Long-Term Course of Hypothyroidism Detected through Neonatal TSH Screening in a Population-Based Cohort of Very Preterm Infants Born at Less than 32 Weeks of Gestation
Abstract
1. Introduction
2. Materials and Methods
- Conclusive confirmation of thyroid dysgenesis, thyroid ectopy, or athyreosis, on imaging.
- Confirmation of severe dyshormonogenesis by molecular genetic testing.
- Positive result of off-treatment re-evaluation of the thyroid axis, or positive result of LT4 dose decreasing trial (= increase in TSH concentration to ≥10 mU/L after 4 weeks off-treatment or after 2 to 3 weeks of LT4 dose decreased by 30%).
- Rise of venous TSH concentration under ongoing LT4 treatment after the first year of life, in combination with repeated increases of LT4 dosage, age and weight appropriate, over years with regular controls of thyroid function.
- Severe permanent hypothyroidism when there was evidence of a significant morphologic abnormality of the thyroid gland or evidence of a decreased FT4 on re-evaluation of thyroid function.
- Mild permanent hypothyroidism when there was no evidence of a morphologic abnormality of the thyroid gland and a TSH increase to above 10 mU/L with FT4 in the normal range was detected on re-evaluation.
3. Results
3.1. Permanent Hypothyroidism
3.2. Transient Hypothyroidism
3.3. Comparison of Permanent and Transient Groups
3.4. Inconclusive Cases
3.5. Comparison of Prevalences between the Study Group and Infants Born ≥ 32 Weeks of Gestation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Trotsenburg, A.S.; Stoupa, A.; Léger, J.; Rohrer, T.R.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital hypothyroidism: A 2020 consensus guidelines update An ENDO-EUROPEAN REFERENCE NETWORK (ERN) initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef]
- Krude, H.; Kühnen, P.; Biebermann, H. Treatment of congenital thyroid dysfunction: Achievements and challenges. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 399–413. [Google Scholar] [CrossRef]
- Léger, J.; Olivieri, A.; Donaldson, M.; Torresani, T.; Krude, H.; van Vliet, G.; Polak, M.; Butler, G. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm. Res. Paediatr. 2014, 81, 80–103. [Google Scholar] [CrossRef]
- Chung, H.R. Screening and management of thyroid dysfunction in preterm infants. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A. Thyroid system immaturities in very low birth weight premature infants. Semin. Perinatol. 2008, 32, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.L.R.; Ogston, S.A.; van Toor, H.; Visser, T.J.; Hume, R. Serum thyroid hormones in preterm infants: Associations with postnatal illnesses and drug usage. J. Clin. Endocrinol. Metab. 2005, 90, 5954–5963. [Google Scholar] [CrossRef]
- Golombek, S.G. Nonthyroidal illness syndrome and euthyroid sick syndrome in intensive care patients. Semin. Perinatol. 2008, 32, 413–418. [Google Scholar] [CrossRef]
- Zung, A.; Bier Palmon, R.; Golan, A.; Troitzky, M.; Eventov-Friedman, S.; Marom, R.; Keidar, R.; Kats, N.; Almashanu, S.; Flidel-Rimon, O. Risk Factors for the Development of Delayed TSH Elevation in Neonatal Intensive Care Unit Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 3050–3055. [Google Scholar] [CrossRef]
- Chaudhari, M.; Slaughter, J.L. Thyroid Function in the Neonatal Intensive Care Unit. Clin. Perinatol. 2018, 45, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Grob, F.; van Vliet, G. Avoiding the Overdiagnosis of Congenital Hypothyroidism in Premature Newborns. Pediatrics 2019, 144, e20191706. [Google Scholar] [CrossRef] [PubMed]
