: The Australian experience. Genet. Med. 2020, 22, 557–565. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.; D’Silva, A.M.; Vetsch, J.; Wakefield, C.E.; Wiley, V.; Farrar, M.A. “We needed this”: Perspectives of parents and healthcare professionals involved in a pilot newborn screening program for spinal muscular atrophy. eClinicalMedicine 2021, 33, 100742. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.T.F.; Keller, E.; Wiley, V.; Wong, M.; Farrar, M.A.; Chambers, G.M. Economic Evaluation of Newborn Screening for Severe Combined Immunodeficiency. Int. J. Neonatal Screen. 2022, 8, 44. [Google Scholar] [CrossRef]
- Gutierrez-Mateo, C.; Timonen, A.; Vaahtera, K.; Jaakkola, M.; Hougaard, D.M.; Bybjerg-Grauholm, J.; Baekvad-Hansen, M.; Adamsen, D.; Filippov, G.; Dallaire, S.; et al. Development of a Multiplex Real-Time PCR Assay for the Newborn Screening of SCID, SMA, and XLA. Int. J. Neonatal Screen. 2019, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.L.; Lee, F.K.; Yazdanpanah, G.K.; Staropoli, J.F.; Liu, M.; Carulli, J.P.; Sun, C.; Dobrowolski, S.F.; Hannon, W.H.; Vogt, R.F. Newborn Blood Spot Screening Test Using Multiplexed Real-Time PCR to Simultaneously Screen for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Clin. Chem. 2015, 61, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute for Clinical and Economic Review (ICER). Modifications to the ICER Value Assessment Framework for Treatments for Ultra-Rare Diseases. Available online: https://icer.org/wp-content/uploads/2020/10/ICER-Adaptations-of-Value-Framework-for-Rare-Diseases.pdf (accessed on 16 December 2019).
- Arjunji, R.; Zhou, J.; Patel, A.; Edwards, M.; Harvey, M.; Soverino, M.; Dabbous, O. PMU30 cost-effectiveness analysis of newborn screening for spinal muscular atrophy (SMA) in the United States. Value Health 2020, 23, S238. [Google Scholar] [CrossRef]
- Chen, H.; Hutton, D.; Lavieri, M.; Prosser, L. Cc2 cost-effectiveness analysis of newborn screening and treatment for spinal muscular atrophy. Value Health 2020, 23, S2. [Google Scholar] [CrossRef]
- D’Silva, A.M.; Kariyawasam, D.S.T.; Best, S.; Wiley, V.; Farrar, M.A.; Ravine, A.; Mowat, D.; Sampaio, H.; Alexander, I.E.; Russell, J.; et al. Integrating newborn screening for spinal muscular atrophy into health care systems: An Australian pilot programme. Dev. Med. Child Neurol. 2021, 64, 625–632. [Google Scholar] [CrossRef]
- Chambers, G.M.; Settumba, S.N.; Carey, K.A.; Cairns, A.; Menezes, M.P.; Ryan, M.; Farrar, M.A. Prenusinersen economic and health-related quality of life burden of spinal muscular atrophy. Neurology 2020, 95, e1–e10. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Mauskopf, J.A.; Augustovski, F.; Caro, J.J.; Lee, K.M.; Minchin, M.; Orlewska, E.; Penna, P.; Barrios, J.-M.R.; Shau, W.-Y. Budget Impact Analysis—Principles of Good Practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014, 17, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Zolgensma—One-time gene therapy for spinal muscular atrophy. Med. Lett. Drugs Ther. 2019, 61, 113–114.
- Commonwealth of Australian Department of Health. Medicare Benefit Schedule Book; Department of Health: Canberra, Australia, 2018.
- Independent Hospital Pricing Authority. National Efficient Price Determination 2019–2020; Independent Hospital Pricing Authority: Sydney, Australia, 2019.
- Commonwealth of Australian Department of Health. Schedule of Pharmaceutical Benefits; Department of Health: Canberra, Australia, 2018.
