Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis
Abstract
1. Introduction
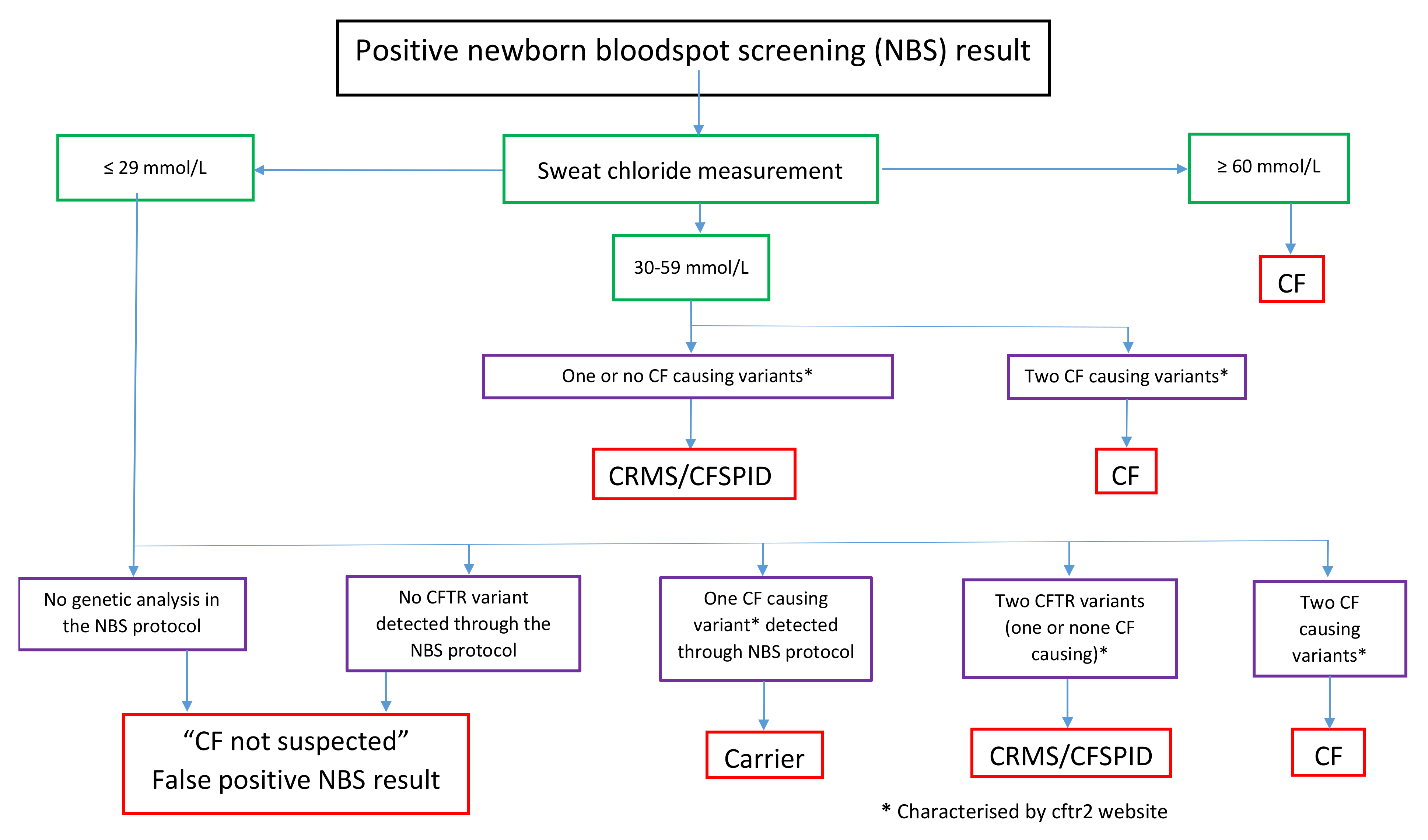
2. Inconclusive Diagnosis after Newborn Screening
2.1. Definition of CRMS/CFSPID
2.2. Incidence of CRMS/CFSPID across Europe
2.3. Assessment of CFTR Protein Function
3. Monitoring Infants Designated CRMS/CFSPID
3.1. Outcomes and Conversion to a Final Diagnosis of CF in Infants Designated CRMS/CFSPID
3.2. Management of Infants with CRMS/CFSPID Designation
4. CRMS/CFSPID Registry Database
Funding
Acknowledgments
Conflicts of Interest
References
- Dijk, F.N.; Fitzgerald, D.A. The impact of newborn screening and earlier intervention on the clinical course of cystic fibrosis. Paediatr. Respir. Rev. 2012, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Yen, E.H.; Quinton, H.; Borowitz, D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J. Pediatr. 2013, 162, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Tridello, G.; Castellani, C.; Meneghelli, I.; Tamanini, A.; Assael, B.M. Early diagnosis from newborn screening maximises survival in severe cystic fibrosis. ERJ Open Res. 2018, 4, 00109–2017. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef]
- Borowitz, D.; Parad, R.B.; Sharp, J.K.; Sabadosa, K.A.; Robinson, K.A.; Rock, M.J.; Farrell, P.M.; Sontag, M.K.; Rosenfeld, M.; Davis, S.D.; et al. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J. Pediatr. 2009, 155 (Suppl. S6), S106–S116. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W. ECFS Neonatal Screening Working Group. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef]
- Ren, C.L.; Borowitz, D.S.; Gonska, T.; Howenstine, M.S.; Levy, H.; Massie, J.; Milla, C.; Munck, A.; Southern, K.W. Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome and Cystic Fibrosis Screen Positive, Inconclusive Diagnosis. J. Pediatr. 2017, 181, S45–S51. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15. [Google Scholar] [CrossRef]
- Southern, K.W.; Barben, J.; Gartner, S.; Munck, A.; Castellani, C.; Mayell, S.J.; Davies, J.C.; Winters, V.; Murphy, J.; Salinas, D.; et al. Inconclusive diagnosis after a positive newborn bloodspot screening result for cystic fibrosis; clarification of the harmonised international definition. J. Cyst. Fibros. 2019, 18, 778–780. [Google Scholar] [CrossRef]
- Sosnay, P.R.; Salinas, D.B.; White, T.B.; Ren, C.L.; Farrell, P.M.; Raraigh, K.S.; Girodon, E.; Castellani, C. Applying Cystic Fibrosis Transmembrane Conductance Regulator Genetics and CFTR2 Data to Facilitate Diagnoses. J. Pediatr. 2017, 181, S27–S32. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Girodon, E.; Roussel, D.; Deneuville, E.; Bui, S.; Huet, F.; Guillot, M.; Aboutaam, R.; Renouil, M.; Munck, A.; et al. Measurement of nasal potential difference in young children with an equivocal sweat test following newborn screening for cystic fibrosis. Thorax 2010, 65, 539–544. [Google Scholar] [CrossRef]
- Derichs, N.; Sanz, J.; Von Kanel, T.; Stolpe, C.; Zapf, A.; Tümmler, B.; Gallati, S.; Ballmann, M. Intestinal current measurement for diagnostic classification of patients with questionable cystic fibrosis: Validation and reference data. Thorax 2010, 65, 594–599. [Google Scholar] [CrossRef] [PubMed]
