Wisconsin’s Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Dried Blood Specimens
2.2. Reagents and Standards
2.3. Quality Control and Calibrators
2.4. First-Tier Screening Assay for the Quantification of 17-Hydroxyprogesterone
2.5. Second-Tier Screening Assay for Quantification of Five Steroids
2.6. Analysis
3. Results
3.1. Method Validation
3.2. Normal Distribution
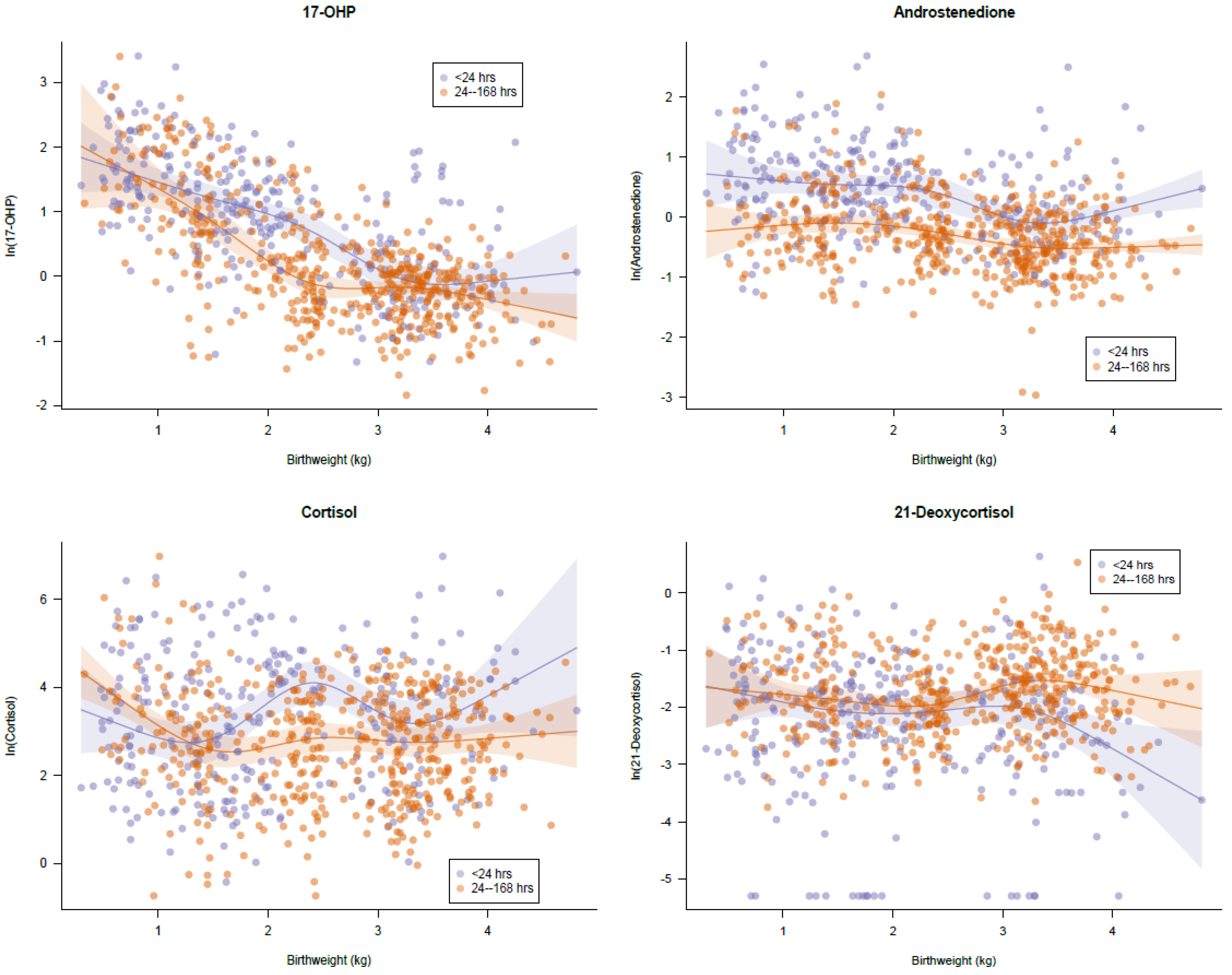
3.3. Retrospective Analysis
3.3.1. Confirmed Cases and False Positives
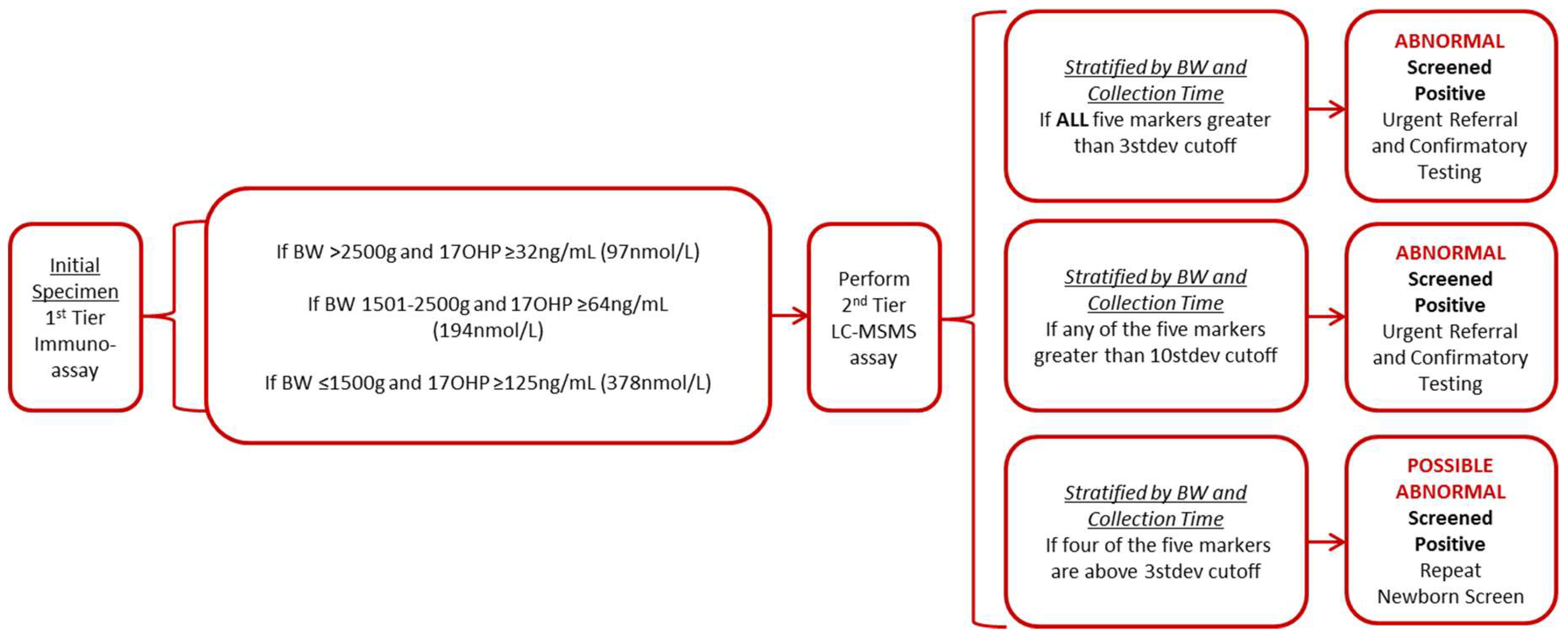
3.3.2. Wisconsin Algorithm
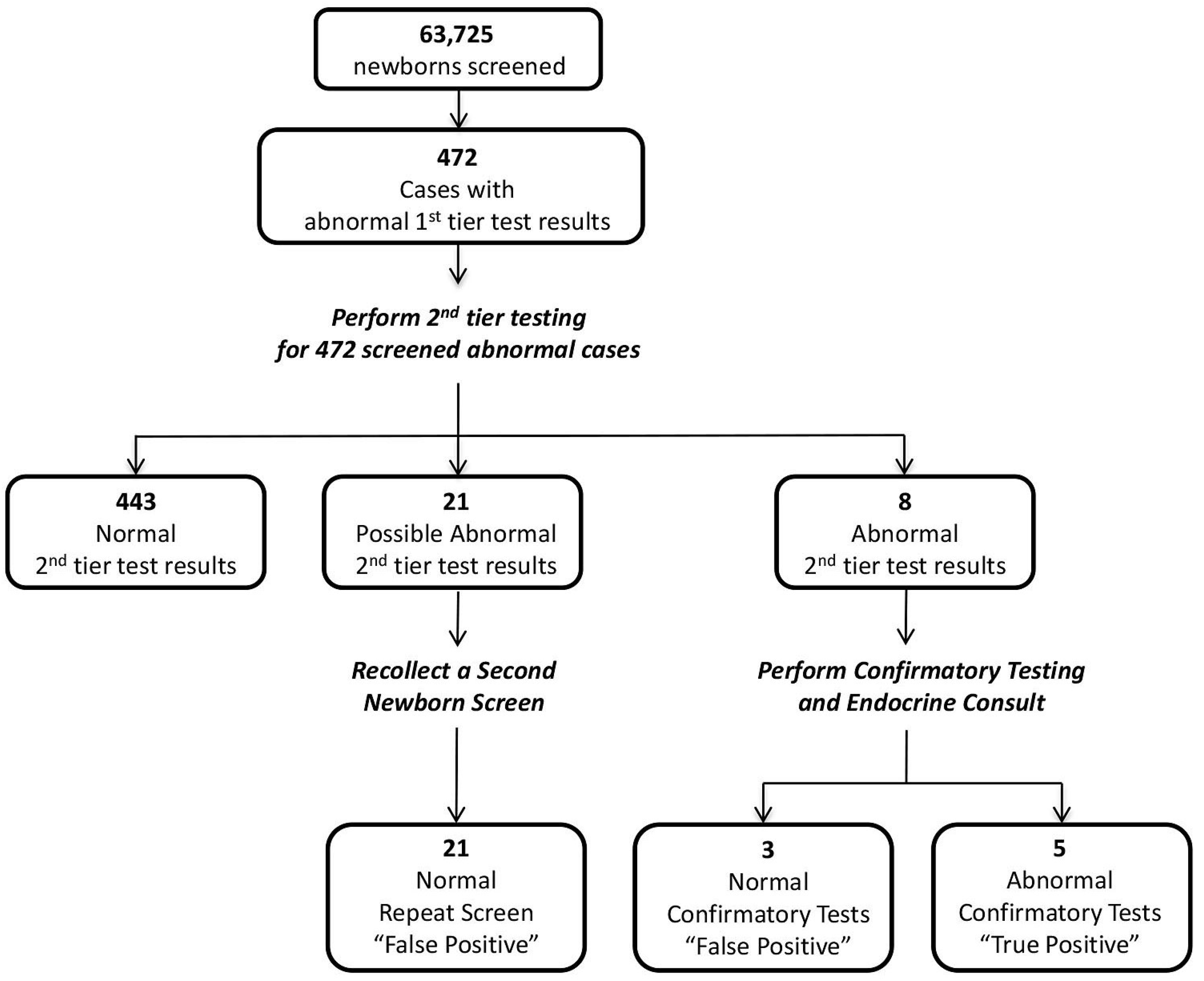
3.4. Prospective Study Using the Proposed Wisconsin Algorithm
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Maouche, D.; Arlt, W.; Merke, D.D. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef]
- Falhammar, H.; Wedell, A.; Nordenstrom, A. Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine 2015, 50, 306–314. [Google Scholar] [CrossRef]
- Speiser, P.; White, P.P. Congenital adrenal hyperplasia. N. Engl. J. Med. 2003, 349, 776–788. [Google Scholar] [CrossRef]
- Pang, S.; Hotchkiss, J.; Drash, A.A.; Levine, L.L.; New, M.M. Microfilter paper method for 17α-hydroxyprogesterone radioimmunoassay: Its application for rapid screening for congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 1977, 45, 1003–1008. [Google Scholar] [CrossRef]
- White, P.P. Neonatal screening for congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 2009, 5, 490–498. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, H.H.; White, P.C.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practic guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- White, P.P. Optimizing newborn screening for congenital adrenal hyperplasia. J. Pediatr. 2013, 163, 10–12. [Google Scholar] [CrossRef]
- Wong, T.; Shackleton, C.H.L.; Covey, T.T.; Ellis, G. Identification of the steroids in neonatal plasma that interfere with 17α-hydroxyprogesterone radioimmunoassays. Clin. Chem. 1992, 38, 1830–1837. [Google Scholar] [PubMed]