- Reserve Bank of Australia. Measures of Consumer Price Inflation. Available online: https://www.rba.gov.au/inflation/measures-cpi.html#year_ended (accessed on 20 October 2020).
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2011, 20, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; McDermott, M.P.; Kaufmann, P.; Darras, B.; Chung, W.K.; Sproule, D.M.; Kang, P.; Foley, A.R.; Yang, M.L.; Martens, W.B.; et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 2014, 83, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Vucic, S.; Johnston, H.M.; du Sart, D.; Kiernan, M.C. Pathophysiological Insights Derived by Natural History and Motor Function of Spinal Muscular Atrophy. J. Pediatr. 2013, 162, 155–159. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 1–8. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Yee, A.; De Ravin, S.S.; Elliott, E.; Ziegler, J.B.; Unit, C.T.T.A.P.S. Severe combined immunodeficiency: A national surveillance study. Pediatr. Allergy Immunol. 2008, 19, 298–302. [Google Scholar] [CrossRef]
- Pai, S.-Y.; Logan, B.R.; Griffith, L.M.; Buckley, R.H.; Parrott, R.E.; Dvorak, C.C.; Kapoor, N.; Hanson, I.C.; Filipovich, A.H.; Jyonouchi, S.; et al. Transplantation Outcomes for Severe Combined Immunodeficiency, 2000–2009. N. Engl. J. Med. 2014, 371, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Belter, L.; Cruz, R.; Jarecki, J. Quality of life data for individuals affected by spinal muscular atrophy: A baseline dataset from the Cure SMA Community Update Survey. Orphanet J. Rare Dis. 2020, 15, 217. [Google Scholar] [CrossRef]


| Strategy | Cost (US$) | Incremental Cost (US$) | QALY | Incremental QALY | ICER (US$/QALY) |
|---|---|---|---|---|---|
| 5 Years | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Current NBS without SMA and SCID | $124.72 ($97.74, $156.36) | 0.00013 (0.00010, 0.00018) | |||
| Future NBS Add SMA and SCID | $170.33 ($104.90, $254.52) | $45.61 (−$25.96, $136.88) | 0.00022 (0.00015, 0.00032) | 0.00009 (0.00002, 0.00018) | $495,506 (dominant, $952,608) |
| 60 Years | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Current NBS Without SMA and SCID | $362.58 ($285.53, $459.35) | 0.00077 (0.00056, 0.00099) | |||
| Future NBS Add SMA and SCID | $276.59 ($160.64, $441.65) | −$86.00 (−$230.88, $82.59) | 0.00172 (0.00103, 0.00263) | 0.00095 (0.00029, 0.00182) | Dominant (dominant, $46,753) |
| Strategy | Cost (US$) | Incremental Cost (US$) | LY | Incremental LY | ICER (US$/LY) |
| 5 Years | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Current NBS without SMA and SCID | $124.72 ($97.74, $156.36) | 0.00033 (0.00027, 0.00040) | |||
| Future NBS Add SMA and SCID | $170.33 ($104.90, $ 254.52) | $45.61 (−$25.96, $136.88) | 0.00042 (0.00029, 0.00061) | 0.00009 (−0.00006, 0.00028) | $514,844 (dominant, $1,145,110) |
| 60 Years | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Current NBS without SMA and SCID | $362.58 ($285.53, $459.35) | 0.00130 (0.00108, 0.00155) | |||
| Future NBS Add SMA and SCID | $276.59 ($160.64, $441.65) | −$86.00 (−$230.88, $82.59) | 0.00267 (0.00164, 0.00397) | 0.00137 (0.00035, 0.00268) | Dominant (dominant, $37,280) |