- De Winter-de Groot, K.M.; Berkers, G.; Marck-van der Wilt, R.E.P.; van der Meer, R.; Vonk, A.; Dekkers, J.F.; Geerdink, M.; Michel, S.; Kruisselbrink, E.; Vries, R.; et al. Forskolin-induced swelling of intestinal organoids correlates with disease severity in adults with cystic fibrosis and homozygous F508del mutations. J. Cyst. Fibros. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, M.; Yang, J.; Bishop, T.; Lessing, S.; Young, S.; Graham, S.; Pearl, M.; Chow, H.; Ho, T.; Currier, R.; et al. California Cystic Fibrosis Newborn Screening Consortium. Newborn Screening for Cystic Fibrosis in California. Pediatrics 2015, 136, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Groves, T.; Robinson, P.; Wiley, V.; Fitzgerald, D.A. Long-term outcomes of children with intermediate sweat chloride values in infancy. J. Pediatr. 2015, 166, 1469–1474. [Google Scholar] [CrossRef]
- Ren, C.L.; Fink, A.K.; Petren, K.; Borowitz, D.S.; McColley, S.A.; Sanders, D.B.; Rosenfeld, M.; Marshall, B.C. Outcomes of infants with indeterminate diagnosis detected by cystic fibrosis newborn screening. Pediatrics 2015, 135, e1386–e1392. [Google Scholar] [CrossRef]
- Levy, H.; Nugent, M.; Schneck, K.; Stachiw-Hietpas, D.; Laxova, A.; Lakser, O.; Rock, M.; Dahmer, M.K.; Biller, J.; Nasr, S.Z.; et al. Refining the continuum of CFTR-associated disorders in the era of newborn screening. Clin. Genet. 2016, 89, 539–549. [Google Scholar] [CrossRef]
- Terlizzi, V.; Mergni, G.; Buzzetti, R.; Centrone, C.; Zavataro, L.; Braggion, C. Cystic fibrosis screen positive inconclusive diagnosis (CFSPID): Experience in Tuscany, Italy. J. Cyst. Fibros. 2019, 18, 484–490. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Castellani, C.; Keenan, K.; Avolio, J.; Volpi, S.; Boland, M.; Kovesi, T.; Bjornson, C.; Chilvers, M.A.; Morgan, L.; et al. Inconclusive diagnosis of cystic fibrosis after newborn screening. Pediatrics 2015, 135, e1377–e1385. [Google Scholar] [CrossRef]
- Salinas, D.B.; Azen, C.; Young, S.; Keens, T.G.; Kharrazi, M.; Parad, R.B. Phenotypes of California CF Newborn Screen-Positive Children with CFTR 5T Allele by TG Repeat Length. Genet. Test. Mol. Biomark. 2016, 20, 496–503. [Google Scholar] [CrossRef]
- Munck, A.; Bourmaud, A.; Bellon, G.; Picq, P.; Farrell, P.M.; DPAM Study Group. Phenotype of children with inconclusive cystic fibrosis diagnosis after newborn screening. Pediatr. Pulmonol. 2016, 20, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Sutherland, R.; Castellani, C.; Keenan, K.; Boland, M.; Reisman, J.; Bjornson, C.; Chilvers, M.A.; van Wylick, R.; Kent, S.; et al. Immunoreactive trypsinogen levels in newborn screened infants with an inconclusive diagnosis of cystic fibrosis. BMC Pediatr. 2019, 19, 369. [Google Scholar] [CrossRef] [PubMed]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Carroll, J.C.; Tam, K.; Kerr, E.; Chakraborty, P.; Potter, B.K.; Patton, S. Psychosocial Response to Uncertain Newborn Screening Results for Cystic Fibrosis. J. Pediatr. 2017, 184, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mayell, S.J.; Munck, A.; Craig, J.V.; Sermet, I.; Brownlee, K.G.; Schwarz, M.J.; Castellani, C.; Southern, K.W. European Cystic Fibrosis Society Neonatal Screening Working Group. A European consensus for the evaluation and management of infants with an equivocal diagnosis following newborn screening for cystic fibrosis. J. Cyst. Fibros. 2009, 8, 71–78. [Google Scholar] [CrossRef]
