Abstract
Newborn screening (NBS) in Alberta is delivered by a number of government and health service entities who work together to provide newborn screening to infants born in Alberta, the Northwest Territories, and the Kitikmeot region of the Nunavut territory. The Alberta panel screens for 21 disorders (16 metabolic, two endocrine, cystic fibrosis, severe combined immunodeficiency, and sickle cell disease). NBS is a standard of care, but is not mandatory. NBS performance is monitored by the Alberta Newborn Metabolic Screening (NMS) Program and NMS Laboratory, who strive for continuous quality improvement. Performance analysis found that over 99% of registered infants in Alberta received a newborn screen and over 98% of these infants received a screen result within 10 days of age.
1. Introduction
Newborn screening (NBS) has dramatically improved the morbidity and mortality associated with the screened disorders. NBS is the first and largest example of systematic, population-wide genetic testing [1]. The history of newborn screening began in the 1960s when Robert Guthrie introduced the Guthrie card and a bacterial inhibition assay to screen for phenylketonuria (PKU) in dried blood spots [2]. During the 1970s and the 1980s, the additions of new screening conditions were limited by the available technology, which restricted screening to one-analysis, one-metabolite, one-disease assays. In the 1990s, the introduction of tandem mass spectrometry (MS/MS) into NBS laboratories revolutionized the NBS field, as this technology had the ability to screen for several different disorders by simultaneous amino acid and acylcarnitine analyses in a single blood spot [3,4,5,6,7,8,9]. As a result, NBS panels began rapidly expanding to include additional inborn errors of amino acid, organic acid, and fatty acid metabolism. Further expansion of screening panels in the 2000s introduced molecular genetic methods and point-of-care testing into newborn screening [10,11,12].
NBS in Canada originated in the 1960s, shortly after Guthrie introduced the concept of PKU screening. Canada is the second largest geographical country in the world and has a population of 35.19 million [13]. The country is divided into 10 provinces and three territories. Health care is publicly funded and falls under the provincial or territorial jurisdiction; therefore, newborn screening is managed provincially or territorially. Unfortunately, neither the screening panels nor the approaches to calculating the number of conditions included in the screening panels are harmonized within the country. Consequently, the disorders screened depend on where an infant is born and can vary from 12 to 40 disorders [14,15,16,17,18,19,20,21,22].
The province of Alberta is approximately 2.5 times greater in area than the United Kingdom of Great Britain and Northern Ireland (UK) (Figure 1); however, Alberta’s population (4.07 million) is approximately 16 times less than that of the UK [13,23]. Access to health care in Alberta has geographical challenges, with regions that are northern and rural.
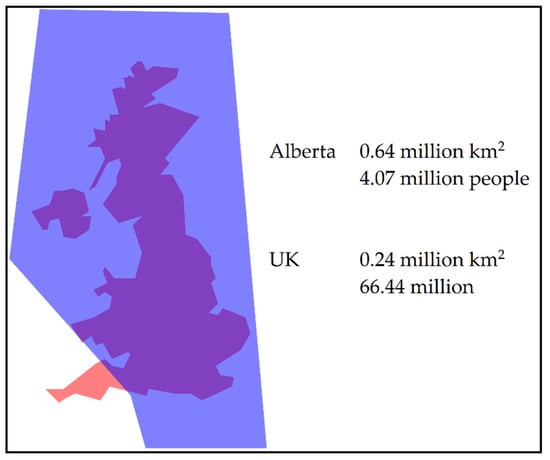
Figure 1.
Geographical comparison of the province of Alberta, Canada (blue) to the United Kingdom of Great Britain and Northern Ireland (orange) [13,23,24].
2. Alberta Newborn Metabolic Screening
The Alberta Newborn Metabolic Screening (NMS) Program is delivered by Alberta Health Services (AHS), including Alberta Public Laboratories (APL), in partnership with Alberta Health, Indigenous Services Canada’s First Nations Inuit Health Branch, physicians and midwives, as well as parents or guardians. Although referred to as Newborn Metabolic Screening in Alberta, the disorders screened are not exclusively metabolic disorders.
The Alberta NMS Laboratory is located at the University of Alberta Hospital (UAH) in the province’s capital, Edmonton, and provides screening to infants born in Alberta, the Northwest Territories, and the Kitikmeot region of the Nunavut territory. The AHS Provincial NMS Program coordinates the operational aspects of newborn screening in Alberta and utilizes a quality management framework to support the screening process. To assist with this process, Alberta launched a secure, web-based application in 2000, called NMS Application, for electronic tracking of the screening status.