- Zung, A.; Radi, A.; Almashanu, S. The natural history of congenital hypothyroidism with delayed TSH elevation in neonatal intensive care newborns. Clin. Endocrinol. 2020, 92, 443–449. [Google Scholar] [CrossRef]
- Hijman, A.-I.; Konrad, D.; Fingerhut, R. Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.L.R.; Lindgren, A.; Watson, J.; Boelen, A.; Cheetham, T. Thyroid function in preterm infants and neurodevelopment at 2 years. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Hunt, R.W. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2007, 1, CD005948. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Iglesias Fernández, C.; Rodríguez Sánchez, A.; Dulín Lñiguez, E.; Rodríguez Arnao, M.D. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm. Res. Paediatr. 2010, 74, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bongers-Schokking, J.J.; Resing, W.C.M.; de Rijke, Y.B.; de Ridder, M.A.J.; de Muinck Keizer-Schrama, S.M.P.F. Cognitive development in congenital hypothyroidism: Is overtreatment a greater threat than undertreatment? J. Clin. Endocrinol. Metab. 2013, 98, 4499–4506. [Google Scholar] [CrossRef]
- Eugster, E.A.; LeMay, D.; Zerin, J.M.; Pescovitz, O.H. Definitive diagnosis in children with congenital hypothyroidism. J. Pediatr. 2004, 144, 643–647. [Google Scholar] [CrossRef]
- LaFranchi, S.H. Screening preterm infants for congenital hypothyroidism: Better the second time around. J. Pediatr. 2014, 164, 1259–1261. [Google Scholar] [CrossRef]
- Hashemipour, M.; Hovsepian, S.; Ansari, A.; Keikha, M.; Khalighinejad, P.; Niknam, N. Screening of congenital hypothyroidism in preterm, low birth weight and very low birth weight neonates: A systematic review. Pediatr. Neonatol. 2018, 59, 3–14. [Google Scholar] [CrossRef]
- Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krankheiten bei Kindern (Kinder-Richtlinie): Kinder-Richtlinie. 2020. Available online: https://www.g-ba.de/downloads/62-492-2432/Kinder-RL_2020-12-17_iK-2021-04-01.pdf (accessed on 28 April 2021).
- Nennstiel-Ratzel, U.; Lüders, A.; Fusch, C.; Mohnike, K. Neugeborenen-Screening bei Frühgeborenen: Noch Raum für Verbesserungen. Z. Geburtshilfe Neonatol 2013, 217, V22_4. [Google Scholar] [CrossRef]
- DGNS e.V.—Deutsche Gesellschaft für Neugeborenenscreening. Nationaler Screeningreport. Available online: https://www.screening-dgns.de/reports.php (accessed on 25 February 2021).
- Bijarnia, S.; Wilcken, B.; Wiley, V.C. Newborn screening for congenital hypothyroidism in very-low-birth-weight babies: The need for a second test. J. Inherit. Metab. Dis. 2011, 34, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.C.; Lizarda, A.; Tucker, R.; Mitchell, M.L.; Vohr, B.; Oh, W.; Phornphutkul, C. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: Incidence and growth and developmental outcomes. J. Pediatr. 2011, 158, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lee, Y.K.; Han, H.-S. Early discontinuation of thyroxine therapy is possible in most very low-birthweight infants with hypothyroidism detected by screening. Acta Paediatr. 2014, 103, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vigone, M.C.; Caiulo, S.; Di Frenna, M.; Ghirardello, S.; Corbetta, C.; Mosca, F.; Weber, G. Evolution of thyroid function in preterm infants detected by screening for congenital hypothyroidism. J. Pediatr. 