Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences
Abstract
1. Introduction
2. Material and Methods
2.1. NewSTEPs and State Implementation of New Disorders
2.2. Data Collection
2.2.1. New Disorder Readiness Tool
2.2.2. New Disorder Awardee Reporting and Interviews
2.3. Data Analysis
2.3.1. Descriptive Statistics
2.3.2. Survival Analysis
2.3.3. Qualitative Analysis
3. Findings
3.1. Time to Implementation of Statewide Screening
3.2. Time from Approval/Mandate to Implementation of Statewide Screening
3.3. Time to Complete Authority to Screen and Program Readiness (Laboratory/Follow-Up/IT) in the Implementation Process
3.4. Understanding Which Readiness Phase Activities Take the Longest Time to Complete
3.5. Time from Addition to RUSP to Initiation of First Activity
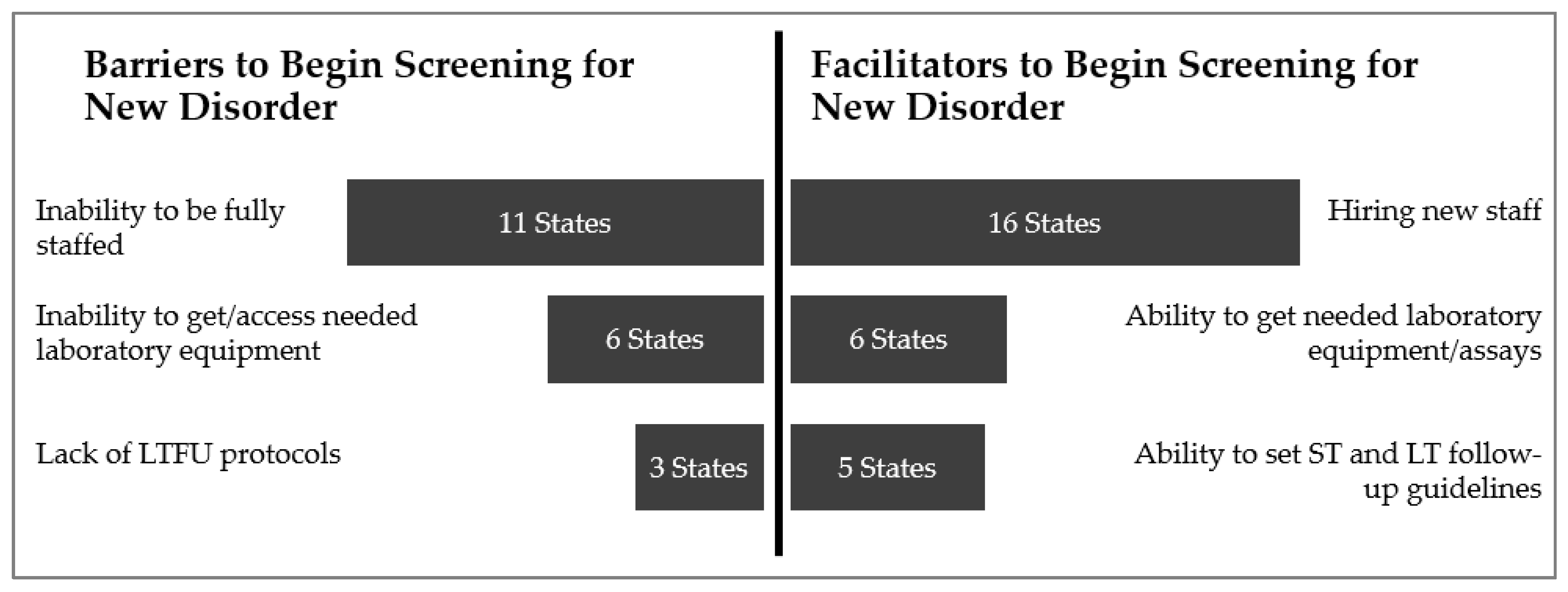
3.6. Barriers and Facilitators to Implementing Statewide Screening
“[Program is] not making headway on training and keeping new staff. There are no simplified reviews for new positions. It is needlessly hard to hire.”
“Getting well-qualified people with what the state pays is the challenge. [We] get them green, train them, and they leave.”
“[Our program is] given six months after getting equipment to begin screening. During this time, we meet with vendors; ask follow-up, laboratory, specialists, and the [advisory group] to work together on what needs to be reported out and how to treat special baby population populations. Decisions are made on what to do for different testing results and special groups during this time [through collaboration].”