Newborn screening in Alberta was implemented in 1967 with the introduction of screening for PKU, which was supplemented in 1977 and in 1990 with congenital hypothyroidism (CH) and biotinidase deficiency (BIOT) screening, respectively. In 2007, 14 disorders were added to the NMS panel: carnitine uptake defect (CUD), citrullinemia (CIT), congenital adrenal hyperplasia (CAH), cystic fibrosis (CF), glutaric acidemia type 1 (GA1), 3-hydroxy-3-methylglutaryl-CoA lyase (HMG) deficiency, isovaleric acidemia (IVA), long chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency, maple syrup urine disease (MSUD), medium chain acyl-CoA dehydrogenase (MCAD) deficiency, methylmalonic acidemia (MMA), propionic acidemia (PA), trifunctional protein (TFP) deficiency, and very long chain acyl-CoA dehydrogenase (VLCAD) deficiency. Alberta was the first jurisdiction in Canada to introduce CF to its screening panel [25]. Further additions to the panel occurred recently in 2019 with the addition of four disorders: classic galactosemia (GALT), tyrosinemia type 1 (TYR1), severe combined immunodeficiency (SCID), and sickle cell disease (SCD). Alberta is the third jurisdiction in Canada to offer newborn screening for SCID, following Ontario in 2013 and the Maritimes (New Brunswick, Nova Scotia, and Prince Edward Island) in 2016 [19,21]. As of 2019, Alberta screens for 21 treatable disorders: 16 metabolic disorders, two endocrine disorders, CF, SCID, and SCD. The disorders are screened by the NMS Laboratory, with the exception of SCID and second-tier testing for CF, which are performed by the Molecular Diagnostics Laboratory at the UAH. CF was the first assay on Alberta’s NMS panel to utilize a two-tier screen. The first tier is immunoreactive trypsinogen and the second tier is a molecular test for common pathogenic variants in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. A summary of the screened conditions and methodologies is shown in Table 1.

Table 1.
Alberta’s newborn screening panel.
3. Screening Process
In Alberta, newborn screening is voluntary; parents or guardians can exercise refusal of screening. Consent for newborn screening is verbal; however, refusal of screening must be documented by the health professional in accordance with the NMS Program policy.
The newborn screening process begins with infant registration and concludes with a negative screen result or referral to diagnostic and treatment services when a positive screen is identified (Figure 2). All infants born in Alberta are registered immediately after birth in the Person Directory (PD) application, which provides a unique lifetime identifier (ULI). The infant’s ULI is provided on the NMS requisition and is used to track the sample through the NMS Application. Both the PD and the NMS Application are maintained by Alberta Health. The NMS Application is integrated with the PD and the NMS Laboratory Information System (LIS) in order to track infants that are born in Alberta and their subsequent newborn screens. Furthermore, it is used to coordinate follow-up, both screening and clinical.
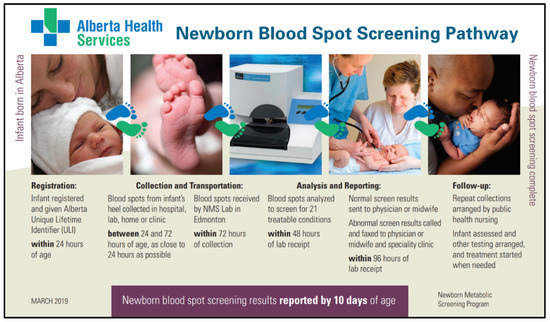
Figure 2.
Example of educational material describing the newborn blood spot screening pathway.