2014, 164, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Jin, H.Y.; Chung, M.L. Feasibility of an Early Discontinuation of Thyroid Hormone Treatment in Very-Low-Birth-Weight Infants at Risk for Transient or Permanent Congenital Hypothyroidism. Horm. Res. Paediatr. 2016, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Janjindamai, W.; Sriplung, H. Congenital hypothyroidism in preterm infants: A 3- to 8-year longitudinal study in southern Thailand. J. Pediatr. Endocrinol. Metab. 2019, 32, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Harigopal, S.; Turner, S.; Cheetham, T. Permanent and transient congenital hypothyroidism in preterm infants. Acta Paediatr. 2012, 101, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.; Trumpff, C.; Grosse, S.D.; Olivieri, A.; van Vliet, G. Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur. J. Endocrinol. 2017, 177, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A. Reevaluation des TSH-Neugeborenen-Screenings für Frühgeborene Kinder unter 32 Vollendeten Schwangerschaftswochen in Bayern im Zeitraum 1999 bis 2013. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2020. [Google Scholar]
- Tylek-Lemanska, D.; Kumorowicz-Kopiec, M.; Starzyk, J. Screening for congenital hypothyroidism: The value of retesting after four weeks in neonates with low and very low birth weight. J. Med. Screen. 2006, 12, 166–169. [Google Scholar] [CrossRef]
- McGrath, N.; Hawkes, C.P.; Mayne, P.; Murphy, N.P. Optimal Timing of Repeat Newborn Screening for Congenital Hypothyroidism in Preterm Infants to Detect Delayed Thyroid-Stimulating Hormone Elevation. J. Pediatr. 2019, 205, 77–82. [Google Scholar] [CrossRef]
- Scavone, M.; Giancotti, L.; Anastasio, E.; Pensabene, L.; Sestito, S.; Concolino, D. Evolution of congenital hypothyroidism in a cohort of preterm born children. Pediatr. Neonatol. 2020, 61, 629–636. [Google Scholar] [CrossRef]
- Olivieri, A.; Medda, E.; de Angelis, S.; Valensise, H.; de Felice, M.; Fazzini, C.; Cascino, I.; Cordeddu, V.; Sorcini, M.; Stazi, M.A. High risk of congenital hypothyroidism in multiple pregnancies. J. Clin. Endocrinol. Metab. 2007, 92, 3141–3147. [Google Scholar] [CrossRef][Green Version]
- De Zegher, F.; Vanderschueren-Lodeweyckx, M.; Heinrichs, C.; van Vliet, G.; Malvaux, P. Thyroid dyshormonogenesis: Severe hypothyroidism after normal neonatal thyroid stimulating hormone screening. Acta Paediatr. 1992, 81, 274–276. [Google Scholar] [CrossRef]
- Heather, N.L.; Hofman, P.L.; de Hora, M.; Carll, J.; Derraik, J.G.B.; Webster, D. Evaluation of the revised New Zealand national newborn screening protocol for congenital hypothyroidism. Clin. Endocrinol. 2017, 86, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Nicholas, A.K.; Schoenmakers, E.; Lyons, G.; Langham, S.; Serra, E.G.; Sebire, N.J.; Muzza, M.; Fugazzola, L.; Schoenmakers, N. DUOX2/DUOXA2 Mutations Frequently Cause Congenital Hypothyroidism that Evades Detection on Newborn Screening in the United Kingdom. Thyroid 2019, 29, 790–801. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung. Iodine Intake in Germany on the Decline Again: Tips for a Good Iodine Supply. Questions and Answers on Iodine Supply and the Prevention of Iodine Deficiency. Available online: https://www.bfr.bund.de/en/iodine_intake_in_germany_on_the_decline_again___tips_for_a_good_iodine_supply-128779.html (accessed on 5 January 2021).
