Processing Newborn Bloodspot Screening Results for CF
Abstract
1. Introduction
2. Sweat Test—The Gold Standard Confirmation Test of a Positive NBS Result
3. Presumptive Diagnosis or How to Proceed if a Sweat Test Fails
4. Unintended Effects of Newborn Screening for CF
5. Consequences for Other Family Members
6. Communicating Positive NBS Results for CF
7. Feedback and Tracking
8. Conclusions
Funding
Conflicts of Interest
References
- Castellani, C.; Massie, J.; Sontag, M.; Southern, K.W. Newborn screening for cystic fibrosis. Lancet Respir. Med. 2016, 4, 653–661. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Southern, K.W.; Brownlee, K.; Roelse, J.D.; Duff, A.; Farrell, M.; Mehta, A.; Munck, A.; Pollitt, R.; Sermet-Gaudelus, I.; et al. European best practice guidelines for cystic fibrosis neonatal screening. J. Cyst. Fibros. 2010, 8, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; Public Health papers, No.34; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Rueegg, C.S.; Barben, J.; Hafen, G.M.; Moeller, A.; Jurca, M.; Fingerhut, R.; Kuehni, C.E.; Group, T.S.C.F.S. Newborn screening for cystic fibrosis—The parent perspective. J. Cyst. Fibros. 2016, 15, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chudleigh, J.; Ren, C.L.; Barben, J.; Southern, K.W. International approaches for delivery of positive newborn bloodspot screening results for CF. J. Cyst. Fibros. 2019, 18, 614–621. [Google Scholar] [CrossRef]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B.; Accurso, F.J.; Castellani, C.; Cutting, G.R.; Durie, P.R.; LeGrys, V.A.; Massie, J.; Parad, R.B.; et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar] [CrossRef]
- LeGrys, V.A.; Yankaskas, J.R.; Quittell, L.M.; Marshall, B.C.; Mogayzel, P.J., Jr.; Cystic Fibrosis Foundation. Diagnostic sweat testing: The Cystic Fibrosis Foundation guidelines. J. Pediatr. 2007, 151, 85–89. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15. [Google Scholar] [CrossRef]
- Bergougnoux, A.; Boureau-Wirth, A.; Rouzier, C.; Altieri, J.P.; Verneau, F.; Larrieu, L.; Koenig, M.; Claustres, M.; Raynal, C. A false positive newborn screening result due to a complex allele carrying two frequent CF-causing variants. J. Cyst. Fibros. 2016, 15, 309–312. [Google Scholar] [CrossRef][Green Version]
- Southern, K.W.; Barben, J.; Gartner, S.; Munck, A.; Castellani, C.; Mayell, S.J.; Davies, J.C.; Winters, V.; Murphy, J.; Salinas, D.; et al. Inconclusive diagnosis after a positive newborn bloodspot screening result for cystic fibrosis; clarification of the harmonised international definition. J. Cyst. Fibros. 2019, 18, 780. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Sweat Testing: Sample Collection and Quantitative Chloride Analysis Approved Guideline, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Royal College of Paediatrics and Child Health. Guidelines for the Performance of the Sweat Test for the Investigation of Cystic Fibrosis in the UK (Version 2). An Evidence Based Guideline 2014. Available online: http://www.acb.org.uk/docs/default-source/committees/scientific/guidelines/acb/sweat-guideline-v2-1.pdf (accessed on 25 March 2020).
