Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic
Abstract
:1. Introduction
2. Methods
2.1. Pre-Pandemic
NYSDOH NBS Program Protocol
2.2. Remote Recruitment
2.2.1. Participant Facing Protocol
2.2.2. NYSDOH NBS Program Protocol
2.3. Hybrid In-Person and Remote Recruitment
2.3.1. Participant Facing Protocol
2.3.2. NYSDOH NBS Program Protocol
2.4. Calculation of Saturation, Enrollment and Decline Rates
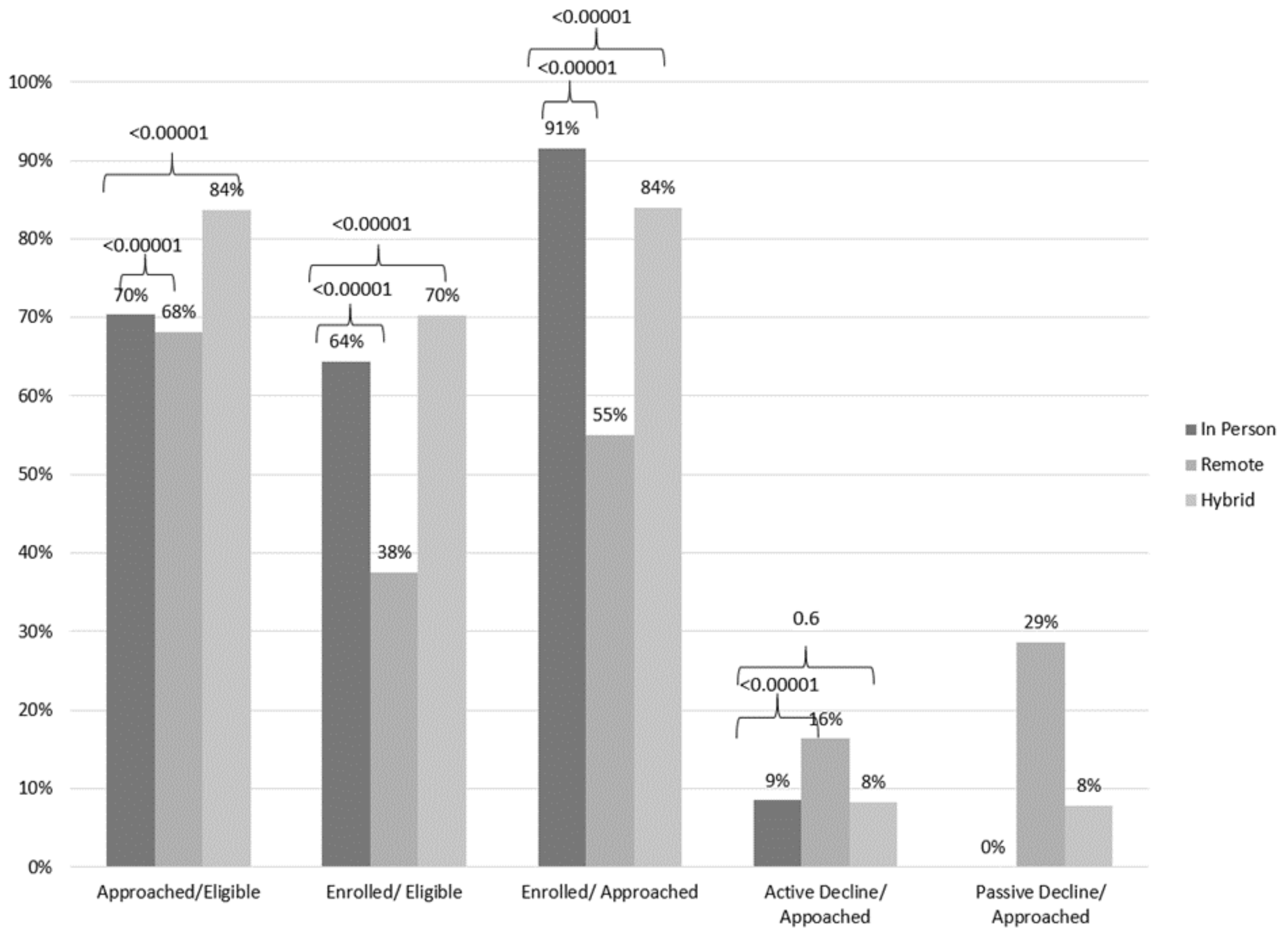
3. Results
Language of Consent
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahimaz, P.; Giordano, J.; Disco, M.; Harrington, E.; Levinson, E.; Spiegel, E.; Andrews, C.; Griffin, E.; Hernan, R.; Wynn, J. COVID contingencies: Early epicenter experiences of different genetics clinics at a New York City institution inform emergency adaptation strategies. J. Genet. Couns. 2021, 30, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.; Fuchs, K.; D’Alton, M.; Goffman, D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. NEJM 2020, 382, 2163–2164. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.; Korpimäki, T.; Furuk, P.; Hakala, H.; Polari, H.; Meriö, L.; Mäkinen, P.; Weeks, I. Characterization of a Blood Spot Creatine Kinase Skeletal Muscle Isoform Immunoassay for High-Throughput Newborn Screening of Duchenne Muscular Dystrophy. Clin. Chem. 2017, 63, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Kraszewski, J.N.; Kay, D.M.; Stevens, C.F.; Koval, C.; Haser, B.; Ortiz, V.; Albertorio, A.; Cohen, L.L.; Jain, R.; Andrew, S.P.; et al. Pilot study of population-based newborn screening for spinal muscular atrophy in New York state. Genet. Med. 2017, 20, 608–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nominate a Condition. Official Web Site of the U.S. Health Resources & Services Administration. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/nominate.html (accessed on 6 December 2021).
- Van Teijlingen, E.; Hundley, V. The importance of pilot studies. Nurs. Stand. 2002, 16, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Institute Inc. SAS 9.4 [Computer Program]. 2014. [Google Scholar]