- Yoon, S.A.; Chang, Y.S.; Ahn, S.Y.; Sung, S.I.; Park, W.S. Incidence and severity of transient hypothyroxinaemia of prematurity associated with survival without composite morbidities in extremely low birth weight infants. Sci. Rep. 2019, 9, 9628. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, D.N.; McGovern, M.; Greene, C.M.; Molloy, E.J. Gender disparities in preterm neonatal outcomes. Acta Paediatr. 2018, 107, 1494–1499. [Google Scholar] [CrossRef]
- Park, E.S.; Yoon, J.Y. Factors associated with permanent hypothyroidism in infants with congenital hypothyroidism. BMC Pediatr. 2019, 19, 453. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Yoon, J.S.; So, C.H.; Lee, H.S.; Hwang, J.S. Predictors of transient congenital hypothyroidism in children with eutopic thyroid gland. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 115–118. [Google Scholar] [CrossRef][Green Version]
- Scavone, M.; Carboni, E.; Stefanelli, E.; Romano, G.; Vero, A.; Giancotti, L.; Miniero, R.; Talarico, V. Prediction of Transient or Permanent Congenital Hypothyroidism from Initial Thyroid Stimulating Hormone Levels. Indian Pediatr. 2018, 55, 1059–1061. [Google Scholar] [CrossRef]
- Zdraveska, N.; Zdravkovska, M.; Anastasovska, V.; Sukarova-Angelovska, E.; Kocova, M. Diagnostic re-evaluation of congenital hypothyroidism in Macedonia: Predictors for transient or permanent hypothyroidism. Endocr. Connect. 2018, 7, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.A.; Denniston, S.; Sesser, D.; Skeels, M.R.; LaFranchi, S.H. Transient versus Permanent Congenital Hypothyroidism after the Age of 3 Years in Infants Detected on the First versus Second Newborn Screening Test in Oregon, USA. Horm. Res. Paediatr. 2016, 86, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.L.; Hsu, H.-W. Unresolved Issues in the Wake of Newborn Screening for Congenital Hypothyroidism. J. Pediatr. 2016, 173, 228–231.e1. [Google Scholar] [CrossRef][Green Version]
- Kwak, M.J. Clinical genetics of defects in thyroid hormone synthesis. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Vigone, M.C.; Di Frenna, M.; Guizzardi, F.; Gelmini, G.; de Filippis, T.; Mora, S.; Caiulo, S.; Sonnino, M.; Bonomi, M.; Persani, L.; et al. Mild TSH resistance: Clinical and hormonal features in childhood and adulthood. Clin. Endocrinol. 2017, 87, 587–596. [Google Scholar] [CrossRef]