- Eng, W.; LeGrys, V.A.; Schechter, M.S.; Laughon, M.M.; Barker, P.M. Sweat-testing in preterm and full-term infants less than 6 weeks of age. Pediatric Pulmonol. 2005, 40, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Rueegg, C.S.; Kuehni, C.E.; Gallati, S.; Jurca, M.; Jung, A.; Casaulta, C.; Barben, J. Swiss Cystic Fibrosis Screening Group Comparison of two sweat test systems for the diagnosis of cystic fibrosis in newborns. Pediatric Pulmonol. 2019, 54, 264–272. [Google Scholar] [CrossRef]
- LeGrys, V.A.; McColley, S.A.; Li, Z.; Farrell, P.M. The need for quality improvement in sweat testing infants after newborn screening for cystic fibrosis. J. Pediatr. 2010, 157, 1035–1037. [Google Scholar] [CrossRef]
- Kleyn, M.; Korzeniewski, S.; Grigorescu, V.; Young, W.; Homnick, D.; Goldstein-Filbrun, A.; Schuen, J.; Nasr, S. Predictors of insufficient sweat production during confirmatory testing for cystic fibrosis. Pediatric Pulmonol. 2011, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Laguna, T.A.; Lin, N.; Wang, Q.; Holme, B.; McNamara, J.; Regelmann, W.E. Comparison of quantitative sweat chloride methods after positive newborn screen for cystic fibrosis. Pediatric Pulmonol. 2012, 47, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Parad, R.B.; Comeau, A.M.; Dorkin, H.L.; Dovey, M.; Gerstle, R.; Martin, T.; O’Sullivan, B.P. Sweat testing infants detected by cystic fibrosis newborn screening. J. Pediatr. 2008, 147, S69–S72. [Google Scholar] [CrossRef] [PubMed]
- Mastella, G.; Di Cesare, G.; Borruso, A.; Menin, L.; Zanolla, L. Reliability of sweat-testing by the Macroduct collection method combined with conductivity analysis in comparison with the classic Gibson and Cooke technique. Acta Paediatr. 2000, 89, 933–937. [Google Scholar] [CrossRef]
- Baumer, J.H. Evidence based guidelines for the performance of the sweat test for the investigation of cystic fibrosis in the UK. Arch. Dis. Child. 2003, 88, 1126–1127. [Google Scholar] [CrossRef]
- Cole, D.E.; Boucher, M.J. Use of a new sample-collection device (Macroduct) in anion analysis of human sweat. Clin. Chem. 1986, 32, 1375–1378. [Google Scholar] [CrossRef]
- Coakley, J.; Scott, S.; Doery, J.; Greaves, R.; Talsma, P.; Whitham, E.; Winship, J. Australasian Guideline (2nd Edition): An Annex to the CLSI and UK Guidelines for the Performance of the Sweat Test for the Diagnosis of Cystic Fibrosis. Clin. Biochem. Rev. 2017, 38, 115–130. [Google Scholar]