“[To] create education materials for the new disorders, [we] used the lysosomal disorder workgroup..., had a neonatologist, parent, people from laboratory and follow up on workgroup. [We also] had a pediatrician consultant help. [The workgroup] gave edits and comments. When [the education materials were] approved [program leadership] took it before the director and the commissioner and got final approval with it.”
4. Discussion
4.1. Time to Statewide Screening
“[It is] easy and cheap to add SMA, but [testing for SMA] puts follow-up in a tough situation sometimes. [Our program has] specialists who don’t know what happens yet, [don’t know] what questions they have yet. [Newborn screening program] is not ready to bring a child through the process [of having positive newborn screen], especially if [the screen] is a false positive?”
4.2. Time from Addition of the RUSP to Statewide Screening
“The primary reason we put testing on hold is because we didn’t have the reagent to screen for it [ALD].”
“We did have staff, but we did not have instrument time. [We had] an agreement [with another agency to access their lab instrumentation], but by [the time of the addition to the RUSP for] ALD, they had too little [available instrument] time to help.”
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 619–623.
- Wilcken, B. Expanded newborn screening: Reducing harm, assessing benefit. J. Inherit. Metab. Dis. 2010, 33, 205–210. [Google Scholar] [CrossRef] [PubMed]
- American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: Toward a uniform screening panel and system—Executive summary. Pediatrics 2006, 117, S296–S307. [Google Scholar] [CrossRef]
- Kemper, A.R.; Green, N.S.; Calonge, N.; Lam, W.K.; Comeau, A.M.; Goldenberg, A.J.; Ojodu, J.; Prosser, L.A.; Tanksley, S.; Bocchini, J.A., Jr. Decision-making process for conditions nominated to the recommended uniform screening panel: Statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 2014, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.I.; Fix, A. As Advancements In Treatment Drive A Newborn Screening Evolution, Will States And The Federal Government Be Able To Keep Up? Available online: https://www.healthaffairs.org/do/10.1377/hblog20190816.174106/full/ (accessed on 17 April 2020).
- Kemper, A.R.; Brosco, J.; Comeau, A.M.; Green, N.S.; Grosse, S.; Kwon, J.; Prosser, L.A.; Ojodu, J.; Jones, E.; Tanksley, S.; et al. Newborn Screening for X-Linked Adrenoleukodystrophy (X-ALD): A Systematic Review of Evidence Final Report (v5. 01); Maternal Child Health Bureau, United States Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children: Rockville, MD, USA, 2015.
- NewSTEPs: Newborn Screening Technical assistance and Evaluation Program [Homepage on the Internet]. Available online: www.newsteps.org (accessed on 2 January 2020).
- Calonge, N.; Green, N.S.; Rinaldo, P.; Lloyd-Puryear, M.; Dougherty, D.; Boyle, C.; Watson, M.; Trotter, T.; Terry, S.F.; Howell, R.R. Committee report: Method for evaluating conditions nominated for population-based screening of newborns and children. Genet. Med. 2010, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Botkin, J.R.; Clayton, E.W.; Fost, N.C.; Burke, W.; Murray, T.H.; Baily, M.A.; Wilfond, B.; Berg, A.; Ross, L.F. Newborn screening technology: Proceed with caution. Pediatrics 2006, 117, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- The Advisory Committee on Heritable Disorders in Newborns and Children Report to Congress 2013–2017; Health Resources and Services Administration (HRSA): Rockville, MD, USA, 2017.
- Kemper, A.; Comeau, A.; Green, N.; Goldenberg, A.; Ojodu, J.; Prosser, L.; Tanksley, S.; Weinreich, S.; Lam, K. Evidence Report: Newborn Screening for Pompe Disease; Maternal Child Health Bureau, United States Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children: Rockville, MD, USA, 2013.
- Kemper, A.R.; Brosco, J.; Comeau, A.M.; Green, N.S.; Grosse, S.; Kwon, J.; Prosser, L.A.; Ojodu, J.; Jones, E.; Tanksley, S.; et al. Newborn Screening for MPS I: Final Report from the Condition Review Workgroup. Public Health System Impact Assessment for MPS I; Maternal Child Health Bureau, United States Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children: Rockville, MD, USA, 2015.