Collection of the blood specimens is recommended at 24 to 72 h of age, ideally closer to 24 h. The upper age limit for screening is 24 months, allowing the inclusion of infants from international adoptions and infants from new immigrant and refugee families. In addition to demographic data, the NMS requisition includes a field for information on gestational age, birth weight, feeding type, and transfusion status (Figure 3). If a newborn requires a blood transfusion, the recommendation is to collect the specimen before the transfusion, even if the infant is less than 24 h old. Blood from the infant’s heel is collected on filter paper attached to the NMS requisition and the samples are air-dried for a minimum of three hours before transport. Each NMS requisition has a fold-over cover that protects the blood spots from contamination. One of the blood spots is located on a perforated portion of the card and this portion has a barcode identical to the requisition, allowing it to be separated from the original card and transported to the Molecular Diagnostics Laboratory for SCID testing. NMS samples are collected in a variety of health care centers such as postpartum hospital units, neonatal intensive care units, special care nurseries, outpatient laboratory services, and at home by midwives or public health nursing. Often, samples require recollection due to illegible demographic information, quality of sample collection, or collection before 24 h of age. The NMS Application will generate alerts for these cases, which allows for the subsequent tracking and recollection of these samples. In the 2018–2019 reporting year, 1038 inadequate sample alerts (1.88%) were generated for 55,316 samples received, the latter including out-of-province samples and follow-up samples (Table 2). Nine hundred sixty-four (92.87%) of the inadequate samples required a repeat collection, of which 809 (89.92%) repeat collections occurred within 96 h of notification of the inadequate sample. The NMS Program provides feedback to collection sites in the form of quarterly sample quality reports to reduce the number of inadequate sample alerts.

Figure 3.
Newborn blood spot screening requisition with a detachable section, allowing for simultaneous testing in two laboratories.

Table 2.
Summary of Newborn Metabolic Screening (NMS) Application inadequate sample alerts for the 2018–2019 reporting year. Reporting year is April 1 to March 31. Samples received, including out-of-province samples and follow-up samples, were 55,316.
Samples are transported from across the province at ambient temperature in a sealed newborn screening envelope to the NMS Laboratory in Edmonton. The screening envelope is bright pink in colour for easy identification and has a space to indicate the number of specimens in the envelope, and that number is reconciled by the NMS Laboratory. A laboratory courier system and contracted courier services are used for transportation; standard mail is also occasionally used. Transport to the NMS Laboratory is to occur within 72 h of sample collection. Individual samples are not tracked during transport, but if a sample is not received by the NMS Laboratory within ten days of an infant’s birth, an alert is generated in the NMS Application.
Upon receipt in the NMS Laboratory, the specimens are barcoded and the demographic information, feeding type, and transfusion status from the requisition are entered into the LIS. The LIS communicates the demographic information to the NMS Application, where the birth registration is linked to the sample in order to track screening. The NMS Program monitors alerts and coordinates the collection of any missing samples. If a sample is received by the laboratory and a ULI has not been assigned to the infant, an alert is generated in the NMS Application and the NMS Program follows up with Alberta Health to ensure that the infant is registered. Samples are to be analyzed within 48 h and both normal and abnormal results reported within 96 h of receipt (including weekends and holidays) in the laboratory. For those samples requiring second-tier molecular genetic testing, results are to be reported within 21 days of receipt in the laboratory.
Samples with abnormal initial results are re-tested the next day for confirmation, with the exception of CF and GALT. There is no confirmation testing for CF. The confirmation and second-tier test for GALT is performed on the same day. The screening algorithms have two types of abnormal results: borderline or critical; however, not all disorders have borderline results. Borderline results on the initial specimen for BIOT, CUD, organic acidemias, and endocrine disorders are followed up with a second NMS collection. The NMS Application generates an alert for samples requiring recollection due to borderline results. If the second collection confirms the initial borderline result, the screen is called out as critical. Critical results require immediate follow-up by the responsible health care provider and the respective specialty clinic. Infants on total parenteral nutrition (TPN) may require a recollection 24 h post-discontinuation of TPN. All low birth weight (<2000 g) infants are recommended to have blood spots recollected at 21–28 days, even if the initial screen results are normal. Additionally, some premature infants with inadequate T cell development will be flagged for SCID re-testing 21–28 days after birth.
After completion of the screening, the NMS Laboratory LIS sends screening results to the NMS Application. If a result is not reported within 25 days of sample receipt by the NMS Laboratory, an alert is generated by the NMS Application for the laboratory to follow up on the testing status. Clinical follow-up for critical results is tracked in the NMS Application through alerts. The NMS samples are retained for 15 years, unless otherwise directed by the parent or guardian. Any additional non-newborn screening-related testing requires parent or guardian consent for sample use.
4. Alberta NMS 2019 Panel Expansion
The newborn screening panel was expanded to include GALT, SCD, and TYR1 on April 1, 2019, and SCID on May 31, 2019.