| Positive NBS | And | Or | |
|---|---|---|---|
| CRMS [5] US | Asymptomatic infants with hypertrypsinemia at birth | Persistently intermediate sweat chloride levels 1 and fewer than 2 CF-causing CFTR mutations | Sweat chloride concentration <30 mmol/L and 2 CFTR mutations with 0 or 1 known to be CF-causing |
| CFSPID [6] Europe | Asymptomatic infants with hypertrypsinemia at birth | 0 or 1 CFTR mutation, plus intermediate sweat chloride (30–59 mmol/L) | 2 CFTR mutations, at least 1 of which has unclear phenotypic consequences, plus a normal sweat chloride (<30 mmol/L) |
| CRMS/CFSPID [8] | Infants with positive newborn screening test | Sweat chloride <30 mmol/L and 2 CFTR mutations with 0 or 1 CF-causing CFTR mutation | Sweat chloride 30–59 mmol/L and 0 or 1 CF-causing CFTR mutation |
| Kharrazi et al. [14] | Groves et al. [15] | Ren et al. [16] | Levy et al. [17] | Terlizzi et al. [18] | Ooi et al. [19] | Munck et al. [21] | |
|---|---|---|---|---|---|---|---|
| Study design | Retrospective | Retrospective case control | CFF registry | Cross sectional | Retrospective | Prospective case control | Prospective case control |
| Country | USA California | Australia | US | US Wisconsin | Italy Tuscany | Canada, Italy | France |
| Birth period | 2007–2012 | 1996–2010 | 2010–2012 | 1994–2012 | 2011–2016 | 2007–2013 | 2002–2009 |
| Follow up duration (y) | Mean 4.5 | 10 | 1 | 8 | Median 0.6 | Median 2.2 | Mean 7.4 |
| Number CF | 345 | 225 | 1540 | 300 | 32 | 80 | 63 |
| Number CRMS/CFSPID | 533 | 29 2 | 309 | 57 | 50 | 82 | 63 2 |
| CF:CRMS/CFSPID | 0.65:1 | 7.8:1 | 5:1 | 5.2:1 | 0.64:1 | 1.8:1 6 | 6.3:1 6 |
| Conversion to CF, N (%) | 20 (5.8) | 14/29 (48) matched to CF | NA 4 | NA 4 | 5 (10) | 9 (11) | 28(44) |
| Increased SCC ≥60 mmol/L | 17 | 2 3 | 5 | 2 | 8 | ||
| 2 CF causing mutations | 0 | 0 | 0 | 4 | 12 | ||
| Both criteria | 0 | 0 | 0 | 3 | 8 | ||
| Other criteria | 3 | 12 | 0 | 0 | 0 | ||
| Age at conversion (y) | Mean 2.5 ± 1.4 | Median 2 (0.2–4) | Mean 1.8 ± 1.2 | Unk 1 | |||
| Pseudomonas aeruginosa, N (%) | Unk 1 | 78.6 | 10.7 | 39 | 25 5 | 12 | 24 |
| Pancreatic insufficiency, N (%) | 3/15 (15) | 4/29 (14) | 14/309 (4.5) | 0 | 0 | 0 | 0 |
| F508del/R117H, N (%) | Unk 1 | 4/14 (29) | 80/309 (26) | 37/57 (63) | 0 | 16/82 (19.5) | 27/63 (43) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munck, A. Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis. Int. J. Neonatal Screen. 2020, 6, 19. https://doi.org/10.3390/ijns6010019
Munck A. Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis. International Journal of Neonatal Screening. 2020; 6(1):19. https://doi.org/10.3390/ijns6010019
Chicago/Turabian StyleMunck, Anne. 2020. "Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis" International Journal of Neonatal Screening 6, no. 1: 19. https://doi.org/10.3390/ijns6010019
APA StyleMunck, A. (2020). Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis. International Journal of Neonatal Screening, 6(1), 19. https://doi.org/10.3390/ijns6010019