- Kemper, A.R.; Lam, K.; Comeau, A.M.; Kwon, J.; Green, N.S.; Ojodu, J.; Grosse, S.; Prosser, L.A.; Jones, E.; Tanksley, S. Evidence-Based Review of Newborn Screening for Spinal Muscular Atrophy (SMA): Final Report (v5. 2); Maternal Child Health Bureau, United States Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children: Rockville, MD, USA, 2018.
- Ojodu, J.; Singh, S.; Kellar-Guenther, Y.; Yusuf, C.; Jones, E.; Wood, T.; Baker, M.; Sontag, M. NewSTEPs: The Establishment of a National Newborn Screening Technical Assistance Resource Center. Int. J. Neonatal Screen. 2018, 4, 1. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Wile, M. State Newborn Health Screening Policies; National Conference of State Legislatures: Washington, DC, USA, 2017. [Google Scholar]
- Xu, A.; Ganapathy, V.; Morain, S. Delay in State Adoption of Newborn Screening Tests. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Kellar-Guenther, Y.; Sontag, M.K.; Linder, E.; Singh, S.; Sheller, R.; Ojodu, J. Analyzing Patterns in NewSTEPs Site Review Recommendations: Practical Applications for Newborn Screening Programs. Int. J. Neonatal Screen. 2019, 5, 13. [Google Scholar] [CrossRef]
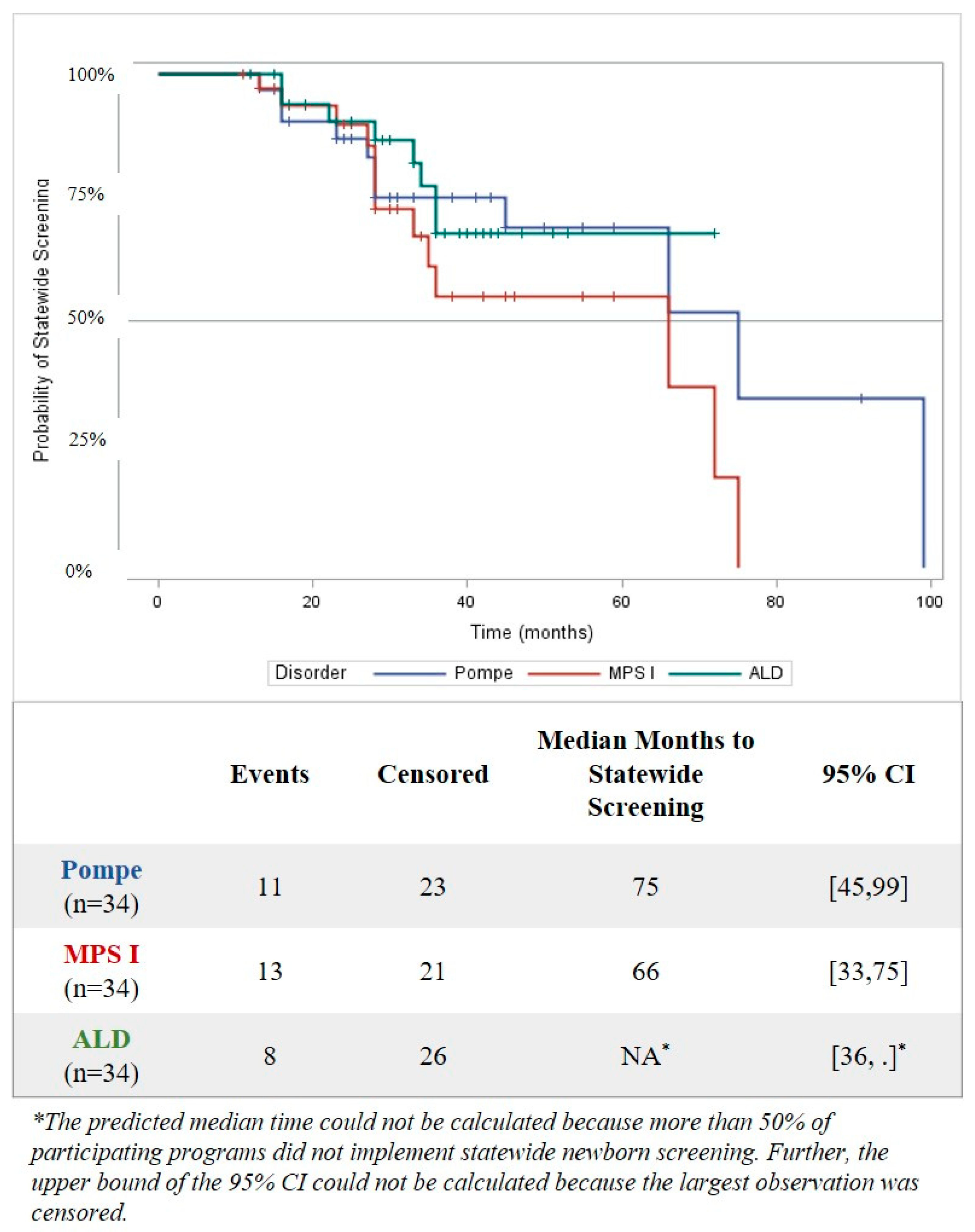
| Number Who Provided Data (n) | Statewide Screening Implemented | In Progress Towards Statewide Screening (i.e., Completed at Least One Activity) | Not Yet Started an Activity | Not Actively Pursuing Statewide Screening | |
|---|---|---|---|---|---|
| Pompe | 39 | 11 (28.2%) | 23 (59.0%) | 4 (10.3%) | 1 (2.6%) |
| MPS I | 38 | 13 (34.2%) | 21 (55.3%) | 4 (10.5%) | 0 (0.0%) |
| ALD | 38 | 8 (21.1%) | 26 (68.4%) | 3 (7.9%) | 1 (2.6%) |
| SMA | 30 | 5 (16.7%) | 20 (66.6%) | 2 (6.6%) | 3 (10.0%) |
| Readiness Phase | Activity | Pompe (n = 11) | MPS I (n = 13) | ALD (n = 8) | SMA (n = 5) |
|---|---|---|---|---|---|
| Approval for Funding | Fee Increase Implemented | 1 (9%) | 1 (8%) | 0 (0%) | 1 (20%) |
| Laboratory Readiness | Add Testing to Outside Laboratory Contract | 2 (18%) | 1 (8%) | 0 (0%) | 0 (0%) |
| Train Laboratory Staff to Begin Testing | 1 (9%) | 1 (8%) | 1 (13%) | 1 (20%) | |
| IT Readiness | Validate Changes to the Follow-up Reporting System | 0 (0%) | 2 (15%) | 0 (0%) | 1 (20%) |
| Validate Changes to Electronic Results Protocol | 0 (0%) | 1 (8%) | 0 (0%) | 1 (20%) | |
| Other IT Activities (e.g., getting DNA sequencing into IT; changing LIMS) | 1 (9%) | 1 (8%) | 1 (13%) | 0 (0%) | |