- Lee, M.; Rajagopalan, L.; Berg, G.G.; Moshang, T., Jr. Serum adrenal steroid concentrations in premature nfants. J. Clin. Endocrinol. Metab. 1989, 69, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Betts, P.; Wood, P.P. The interpretation of bloodspot 17-α-hydroxyprogesterone levels in term and pre-term neonates. Ann. Clin. Biochem. 1986, 23, 546–551. [Google Scholar] [CrossRef]
- Allen, D.D.; Hoffman, G.G.; Fitzpatrick, P.; Laessig, R.; Maby, S.; Slyper, A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J. Pediatr. 1997, 130, 128–133. [Google Scholar] [CrossRef]
- Van der Kamp, H.J.; Oudshoorn, C.G.; Elvers, B.H.; van Baarle, M.; Otten, B.J.; Wit, J.M.; Verkerk, P.H. Cutoff levels of 17-α-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J. Clin. Endocrinol. Metab. 2005, 90, 3904–3907. [Google Scholar] [CrossRef] [PubMed]
- Olgemoller, B.; Roscher, A.A.; Liebl, B.; Fingerhut, R. Screening for congenital drenal hyperplasia; adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J. Clin. Endocrinol. Metab. 2003, 88, 5790–5794. [Google Scholar] [CrossRef]
- Held, P.K.; Shapira, S.K.; Hinton, C.F.; Jones, E.; Hannon, W.H.; Ojodu, J. Congenital adrenal hyperplasia cases identified by newborn screening in one- or two-screen states. Mol. Genet. Metab. 2015, 116, 133–138. [Google Scholar] [CrossRef]
- Lacey, J.M.; Minutti, C.Z.; Magera, M.J.; Tauscher, A.L.; Casetta, B.; McCann, M.; Lymp, J.; Hahn, S.H.; Rinaldo, P.; Matern, D. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin. Chem. 2004, 50, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Liu, A.; Randall, H.; Haslip, C.; Keune, F.; Murray, M.; Longo, N.; Pasquali, M. Use of steroid profiling by UPLC-MS/MS as a second-tier test in newborn screening for congenital adrenal hyperplasia: The Utah experience. Pediatr. Res. 2009, 66, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.; Ho, T.; Rich, P.; Xu, D.; Lorey, F.; She, J.; Bhandal, A. An automated method on analysis of blood steroids using liquid chromatography–tandem mass spectrometry: Application to population screening for congenital adrenal hyperplasia in newborns. Clin. Chim. Acta 2011, 412, 2076–2084. [Google Scholar] [CrossRef]