- Hammond, K.B.; Nelson, L.; Gibson, L.E. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J. Pediatr. 1994, 124, 255–260. [Google Scholar] [CrossRef]
- Heeley, M.E.; Woolf, D.A.; Heeley, A.F. Indirect measurements of sweat electrolyte concentration in the laboratory diagnosis of cystic fibrosis. Arch. Dis. Child. 2000, 82, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Lezana, J.L.; Vargas, M.H.; Karam-Bechara, J.; Aldana, R.S.; Furuya, M.E.Y. Sweat conductivity and chloride titration for cystic fibrosis diagnosis in 3834 subjects. J Cyst. Fibros. 2003, 2, 1–7. [Google Scholar] [CrossRef]
- Webster, H.L.; Quirante, C.G. Micro-flowcell conductometric sweat analysis for cystic fibrosis diagnosis. Ann. Clin. Biochem. 2000, 37, 399–407. [Google Scholar] [CrossRef]
- Barben, J.; Ammann, R.A.; Metlagel, A.; Schöni, M.H. Conductivity determined by a new sweat analyzer compared with chloride concentrations for the diagnosis of cystic fibrosis. J. Pediatr. 2005, 146, 183–188. [Google Scholar] [CrossRef]
- Desax, M.C.; Ammann, R.A.; Hammer, J.; Schoeni, M.H.; Barben, J. Nanoduct sweat testing for rapid diagnosis in newborns, infants and children with cystic fibrosis. Eur. J. Pediatr. 2008, 167, 299–304. [Google Scholar] [CrossRef]
- Sands, D.; Oltarzewski, M.; Nowakowska, A.; Zybert, K. Bilateral sweat tests with two different methods as a part of cystic fibrosis newborn screening (CF NBS) protocol and additional quality control. Folia Histochem. Cytobiol. 2010, 48, 358–365. [Google Scholar] [CrossRef]
- Sezer, R.G.; Aydemir, G.; Akcan, A.B.; Paketci, C.; Karaoglu, A.; Aydinoz, S.; Bozaykut, A. Nanoduct sweat conductivity measurements in 2664 patients: Relationship to age, arterial blood gas, serum electrolyte profiles and clinical diagnosis. J. Clin. Med. Res. 2013, 5, 34–41. [Google Scholar] [CrossRef][Green Version]
- Vernooij-van Langen, A.; Dompeling, E.; Yntema, J.B.; Arets, B.; Tiddens, H.; Loeber, G.; Dankert-Roelse, J. Clinical evaluation of the Nanoduct sweat test system in the diagnosis of cystic fibrosis after newborn screening. Eur. J. Pediatr. 2015, 174, 1025–1034. [Google Scholar] [CrossRef]