GALT utilizes a two-tier screen, where the first tier measures GALT enzyme activity and the second tier measures total galactose concentration. The first tier is based on the Beutler spot assay and uses four enzymatic steps, including an initial GALT enzyme step [26]. A deficiency in any of these enzymes can result in an apparent decrease in GALT enzyme activity [27]. An advantage of the second tier, measuring total galactose, is that the incidental finding of galactose-6-phosphate dehydrogenase (G6PD) deficiency is not called out as a positive screen for classic galactosemia.
Screening for SCD includes screening for sickle cell disease (HbSS), hemoglobin SC disease (HbSC), and hemoglobin S/beta thalassemia (HbSβ− or HbSβ+). Sickle cell carriers (HbAS) are reported out as a positive sickle cell trait. All samples collected post-transfusion are sent for molecular genetic analysis.
The SCID screening generally follows the Wisconsin procedure [28]. An initial screen analyzing only the TREC copy number is performed and those that fall below 70 copies per microliter are duplicate screened using a multiplex assay which includes TREC and the RNaseP control gene. Those infants with adequate DNA and a TREC value below 40 copies per microliter are flagged for clinical follow-up.
TYR1 is screened by measuring succinylacetone using MS/MS.
5. Key Performance Measures and Outcomes from Alberta Newborn Screening
The NMS Program monitors and reports on the performance of newborn screening in Alberta using data generated from the NMS Application and the NMS Laboratory. Table 3 includes some of the performance measures reported by the NMS Program for the last three fiscal years. Over 99% of registered infants in Alberta received a newborn screen. The most common reason for not receiving a screen on a registered infant is neonatal death; other reasons include refusal by the parent or guardian or physician, inability to locate the infant, and relocation of the infant out-of-province. Over 98% of registered infants have results reported within 10 days of age. Approximately 29% of infants with abnormal screens receive an abnormal diagnostic outcome. In the 2018–2019 reporting year, 55 of 188 abnormal screens with follow-up completed were confirmed to have the associated disorder (Table 4). Diagnostic outcomes are communicated to the families by the respective specialty clinic, who also follow up with the patients, as required. The NMS Program is working towards implementation of tracking long-term outcomes of newborn screening.

Table 3.
Summary of NMS Program key performance measures. Reporting year is from April 1 to March 31.

Table 4.
2018–2019 Diagnostic Outcome Summary for 52,099 infants screened. 1
6. Conclusions
The framework, design, and execution of the NMS Program is vital in ensuring that all infants born in Alberta are offered screening. Since the inception of newborn screening in Alberta in 1967 with screening for PKU, there have been several expansions, as recent as 2019, resulting in the current panel of 21 disorders. Due to disparity in newborn screening in Canada, infants’ health outcomes can be influenced by their province or territory of birth. The need for a national newborn screening strategy has been recognized. Despite meetings among the provinces and territories to create a national consensus, newborn screening panels remain non-uniform across Canada [29].
Author Contributions
Writing—original draft preparation, A.D.S. and V.W.; writing—review and editing, A.D.S., I.S. and V.W.; review—all authors.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully acknowledge Alberta Health Services, Alberta Health, Indigenous Services Canada’s First Nations Inuit Health Branch, physicians and midwives, and parents or guardians for their participation in the delivery of newborn screening in Alberta.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, N.S.; Dolan, S.M.; Murray, T.H. Newborn screening: Complexities in universal genetic testing. Am. J. Public Health 2006, 96, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.; Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [PubMed]
- Chace, D.H.; Millington, D.S.; Terada, N.; Kahler, S.G.; Roe, C.R.; Hofman, L.F. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin. Chem. 1993, 39, 66–71. [Google Scholar] [PubMed]
- Chace, D.H.; Sherwin, J.H.; Hillman, S.L.; Lorey, F.; Cunningham, G.C. Use of phenylalanine-to-tyrosine ratio determined by tandem mass spectrometry to improve newborn screening for phenylketonuria of early discharge specimens collected in the first 24 hours. Clin. Chem. 1998, 44, 2405–2409. [Google Scholar] [PubMed]