- Janzen, N.; Sander, S.; Terhardt, M.; Pter, M.; Sander, J. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 861, 117–122. [Google Scholar] [CrossRef]
- Boelen, A.; Ruiter, A.F.; Claahsen-van der Grinten, H.L.; Endert, E.; Ackermans, M.T. Determination of a steroid profile in heel prick blood using LC–MSMS. Bioanalysis 2016, 8, 375–384. [Google Scholar] [CrossRef]
- Fiet, J.; Le Bouc, Y.; Guéchot, J.; Hélin, N.; Maubert, M.A.; Farabos, D.; Lamazière, A. A liquid chromatography/Tandem mass spectrometry profile of 16 serum steroids, including 21-deoxycortisol and 21-deoxycorticosterone, for management of congenital adrenal hyperplasia. J. Endocr. Soc. 2017, 1, 186–201. [Google Scholar] [CrossRef]
- Costa-Barbosa, F.A.; Carvalho, V.M.; Nakamura, O.H.; Bachega, T.A.S.S.; Vieira, J.G.H.; Kater, C.E. Zona fasciculata 21-hydroxysteroids and precursor-to-product ratios in 21-hydroxylse deficiency: Further characterization of classic and non-classic patients and heterozygote carriers. J. Endocrinol. Investig. 2011, 34, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.; Peter, M.; Sander, S.; Steuerwald, U.; Terhardt, M.; Holtkamp, U.; Sander, J. Newborn screening for congenital adrenal hyperplasia: Additional steroid profile using liquid chromatography-tandem mass spectrometry. J. Clin. Endocrinol. Metab. 2007, 92, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Güran, T.; Tezel, B.; Gürbüz, F.; Eklioğlu, B.S.; Hatipoğlu, N.; Kara, C.; Şimşek, E.; Çizmecioğlu, F.M.; Ozon, A.; Aydın, M.; et al. Neonatal screening for congenital adrenal hyperplasia in turkey: A pilot study with 38,935 infants. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bodegard, G.; Fyro, K.; Larsson, A. Psychological reactions in 102 families with a newborn who has a falsely positive screening test for congenital hypothyroidism. Acta Paediatr. Scand. Suppl. 1983, 304, 1–21. [Google Scholar] [CrossRef]