- Korada, M.; Pearce, M.S.; Ward Platt, M.P.; Avis, E.; Turner, S.; Wastell, H.; Cheetham, T. Repeat testing for congenital hypothyroidism in preterm infants is unnecessary with an appropriate thyroid stimulating hormone threshold. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, 286–288. [Google Scholar] [CrossRef]
- van Vliet, G.; Diaz Escagedo, P. Redefining Congenital Hypothyroidism? J. Clin. Endocrinol. Metab. 2021, 106, 1463–1465. [Google Scholar] [CrossRef]
- Wassner, A.J. Pediatric Hypothyroidism: Diagnosis and Treatment. Paediatr. Drugs 2017, 19, 291–301. [Google Scholar] [CrossRef]
- Kaluarachchi, D.C.; Allen, D.B.; Eickhoff, J.C.; Dawe, S.J.; Baker, M.W. Thyroid-Stimulating Hormone Reference Ranges for Preterm Infants. Pediatrics 2019, 144, e20190290. [Google Scholar] [CrossRef]
- Pollitt, R.J. Evidence or enthusiasm? Why yields from UK newborn screening programmes for congenital hypothyroidism are increasing. Arch. Dis. Child. 2016, 101, 120–123. [Google Scholar] [CrossRef]
- Krude, H.; Blankenstein, O. Treating patients not numbers: The benefit and burden of lowering TSH newborn screening cut-offs. Arch. Dis. Child. 2011, 96, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.S. Current status of newborn screening: Decision-making about the conditions to include in screening programs. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef] [PubMed]



| Category | n | % | |
|---|---|---|---|
| Gestational age (weeks) | 23–25 | 16 | 31.4 |
| 26–28 | 12 | 23.5 | |
| 29–31 | 23 | 45.1 | |
| Birth weight (BW) category | LBW (<2500 g) | 6 | 11.8 |
| VLBW (<1500 g) | 15 | 29.4 | |
| ELBW (<1000 g) | 30 | 58.8 | |
| SGA | 6 | 11.8 | |
| Sex | Male | 30 | 58.8 |
| Female | 21 | 41.2 | |
| Age at initial screening | <36 h | 5 | 9.8 |
| 36–72 h | 41 | 80.4 | |
| >72 h | 5 | 9.8 | |
| Serum thyroid-stimulation hormone (TSH) category before start of therapy | >100 mU/L | 20 | 39.2 |
| >50–100 mU/L | 12 | 23.5 | |
| >20–50 mU/L | 16 | 31.4 | |
| <20 mU/L | 1 | 2.0 | |
| N/A | 2 | 3.9 | |
| Serum free thyroxine (FT4) category before start of therapy | <5 pmol/L | 9 | 17.6 |
| 5–<10 pmol/L | 19 | 37.3 | |
| 10–<15 pmol/L | 14 | 27.5 | |
| ≥15 pmol/L | 2 | 3.9 | |
| N/A | 7 | 13.7 | |
| Follow-up thyroid care | Paediatric endocrinology | 15 | 29.4 |
| General paediatrician | 36 | 70.6 | |
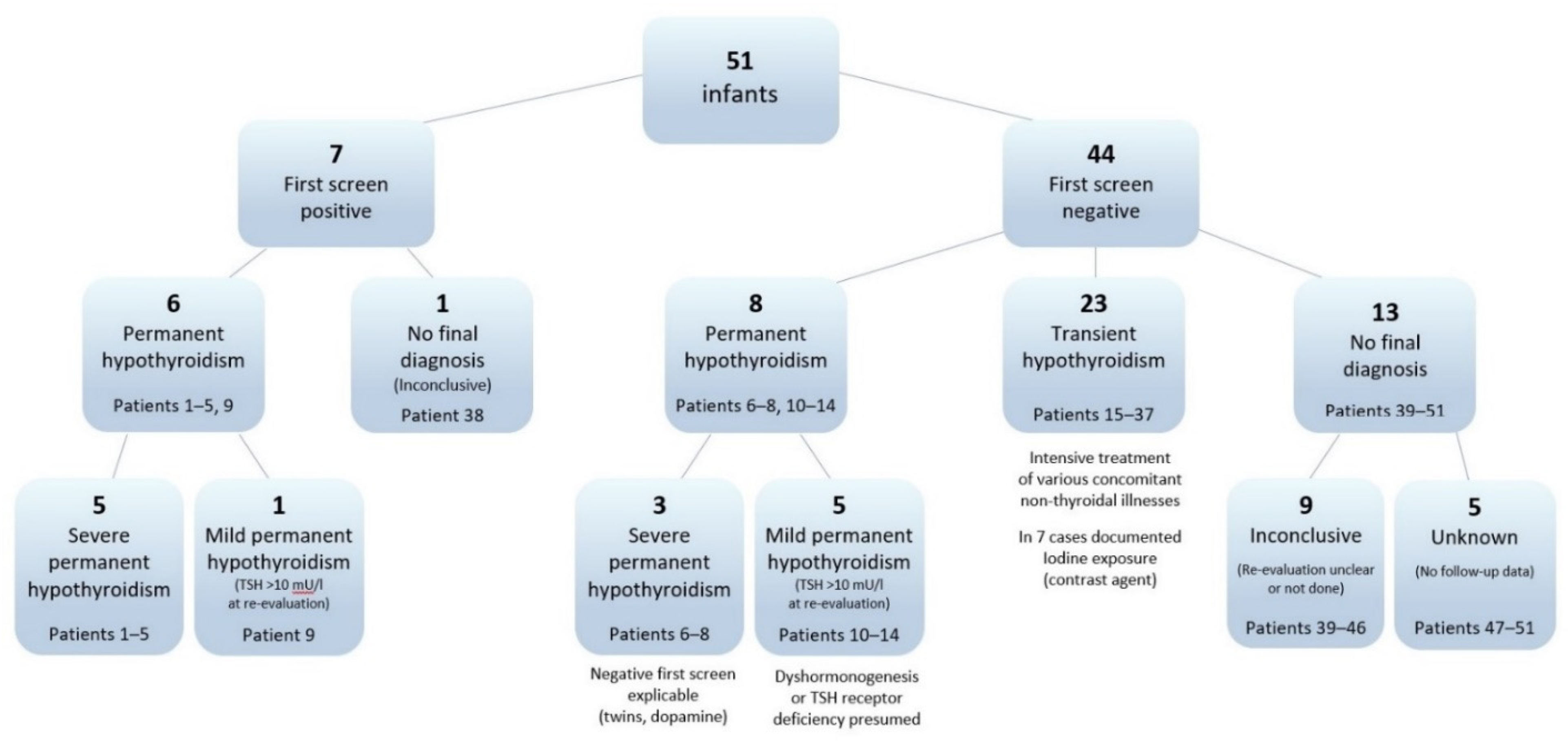
| Final diagnostic category | Permanent hypothyroidism | 14 | 27.5 |
| severe | 8 | 15.7 | |
| mild | 6 | 11.8 | |