- Barben, J.; Rueegg, C.S.; Jurca, M.; Spalinger, J.; Kuehni, C.E.; Swiss Cystic Fibrosis Screening Group. Measurement of fecal elastase improves performance of newborn screening for cystic fibrosis. J. Cyst. Fibros. 2016, 15, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Castellani, C.; Keenan, K.; Avolio, J.; Volpi, S.; Boland, M.; Kovesi, T.; Bjornson, C.; Chilvers, M.A.; Morgan, L.; et al. Inconclusive diagnosis of cystic fibrosis after newborn screening. Pediatrics 2015, 135, e1377–e1385. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Southern, K.W. Cystic Fibrosis Screen Positive, inconclusive Diagnosis (CFSPID). Curr. Opin. Pulm. Med. 2016, 22, 617–622. [Google Scholar] [CrossRef]
- Massie, J.; Gillam, L. Uncertain diagnosis after newborn screening for cystic fibrosis: An ethics-based approach to a clinical dilemma. Pediatric Pulmonol. 2014, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Mischler, E.H.; Farrell, P.M.; Fost, N.; Peterson, N.M.; Carey, P.; Bruns, W.T.; McCarthy, C. Parents’ knowledge of neonatal screening and response to false-positive cystic fibrosis testing. J. Dev. Behav. Pediatr. 1992, 13, 181–186. [Google Scholar] [CrossRef]
- Tluczek, A.; Koscik, R.L.; Farrell, P.M.; Rock, M.J. Psychosocial risk associated with newborn screening for cystic fibrosis: Parents’ experience while awaiting the sweat-test appointment. Pediatrics 2005, 115, 1692–1703. [Google Scholar] [CrossRef]
- Chudleigh, J.; Chinnery, H.; Bonham, J.R.; Olander, E.K.; Moody, L.; Simpson, A.; Morris, S.; Ulph, F.; Bryon, M.; Southern, K.W. A Qualitative Exploration of Health Professionals’ Experiences of Communicating Positive Newborn Bloodspot Screening Results for Nine Conditions in England; 2020. (In Press)
- Dillard, J.P.; Shen, L.; Robinson, J.D.; Farrell, P.M. Parental information seeking following a positive newborn screening for cystic fibrosis. J. Health Commun. 2010, 15, 880–894. [Google Scholar] [CrossRef]
- Ulph, F.; Cullinan, T.; Qureshi, N.; Kai, J. Informing children of their newborn screening carrier result for sickle cell or cystic fibrosis: Qualitative study of parents’ intentions, views and support needs. J. Genet. Couns. 2014, 23, 409–420. [Google Scholar] [CrossRef]
- Miller, A.C.; Comellas, A.P.; Hornick, D.B.; Stoltz, D.A.; Cavanaugh, J.E.; Gerke, A.K.; Welsh, M.J.; Zabner, J.; Polgreen, P.M. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc. Natl. Acad. Sci. USA 2020, 117, 1621–1627. [Google Scholar] [CrossRef]
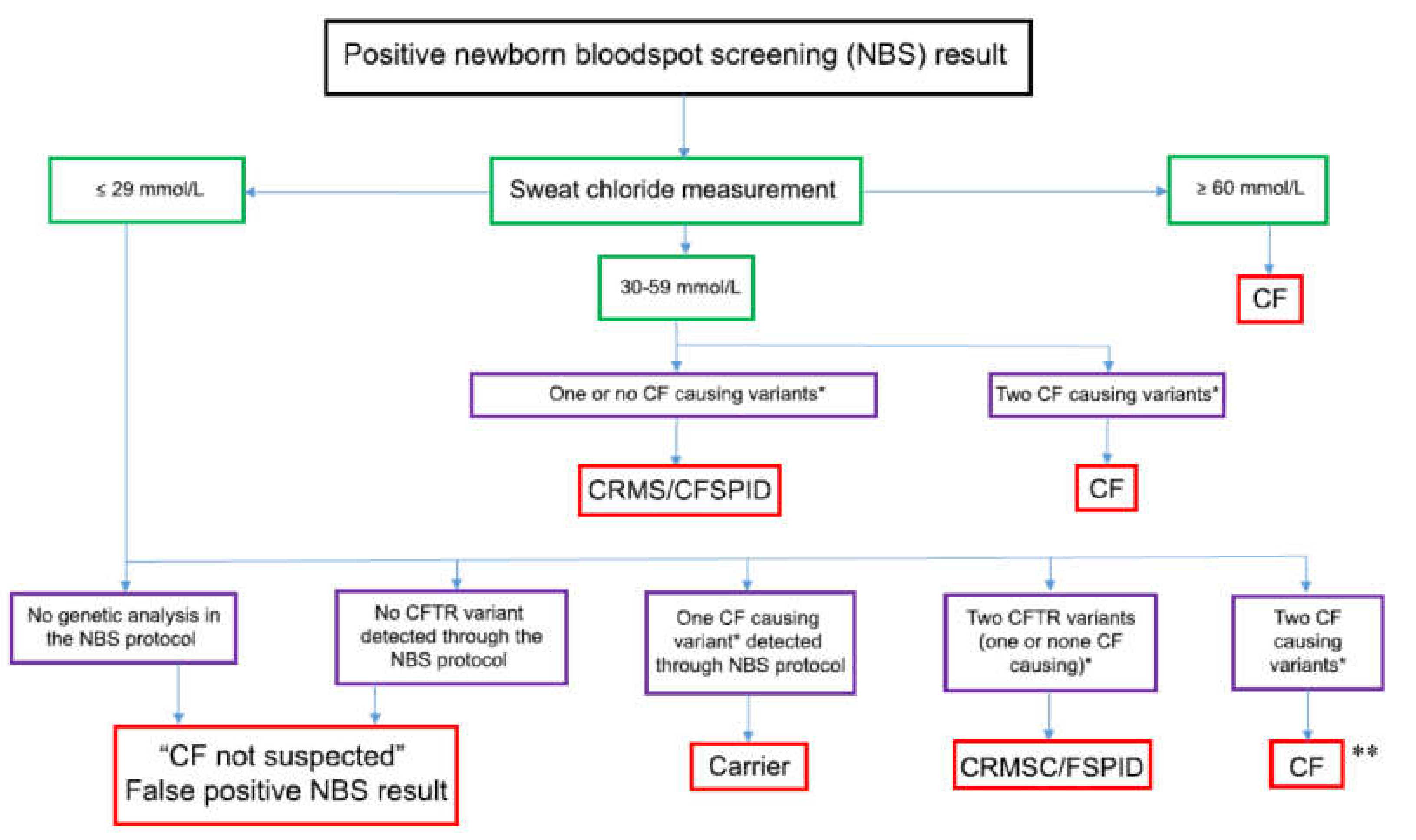
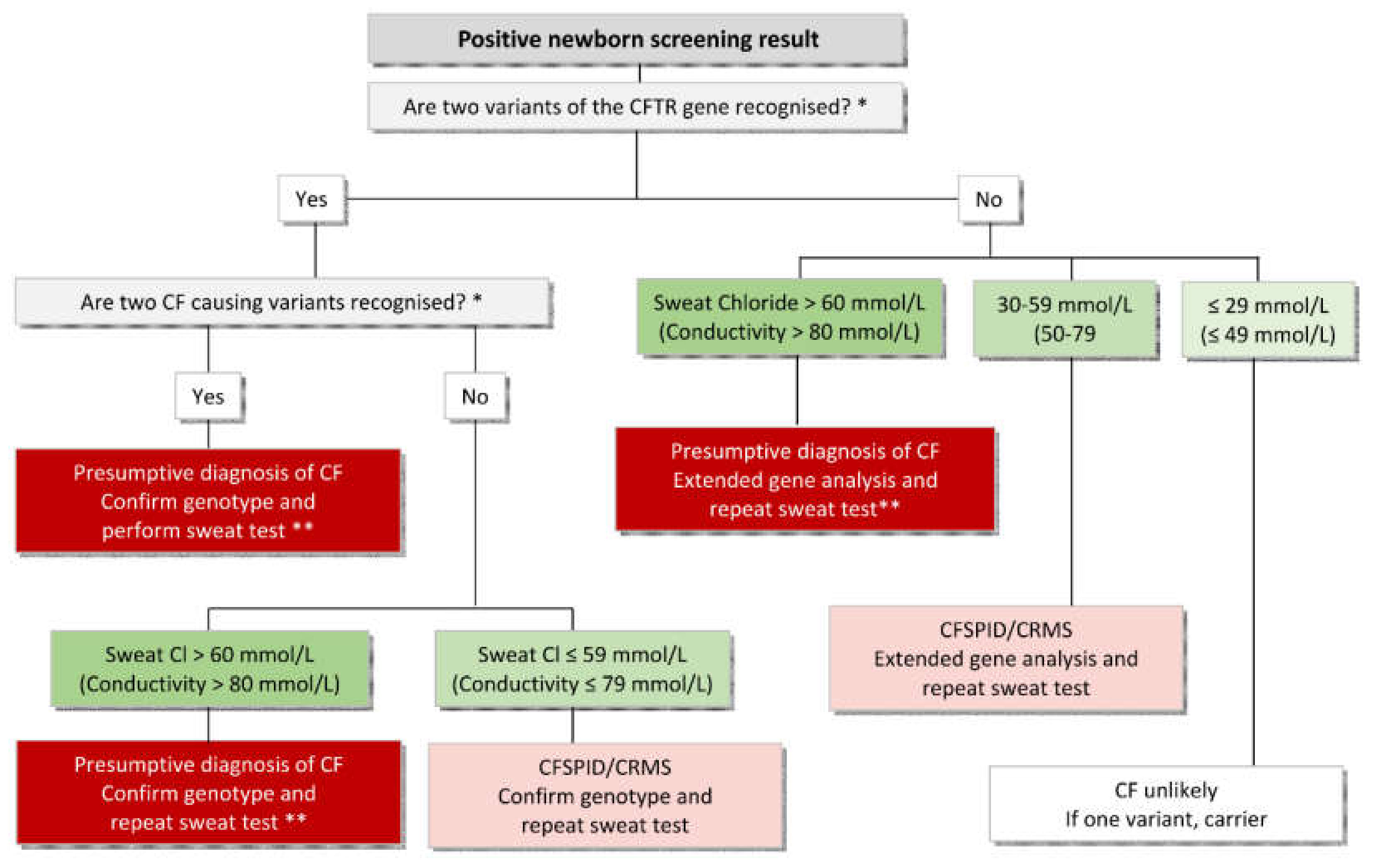