- Kwon, C.; Farrell, P.P. The magnitude and challenge of false-positive newborn screening test results. Arch. Pediatr. Adolesc. Med. 2000, 154, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Favrilov, D.; Rinaldo, P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: The Mayo Clinic experience (2004–2007). J. Inherit. Metab. Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.Z.; Lacey, J.M.; Magera, M.J.; Hahn, S.H.; McCann, M.; Schulze, A.; Cheillan, D.; Dorche, C.; Chace, D.H.; Zimmerman, D.; et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2004, 89, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Varness, T.T.; Allen, D.D.; Hoffman, G.G. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. J. Pediatr. 2005, 147, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Berenbaum, S.A.; Manter-Kapanke, V.; Simmank, J.; Korman, K.; Prentice, L.; Prentice, L.; Gonzalez, J.; Gunn, S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 1998, 101 Pt 1, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Sarafoglou, K.; Banks, K.; Kyllo, J.; Pittock, S.; Thomas, W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA 2012, 307, 2371–2374. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Banks, K.; Gaviglio, A.; Hietala, A.; McCann, M.; Thomas, W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics 2012, 130, e1261–e1268. [Google Scholar] [CrossRef] [PubMed]
- Sarafoglou, K.; Gaviglio, A.; Hietala, A.; Forgner, G.; Banks, K.; McCann, M.; Thomas, W. Comparison of Newborn Screening Protocols for Congenital Adrenal Hyperplasia in Preterm Infants. J. Pediatr. 2014, 164, 1136–1140. [Google Scholar] [CrossRef]
- Tang, H.; Feuchtbaum, L.; Neogi, P.; Ho, T.; Gaffney, L.; Currier, R.R. Damaged goods? An empirical cohort study of blood specimens collected 12 to 23 hours after birth in newborn screening in California. Genet. Med. 2016, 18, 259–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, J.Y.; Park, H.D.; Kim, J.W.; Oh, H.J.; Yang, J.S.; Chang, Y.S.; Park, W.S.; Lee, S.Y. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduced follow-up burdens in a tertiary care hospital; A retrospective and prospective evaluation. J. Perinat. Med. 2014, 42, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, G.; Currier, R.; McHugh, D.M.; Gavrilov, D.; Magera, M.J.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S.; et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet. Med. 2012, 14, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kopacek, C.; Prado, M.J.; da Silva, C.M.; de Castro, S.M.; Beltrão, L.A.; Vargas, P.R.; Grandi, T.; Rossetti, M.L.R.; Spritzer, P.M. Clinical and molecular profile of newborns with confirmed or suspicious congenital adrenal hyperplasia detected after a public screening program implementation. J. Pediatr. 2019, 95, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Nordenstrom, A.; Wedell, A.; Hagenfeldt, L.; Marcus, C.; Larsson, A. Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics 2001, 108, E68. [Google Scholar] [CrossRef]
| Steroids and Internal Standards | MRM Transitions | Retention Time (min) | Collision Energy (V) |
|---|---|---|---|
| 17-hydroxyprogesterone (Q) | 331.2→97 | 3.1 | 60 |
| 17-hydroxyprogesterone (I) | 331.2→109 | 60 | |
| Cortisol (Q) | 363.3→121.2 | 2.25 | 30 |
| Cortisol (I) | 363.3→327.1 | 24 | |
| Androstenedione (Q) | 287.2→97 | 2.9 | 28 |
| Androstenedione (I) | 287.2→109 | 31 | |
| 21-Deoxycortisol (Q) | 347.3→311.2 | 2.5 | 25 |
| 21-Deoxycortisol (I) | 347.3→109.01 | 46 | |
| D8-17-hydroxygrogesterone | 339.2→113.1 | 3.1 | 47 |
| D4-Cortisol | 367.1→121.1 | 2.25 | 34 |
| D7-Androstenedione | 294.2→100.1 | 2.9 | 27 |
| D8 21-Deoxycortisol | 355.2→319.2 | 2.5 | 25 |
| Time (min) | %B | Flow Rate (mL/min) |
|---|---|---|
| 0.01 | 42.5 | 0.25 |
| 4.4 | 100 | 0.25 |
| 4.45 | 100 | 0.25 |
| 4.5 | 100 | 0.5 |
| 6.5 | 100 | 0.5 |
| 6.55 | 100 | 0.25 |
| 6.6 | 100 | 0.25 |
| 6.61 | 42.5 | 0.25 |
| 8.7 | 42.5 | 0.25 |