- Amin, M.D.; Bundogji, N.K.; Zamora, S.M.; Magit, A.E. A survey of adult preferences regarding recruitment for pediatric research. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110108. [Google Scholar] [CrossRef]
- Vaughan, S.E.; Misra, D.P.; Wong, A.C.; Rengers, B.; Jablonski, S.; Price, M.; Giurgescu, C. Successful Recruitment Strategies for Engaging Pregnant African American Women in Research. West. J. Nurs. Res. 2022, 44, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Skelton, E.; Drey, N.; Rutherford, M.; Ayers, S.; Malamateniou, C. Electronic consenting for conducting research remotely: A review of current practice and key recommendations for using e-consenting. Int. J. Med. Inform. 2020, 143, 104271. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wynn, J.; Tavakoli, N.P.; Armstrong, N.; Gomez, J.; Koval, C.; Lai, C.; Tang, S.; Quevedo Prince, A.; Quevedo, Y.; Rufino, K.; et al. Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic. Int. J. Neonatal Screen. 2022, 8, 23. https://doi.org/10.3390/ijns8020023
Wynn J, Tavakoli NP, Armstrong N, Gomez J, Koval C, Lai C, Tang S, Quevedo Prince A, Quevedo Y, Rufino K, et al. Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic. International Journal of Neonatal Screening. 2022; 8(2):23. https://doi.org/10.3390/ijns8020023
Chicago/Turabian StyleWynn, Julia, Norma P. Tavakoli, Niki Armstrong, Jacqueline Gomez, Carrie Koval, Christina Lai, Stephanie Tang, Andrea Quevedo Prince, Yeyson Quevedo, Katrina Rufino, and et al. 2022. "Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic" International Journal of Neonatal Screening 8, no. 2: 23. https://doi.org/10.3390/ijns8020023
APA StyleWynn, J., Tavakoli, N. P., Armstrong, N., Gomez, J., Koval, C., Lai, C., Tang, S., Quevedo Prince, A., Quevedo, Y., Rufino, K., Palacio Morales, L., Pena, A., Grossman, S., Monfiletto, M., Ruda, E., Jimenez, V., Verdade, L., Jones, A., Barriga, M. G., ... Gruber, D. (2022). Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic. International Journal of Neonatal Screening, 8(2), 23. https://doi.org/10.3390/ijns8020023