| Education | Identify/Modify Family Education Materials to be State Specific | 0 (0%) | 1 (8%) | 2 (25%) | 1 (20%) |
| Initiate a Strategy for Family Education Materials & Create Own Family Education Materials | 1 (9%) | 2 (15%) | 1 (13%) | 1 (20%) | |
| Identify/Create Measures to Track Impact of Family Education Materials | 5 (46%) | 6 (46%) | 2 (25%) | 2 (40%) | |
| Family Education Materials are Distributed | 1 (9%) | 2 (15%) | 0 (0%) | 1 (20%) | |
| Identify/Modify Provider Education Materials to be State Specific | 0 (0%) | 1 (8%) | 1 (13%) | 0 (0%) | |
| Identify/Create Measures to Track Impact of Provider Education Materials | 6 (55%) | 6 (46%) | 2 (25%) | 2 (40%) | |
| Identify/Modify General Public Education Materials to be State Specific | 1 (9%) | 2 (15%) | 0 (0%) | 1 (20%) | |
| Initiate a Strategy for General Public Education Materials & Create Own General Public Education Materials | 1 (9%) | 2 (15%) | 0 (0%) | 1 (20%) | |
| Identify/Create Measures to Track Impact of General Public Education Materials | 6 (55%) | 7 (54%) | 2 (25%) | 1 (20%) | |
| General Public Education Materials are Distributed | 1 (9%) | 2 (15%) | 0 (0%) | 1 (20%) |
| Mandate/Approval to Start Screening | Fee Increase Implemented | Laboratory Readiness | Follow-Up Readiness | IT Readiness | ||
|---|---|---|---|---|---|---|
| Predictive Median Months from Survival Analysis [95% CI] (number of state NBS programs) | Pompe | NA + | 24 | 39 | 26 | 16 |
| (27) β | (15) β | (24,68) | (18,36) | (7,37) | ||
| (n = 25) | (n = 27) | (n = 28) | (n = 24) | (n = 19) | ||
| MPS I | 36 * | 49 ** | 39 | 24 | 18 | |
| (18) β | (22,49) | (23,60) | (13,42) | (7,37) | ||
| (n = 25) | (n = 30) | (n = 28) | (n = 24) | (n = 20) | ||
| ALD | NA +,* | 27 | NA + | NA + | 14 | |
| (17) β | (24) β | (31) β | (26) β | (7) β | ||
| (n = 24) | (n = 30) | (n = 30) | (n = 26) | (n = 18) | ||
| Observed Median Months to Complete Phase (Min, Max) (number of state NBS programs) | Pompe | 18.0 | 16.5 | 23.0 | 21.5 | 12.5 |
| (9,30) | (4,34) | (4,68) | (1,77) | (2,40) | ||
| (n = 9) | (n = 14) | (n = 14) | (n = 14) | (n = 14) | ||
| MPS I | 13.0 * | 19.5 ** | 24.0 | 18.5 | 13.5 | |
| (3,36) | (4,49) | (14,75) | (5,42) | (2,46) | ||
| (n = 11) | (n = 12) | (n = 14) | (n = 14) | (n = 14) | ||
| ALD | 12.0 * | 21.0 | 20.0 | 18.0 | 8.0 | |
| (0,30) | (1,34) | (13,36) | (2,30) | (2,14) | ||
| (n = 9) | (n = 13) | (n = 9) | (n = 9) | (n = 9) | ||