| 17-Hydroxyprogesterone (ng/mL) | 4-Androstenedione (ng/mL) | (17-OHP + 4-A)/F) | (17-OHP + 21)/F) | 21-Deoxycortisol (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| BW (g) | 24–168 h | <24 h | 24–168 h | <24 h | No Stratification by Timing | No Stratification by Timing | No Stratification by Timing or BW | |
| ≤1500 | count | 103 | 106 | 103 | 106 | 209 | 209 | 783 |
| median | 3.31 | 4.55 | 0.88 | 1.84 | 0.26 | 0.19 | 0.15 | |
| mean | 5.05 | 6.16 | 1.20 | 2.52 | 0.74 | 0.57 | 0.21 | |
| 3stdev | 18.72 | 20.99 | 4.56 | 8.64 | 5.43 | 4.52 | 0.78 | |
| 0.99 | 18.75 | 25.31 | 5.83 | 8.60 | 6.13 | 4.44 | 0.92 | |
| 1501–2499 | count | 121 | 99 | 121 | 99 | 220 | 220 | |
| median | 1.07 | 2.79 | 0.81 | 1.62 | 0.15 | 0.10 | ||
| mean | 1.65 | 3.18 | 1.07 | 2.07 | 0.41 | 0.28 | ||
| 3stdev | 6.26 | 9.05 | 3.80 | 7.92 | 2.59 | 1.86 | ||
| 0.99 | 6.91 | 9.12 | 4.16 | 12.25 | 2.45 | 1.63 | ||
| ≥2500 | count | 256 | 98 | 256 | 98 | 354 | 354 | |
| median | 0.81 | 1.01 | 0.62 | 0.98 | 0.08 | 0.05 | ||
| mean | 0.89 | 1.56 | 0.71 | 1.42 | 0.17 | 0.12 | ||
| 3stdev | 2.30 | 6.26 | 1.92 | 5.98 | 0.85 | 0.62 | ||
| 0.99 | 2.50 | 6.98 | 2.02 | 6.45 | 1.00 | 0.76 | ||
| Patient # | 17-OHP | Birthweight (g) | Age at Collection (h) | 17-OHP | 4-A | 21 | (17-OHP + 4-A)/F) | (17-OHP + 21)/F) | Form of CAH |
|---|---|---|---|---|---|---|---|---|---|
| (ng/mL) | (ng/mL) | (ng/mL) | (ng/mL) | ||||||
| 1st tier | 2nd tier | 2nd tier | 2nd tier | 2nd tier | 2nd tier | ||||
| 1 | >189 | 3780 | 56 | 84.70 | 55.10 | 50.50 | 6.99 | 6.76 | Classical-SW |
| 2 | 116 | 3030 | 53 | 13.90 | 17.20 | 5.75 | 2.16 | 1.36 | Classical-SV |
| 3 | >189 | 3062 | 24 | 60.10 | 8.97 | 38.60 | 12.16 | 17.38 | Classical-SW |
| 4 | >220 | 4190 | 26 | 48.20 | 10.40 | 13.90 | 2.56 | 2.71 | Classical-SW |
| 5 | 125 | 3677 | 25 | 20.40 | 6.19 | 16.00 | 1.44 | 1.97 | Classical |
| 6 | >220 | 3602 | 24 | 33.90 | 9.51 | 5.18 | 1.56 | 1.41 | Classical-SW |
| 7 | >220 | 2840 | 27 | 71.10 | 38.70 | 11.60 | 20.26 | 15.26 | Classical-SW |
| 8 | 62 | 2860 | 24 | 7.23 | 3.00 | 1.63 | 0.99 | 0.86 | Classical-SW |