- Millington, D.S.; Kodo, N.; Norwood, D.L.; Roe, C.R. Tandem mass spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis. 1990, 13, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Millington, D.S.; Kodo, N.; Terada, N.; Roe, D.; Chase, D.H. The analysis of diagnostic markers of genetic disorders in human blood and urine using tandem mass spectrometry with liquid secondary ion mass spectrometry. Int. J. Mass Spectrom. Ion Process. 1991, 111, 211228. [Google Scholar] [CrossRef]
- Chace, D.H.; Hillman, S.L.; Millington, D.S.; Kahler, S.G.; Roe, C.R.; Naylor, E.W. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin. Chem. 1995, 41, 62–68. [Google Scholar] [PubMed]
- Chace, D.H.; Hillman, S.L.; Millington, D.S.; Kahler, S.G.; Adam, B.W.; Levy, H.L. Rapid diagnosis of homocystinuria and other hypermethioninemias from newborns’ blood spots by tandem mass spectrometry. Clin. Chem. 1996, 42, 349–355. [Google Scholar] [PubMed]
- Chace, D.H.; Hillman, S.L.; Van Hove, J.L.; Naylor, E.W. Rapid diagnosis of MCAD deficiency: Quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn blood spots by tandem mass spectrometry. Clin. Chem. 1997, 43, 21062113. [Google Scholar]
- Bobadilla, J.L.; Macek, M., Jr.; Fine, J.P.; Farrell, P.M. Cystic fibrosis: A worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum. Mutat. 2002, 19, 575–606. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.C.; McPhillips, H.; Davis, R.L.; Lieu, T.L.; Homer, C.J.; Helfand, M. Universal newborn hearing screening: Summary of evidence. JAMA 2001, 286, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Mahle, W.T.; Newburger, J.W.; Matherne, G.P.; Smith, F.C.; Hoke, T.R.; Koppel, R.; Gidding, S.S.; Beekman, R.H., 3rd; Grosse, S.D.; American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the AHA and AAP. Circulation 2009, 120, 447–458. [Google Scholar] [PubMed]
- Statistics Canada. Available online: https://www.statcan.gc.ca/eng/start (accessed on 10 July 2019).
- Perinatal Services BC. Available online: http://www.perinatalservicesbc.ca/our-services/screening-programs/newborn-screening-program (accessed on 10 July 2019).
- Newborn Metabolic Screening. Available online: https://www.alberta.ca/newborn-metabolic-screening.aspx (accessed on 10 July 2019).
- Winnipeg Regional Health Authority. Available online: http://www.wrha.mb.ca/prog/genetics/newborn-screening.php (accessed on 10 July 2019).
- Dery, M.C. (Shared Health Manitoba, Winnipeg, Manitoba, Canada). Personal communication, 2019.
- Saskatchewan Health Authority. Available online: https://www.saskhealthauthority.ca/Services-Locations/RRPL (accessed on 10 July 2019).
- Newborn Screening Ontario. Available online: https://www.newbornscreening.on.ca/en (accessed on 10 July 2019).
- Neonatal Blood and Urine Screening Program –Services Québec. Available online: http://www4.gouv.qc.ca/EN/Portail/Citoyens/Evenements/DevenirParent/Pages/progr_depst_neont_sangn.aspx (accessed on 10 July 2019).
- Maritime Newborn Screening Program. Available online: http://www.iwk.nshealth.ca/newbornscreening (accessed on 10 July 2019).
- Newfoundland and Labrador Medical Association. Available online: http://www.nlma.nl.ca/News-And-Events/Notices-And-Advisories/Page/0/Article/238 (accessed on 10 July 2019).
- Office for National Statistics. Available online: https://www.ons.gov.uk/ (accessed on 10 July 2019).
- MapFight. Available online: https://mapfight.appspot.com/ (accessed on 18 July 2017).
- Lilley, M.; Christian, S.; Hume, S.; Scott, P.; Montgomery, M.; Semple, L.; Zuberbuhler, P.; Tabak, J.; Bamforth, F.; Somerville, M.J. Newborn screening for cystic fibrosis in Alberta: Two years of experience. Paediatr. Child Health 2010, 15, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Baluda, M.C. A simple spot screening test for galactosemia. J. Lab. Clin. Med. 1966, 68, 137–141. [Google Scholar] [PubMed]
- Stuhrman, G.; Perez Juanazo, S.J.; Crivelly, K.; Smith, J.; Anderson, H.; Morava, E. False-positive newborn screen using the Beutler spot assay for galactosemia in glucose-6-phosphate dehydrogenase deficiency. JMID Rep. 2017, 36, 1–5. [Google Scholar]
- Baker, M.W.; Grossman, W.J.; Laessig, R.H.; Hoffman, G.L.; Brokopp, C.D.; Kurtycz, D.F.; Cogley, M.F.; Litsheim, T.J.; Katcher, M.L.; Routes, J.M. Development of a routine newborn screening protocol for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2009, 124, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.C.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).