| Longest to Shortest | Pompe (Median Months, n) | MPS I (Median Months, n) | ALD (Median Months, n) | SMA (Median Months, n) |
| Develop follow-up staffing protocols/ensure adequate staffing (12.6, n = 13) | Identify screening methodology/assay for 1st tier testing (14.8, n = 15) | Develop/validate assay for 2nd tier testing (19.7, n = 3) | Obtain approval from the state budget authority (10, n = 6) | |
| Develop/validate assay for 1st tier testing (12.4, n = 12) | Other funding activities * (14.8, n = 4) | Modify laboratory space; install equipment (17.4, n = 8) | Other approval/authority activities (7.8, n = 4) | |
| Identify/modify general public education materials to be state-specific (12.0, n = 8) | Develop/validate assay for 1st tier testing (12.5, n = 14) | Develop/validate assay for 1st tier testing (12.7, n = 6) | Ensure adequate laboratory space for testing (6.4, n = 12) | |
| Identify screening methodology/assay for 1st tier testing (11.9, n = 13) | Procure additional laboratory equipment (11.8, n = 13) | Other funding activities (12.7, n = 3) | Develop budget to show cost for screen (6.2, n = 12) | |
| Identify/modify family education materials to be state-specific (11.6, n = 8) | Identify/modify general public education materials to be state-specific (11.7, n = 9) | Identify medical specialists/treatment center (11.9, n = 13) | Obtain approval from NBS Advisory Committee (5.3, n = 15) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellar-Guenther, Y.; McKasson, S.; Hale, K.; Singh, S.; Sontag, M.K.; Ojodu, J. Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences. Int. J. Neonatal Screen. 2020, 6, 35. https://doi.org/10.3390/ijns6020035
Kellar-Guenther Y, McKasson S, Hale K, Singh S, Sontag MK, Ojodu J. Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences. International Journal of Neonatal Screening. 2020; 6(2):35. https://doi.org/10.3390/ijns6020035
Chicago/Turabian StyleKellar-Guenther, Yvonne, Sarah McKasson, Kshea Hale, Sikha Singh, Marci K. Sontag, and Jelili Ojodu. 2020. "Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences" International Journal of Neonatal Screening 6, no. 2: 35. https://doi.org/10.3390/ijns6020035
APA StyleKellar-Guenther, Y., McKasson, S., Hale, K., Singh, S., Sontag, M. K., & Ojodu, J. (2020). Implementing Statewide Newborn Screening for New Disorders: U.S. Program Experiences. International Journal of Neonatal Screening, 6(2), 35. https://doi.org/10.3390/ijns6020035