| 1 | Sweat collection by experienced personnel (at least 150 sweat tests per annum) following national or international guidelines and subject to regular (at least annual) peer review. |
| 2 | Use of commercially available equipment approved for diagnostic use according to the national regulatory requirements or EU standards if no local ones are available. |
| 3 | Internal quality control (usually three samples) with acceptable limits of agreement for chloride before each sweat analysis. |
| 4 | Regular external quality assurance for the analyses according to national guidelines. |
| 5 | A high number of QNS (Quantity Not Sufficient) rates is a marker of technical issue. This necessitates renewing training for personnel experiencing sweat tests. |
| 1 | The quantity of sweat should indicate an adequate rate of sweat production (15μL for Macroduct™ tube system). |
| 2 | The sweat sample should be processed immediately after sweat collection. |
| 3 | A sweat chloride value >59 mmol/L is consistent with a diagnosis of CF. |
| 4 | A sweat chloride value <30 mmol/L makes the diagnosis of CF unlikely. However, specific CF causing mutations can be associated with a sweat test below 30 mmol/L. These include c.3718-2477C N T (3849 + 10kbC N T) and mutations associated with varied clinical consequence, such as c.617T N G (L206W), c.1040G N A (R347H) and c.3454G N C (D1152H). |
| 5 | Individuals with sweat chloride values in the borderline range (30–59 mmol/L) should undergo a repeat sweat test and further evaluation in a specialist CF centre, including detailed clinical assessment and extensive CFTR gene mutation analysis. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barben, J.; Chudleigh, J. Processing Newborn Bloodspot Screening Results for CF. Int. J. Neonatal Screen. 2020, 6, 25. https://doi.org/10.3390/ijns6020025
Barben J, Chudleigh J. Processing Newborn Bloodspot Screening Results for CF. International Journal of Neonatal Screening. 2020; 6(2):25. https://doi.org/10.3390/ijns6020025
Chicago/Turabian StyleBarben, Jürg, and Jane Chudleigh. 2020. "Processing Newborn Bloodspot Screening Results for CF" International Journal of Neonatal Screening 6, no. 2: 25. https://doi.org/10.3390/ijns6020025
APA StyleBarben, J., & Chudleigh, J. (2020). Processing Newborn Bloodspot Screening Results for CF. International Journal of Neonatal Screening, 6(2), 25. https://doi.org/10.3390/ijns6020025