| Transient hypothyroidism | 23 | 45.1 | |
| Inconclusive | 9 | 17.6 | |
| Unknown | 5 | 9.8 | |
| Final Diagnosis After Long-Term Follow-Up | Patient Number | Sex | Birth Weight (g) | Gestational Age/Postmenstrual Age (Weeks) | Newborn Screening Results | Confirmatory Testing Results (Serum) | L-T4 Dosage at Age 2 Years (µg/kg) | Annotations | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| At Birth | At Diagnosis | First Screen | Max. TSH (mU/L) | TSH (mU/L) | FT4 (pmol/L) | ||||||
| Severe permanent hypothyroidism | 1 | f | 1600 | 29 | 30 | pos. | 323 | >100 | 1.4 | 3.8 | Thyroid dysplasia. Dizygotic twin of euthyroid co-twin. |
| 2 | m | 1550 | 30 | 32 | pos. | 532 | 1365 | 1.7 | 5.8 | Athyreosis. | |
| 3 | m | 960 | 27 | 28 | pos. | 222 | 101 | N/A | 4.6 | Thyroid dysplasia. | |
| 4 | f | 1450 | 30 | 32 | pos. | 218 | 459 | N/A | 3.1 | Thyroid dysplasia. Identical twin of euthyroid co-twin. | |
| 5 | m | 1410 | 29 | 29 | pos. | 341 | >100 | 3.4 | 3.8 | ||
| 6 | f | 1200 | 28 | 34 | neg. | 731 | >100 | <0.3 | 4.4 | Athyreosis. Identical twin of euthyroid co-twin. Delayed re-screening in spite of tracking. | |
| 7 | f | 1115 | 29 | 39 | neg. | 875 | >100 | 1.5 | 5 | Thyroid dysplasia. Identical twin of euthyroid co-twin. Born before implementation of re-screening at 32 weeks. | |
| 8 | m | 460 | 25 | 28 | neg. | 149 | 280 | TT4 low | 3 | First screen under the influence of high-dose dopamine. | |
| Mild permanent hypothyroidism | 9 | f | 1300 | 29 | 29 | pos. | 122 | 92 | 12.9 | 4 | Family history of hypothyroidism. |
| 10 | m | 620 | 25 | 33 | neg. | 306 | 629 | 1.3 | 4.8 | Dyshormonogenesis assumed. | |
| 11 | m | 1730 | 30 | 40 | neg. | 275 | >100 | 6.7 | 2.8 | Negative control at age 2 weeks. | |
| 12 | f | 1200 | 29 | 33 | neg. | 34 | 62 | 7.0 | 4.3 | Identical twin of patient 13. Family history of hypothyroidism. | |
| 13 | f | 1420 | 29 | 33 | neg. | 11 | 30 | 11.1 | 3.7 | Identical twin of patient 12. Family history of hypothyroidism. | |
| 14 | m | 1495 | 30 | 34 | neg. | 23 | 27 | 12.9 | 3.3 | Family history of thyroid disorders | |
| Transient hypothyroidism | 15 | m | 1640 | 31 | 33 | neg. | 30 | 210 | 2.8 | 0 | |
| 16 | f | 1730 | 31 | 34 | neg. | 87 | 64 | 3.3 | 0 | ||
| 17 | m | 870 | 28 | 32 | neg. | 19 | 19 | 5.1 | 1.7 | Identical twin of euthyroid co-twin. End of therapy at 44 months. | |
| 18 | f | 1060 | 30 | 32 | neg. | 26 | 77 | 5.1 | 1.2 | Dizygotic twin of euthyroid co-twin. End of therapy at 27 months. | |