| Future NBS | No. of Newborns (N) | Year 1 (US$) | Year 2 (US$) | Year 3 (US$) | Year 4 (US$) | Year 5 (US$) | Total (US$) |
|---|---|---|---|---|---|---|---|
| Screening * | 99,989 | $558,371 | $558,371 | ||||
| Screened SMA | 8.5 | $13,759,906 | $518,371 | $390,943 | $357,220 | $341,565 | $15,368,005 |
| Clinical SMA | 0.6 | $318,122 | $140,049 | $125,867 | $110,384 | $97,913 | $792,335 |
| Screened SCID | 2 | $245,120 | $7750 | $6870 | $6097 | $5413 | $271,250 |
| late SCID | 0.01 | $2388 | $58 | $51 | $45 | $40 | $2581 |
| Total budget | 100,000 | $14 | $666,227 | $523,731 | $473,746 | $444,931 | $16,992,542 |
| Current NBS | No. of Newborns (N) | Year 1 (US$) | Year 2 (US$) | Year 3 (US$) | Year 4 (US$) | Year 5 (US$) | Total (US$) |
| No Screening * | 99,989 | $0 | |||||
| Clinical SMA | 9.1 | $4,824,852 | $2,124,069 | $1,908,986 | $1,674,157 | $1,485,020 | $12,017,084 |
| Clinical SCID | 1.6 | $382,024 | $9264 | $8167 | $7203 | $6357 | $413,014 |
| Early SCID by family history | 0.4 | $49,024 | $1550 | $1374 | $1219 | $1083 | $54,250 |
| Total budget | 100,000 | $5,255,900 | $2,134,883 | $1,918,527 | $1,682,579 | $1,492,460 | $12,484,349 |
| Future NBS | Year 1 (US$) | Year 2 (US$) | Year 3 (US$) | Year 4 (US$) | Year 5 (US$) | Total (US$) |
|---|---|---|---|---|---|---|
| Cohort 1 | $14,883,907 | $666,227 | $523,731 | $473,746 | $444,931 | $16,992,542 |
| Cohort 2 | $14,883,907 | $666,227 | $523,731 | $473,746 | $16,547,612 | |
| Cohort 3 | $14,883,907 | $666,227 | $523,731 | $16,073,865 | ||
| Cohort 4 | $14,883,907 | $666,227 | $15,550,134 | |||
| Cohort 5 | $14,883,907 | $14,883,907 | ||||
| Total budget | $14,883,907 | $15,550,134 | $16,073,865 | $16,547,612 | $16,992,542 | $80,048,061 |
| Current NBS | Year 1 (US$) | Year 2 (US$) | Year 3 (US$) | Year 4 (US$) | Year 5 (US$) | Total (US$) |
| Cohort 1 | $5,255,900 | $2,134,883 | $1,918,527 | $1,682,579 | $1,492,460 | $12,484,349 |
| Cohort 2 | $5,255,900 | $2,134,883 | $1,918,527 | $1,682,579 | $10,991,889 | |
| Cohort 3 | $5,255,900 | $2,134,883 | $1,918,527 | $9,309,310 | ||
| Cohort 4 | $5,255,900 | $2,134,883 | $7,390,783 | |||
| Cohort 5 | $5,255,900 | $5,255,900 | ||||
| Total budget | $5,255,900 | $7,390,783 | $9,309,310 | $10,991,889 | $12,484,349 | $45,432,231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, S.T.F.; Keller, E.; Wiley, V.; Farrar, M.A.; Wong, M.; Chambers, G.M. Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Int. J. Neonatal Screen. 2022, 8, 45. https://doi.org/10.3390/ijns8030045
Shih STF, Keller E, Wiley V, Farrar MA, Wong M, Chambers GM. Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. International Journal of Neonatal Screening. 2022; 8(3):45. https://doi.org/10.3390/ijns8030045
Chicago/Turabian StyleShih, Sophy T. F., Elena Keller, Veronica Wiley, Michelle A. Farrar, Melanie Wong, and Georgina M. Chambers. 2022. "Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency" International Journal of Neonatal Screening 8, no. 3: 45. https://doi.org/10.3390/ijns8030045
APA StyleShih, S. T. F., Keller, E., Wiley, V., Farrar, M. A., Wong, M., & Chambers, G. M. (2022). Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. International Journal of Neonatal Screening, 8(3), 45. https://doi.org/10.3390/ijns8030045