| 19 | m | 860 | 27 | 34 | neg. | 254 | 264 | 5.2 | 2.2 | End of therapy at 75 months | |
| 20 | m | 670 | 25 | 34 | neg. | 250 | 33 | 5.4 | 0 | Iodinated contrast agent. | |
| 21 | m | 655 | 24 | 27 | neg. | 44 | 77 | 6.2 | 1.3 | End of therapy at 30 months. | |
| 22 | f | 1170 | 28 | 32 | neg. | 165 | 233 | 6.4 | 0 | Iodinated contrast agent. | |
| 23 | m | 1420 | 30 | 32 | neg. | 19 | 43 | 7.7 | 1 | End of therapy at 28 months. | |
| 24 | m | 740 | 25 | 29 | neg. | 51 | 78 | 7.7 | 0 | Iodinated contrast agent. | |
| 25 | f | 890 | 27 | 37 | neg. | 23 | 76 | 9.0 | 0 | Neg. re-screening at 5 weeks. | |
| 26 | m | 760 | 24 | 28 | neg. | 394 | 1004 | TT4 low | 2.5 | End of therapy at 33 months. | |
| 27 | f | 1210 | 29 | 33 | neg. | 13 | 25 | 10.9 | 0 | Iodinated contrast agent. | |
| 28 | m | 935 | 29 | 33 | neg. | 13 | 47 | 10.9 | 2.5 | End of therapy at 43 months. | |
| Transient hypothyroidism | 29 | m | 700 | 24 | 28 | neg. | 13 | 35 | 11.7 | 0 | Iodinated contrast agent. |
| 30 | m | 580 | 24 | 28 | neg. | 11 | 27 | 12.0 | 0 | ||
| 31 | f | 480 | 27 | 32 | neg. | 24 | 65 | 13.1 | 2.1 | Iodinated contrast agent. End of therapy at 43 months. | |
| 32 | f | 1095 | 29 | 37 | neg. | 18 | 51 | 13.1 | 2.5 | Identical twin of euthyroid co-twin. No re-screening at 32 weeks. End of therapy at 27 months. | |
| 33 | m | 410 | 25 | 27 | neg. | 45 | 44 | 14.2 | 0 | ||
| 34 | m | 990 | 30 | 34 | neg. | 28 | 22 | 14.2 | 0 | Identical twin of euthyroid co-twin. | |
| 35 | f | 620 | 24 | 28 | neg. | 26 | 97 | N/A | 0 | Iodinated contrast agent. | |
| 36 | m | 659 | 24 | 26 | neg. | 11 | 45 | N/A | 0 | ||
| 37 | m | 980 | 27 | 29 | neg. | 47 | N/A | N/A | 0 | ||
| Inconclusive | 38 | m | 1780 | 31 | 32 | pos. | 71 | N/A | 11.7 | 3.4 | Mostly extremely low TSH values under treatment, maternal autoimmune thyroiditis mentioned in medical report. |
| 39 | m | 570 | 24 | 27 | neg. | 52 | 336 | 5.1 | 0.9 | Iodinated contrast agent. | |
| 40 | m | 740 | 27 | 32 | neg. | 52 | >100 | 5.1 | 1.8 | ||
| 41 | f | 1190 | 29 | 32 | neg. | 15 | 63 | 9.0 | 2.3 | ||
| 42 | m | 540 | 24 | 47 | neg. | 52 | >100 | 9.0 | 2.1 | Late treatment due to fluctuations or thyroid function tests and presumed transience. | |
| 43 | f | 806 | 27 | 33 | neg. | 20 | 57 | 9.5 | 1.2 | Identical twin of euthyroid co-twin. Family history of hypothyroidism. | |
| 44 | f | 620 | 26 | 35 | neg. | 18 | 29 | 11.7 | 2.5 | Iodinated contrast agent. | |
| 45 | m | 850 | 31 | 33 | neg. | 32 | 81 | 14.5 | 2.7 | Iodinated contrast agent. | |
| 46 | f | 1138 | 31 | 40 | neg. | 24 | 24 | 19.4 | 2.0 | Identical twin of euthyroid co-twin. Re-screening neg. at 2 weeks, pos. at 8 weeks. | |
| Unknown | 47 | m | 740 | 25 | 29 | neg. | 162 | 323 | 2.8 | N/A | |
| 48 | f | 560 | 23 | 33 | neg. | 137 | 157 | 5.1 | N/A | Iodinated contrast agent. | |
| 49 | f | 720 | 25 | 33 | neg. | 60 | 128 | 7.7 | N/A | Iodinated contrast agent. | |
| 50 | m | 841 | 29 | 35 | neg. | 27 | 29 | 7.8 | N/A | ||
| 51 | m | 805 | 27 | 35 | neg. | 15 | 22 | 15.4 | N/A | ||
| Study Group <32 Weeks of Gestation 1 | Comparison Group ≥32 Weeks of Gestation 2 | |||
|---|---|---|---|---|
| n | Per 10,000 births | n | Per 10,000 births | |
| Permanent | 14 | 6.0 | 375 | 2.3 |
| Transient | 23 | 9.9 | 40 | 0.25 |
| Inconclusive | 9 | 3.9 | 7 | 0.04 |
| Unknown | 5 | 2.2 | 22 | 0.14 |
| Total | 51 | 22.0 | 444 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odenwald, B.; Fischer, A.; Röschinger, W.; Liebl, B.; Schmidt, H.; Nennstiel, U. Long-Term Course of Hypothyroidism Detected through Neonatal TSH Screening in a Population-Based Cohort of Very Preterm Infants Born at Less than 32 Weeks of Gestation. Int. J. Neonatal Screen. 2021, 7, 65. https://doi.org/10.3390/ijns7040065
Odenwald B, Fischer A, Röschinger W, Liebl B, Schmidt H, Nennstiel U. Long-Term Course of Hypothyroidism Detected through Neonatal TSH Screening in a Population-Based Cohort of Very Preterm Infants Born at Less than 32 Weeks of Gestation. International Journal of Neonatal Screening. 2021; 7(4):65. https://doi.org/10.3390/ijns7040065
Chicago/Turabian StyleOdenwald, Birgit, Aline Fischer, Wulf Röschinger, Bernhard Liebl, Heinrich Schmidt, and Uta Nennstiel. 2021. "Long-Term Course of Hypothyroidism Detected through Neonatal TSH Screening in a Population-Based Cohort of Very Preterm Infants Born at Less than 32 Weeks of Gestation" International Journal of Neonatal Screening 7, no. 4: 65. https://doi.org/10.3390/ijns7040065
APA StyleOdenwald, B., Fischer, A., Röschinger, W., Liebl, B., Schmidt, H., & Nennstiel, U. (2021). Long-Term Course of Hypothyroidism Detected through Neonatal TSH Screening in a Population-Based Cohort of Very Preterm Infants Born at Less than 32 Weeks of Gestation. International Journal of Neonatal Screening, 7(4), 65. https://doi.org/10.3390/ijns7040065