| 9 | 48 | 4079 | 25 | 6.14 | 2.63 | 0.83 | 1.08 | 0.86 | Classical-SW |
| 10 | 156 | 2730 | 45 | 14.10 | 2.87 | 3.66 | 1.17 | 1.22 | Classical |
| 11 | 72 | 4100 | 36 | 8.56 | 2.37 | 5.69 | 1.64 | 2.14 | Classical |
| 12 | 33 | 3340 | 24 | 3.02 | 1.65 | 4.98 | 0.32 | 0.56 | Non-classical |
| 13 | 425 | 2850 | 48 | 294.00 | 364.00 | 15.80 | 37.82 | 17.80 | Classical-SW |
| 14 | 365 | 3470 | 25 | 78.50 | 43.40 | 11.30 | 9.03 | 6.65 | Classical |
| 15 | 418 | 3157 | 37 | 395.00 | 20.60 | 68.10 | 28.66 | 31.94 | Classical-SW |
| 16 | 53 | 1180 | 2 | 19.10 | 2.68 | 1.14 | 34.57 | 32.13 | Classical-SV |
| 17 | 129 | 3610 | 45 | 19.90 | 4.45 | 22.00 | 1.25 | 2.15 | Classical |
| 18 | 96 | 3735 | 33 | 10.60 | 3.07 | 1.36 | 4.08 | 3.57 | Classical-SV |
| 19 | >200 | 3530 | 24 | 359.00 | 76.60 | 38.90 | 28.10 | 25.67 | Classical |
| 20 | >200 | 3055 | 45 | 135.00 | 275.00 | 2.15 | 31.06 | 10.39 | Classical-SW |
| 21 | >200 | 2650 | 35 | 172.00 | 234.00 | 6.14 | 57.67 | 25.30 | Classical |
| 22 | 123 | 4100 | 63 | 21.30 | 14.60 | 7.48 | 3.55 | 2.85 | Classical |
| 23 | >200 | 2970 | 27 | 74.10 | 64.60 | 23.10 | 8.46 | 5.93 | Classical-SW |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bialk, E.R.; Lasarev, M.R.; Held, P.K. Wisconsin’s Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia. Int. J. Neonatal Screen. 2019, 5, 33. https://doi.org/10.3390/ijns5030033
Bialk ER, Lasarev MR, Held PK. Wisconsin’s Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia. International Journal of Neonatal Screening. 2019; 5(3):33. https://doi.org/10.3390/ijns5030033
Chicago/Turabian StyleBialk, Eric R., Michael R. Lasarev, and Patrice K. Held. 2019. "Wisconsin’s Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia" International Journal of Neonatal Screening 5, no. 3: 33. https://doi.org/10.3390/ijns5030033
APA StyleBialk, E. R., Lasarev, M. R., & Held, P. K. (2019). Wisconsin’s Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia. International Journal of Neonatal Screening, 5(3), 33. https://doi.org/10.3390/ijns5030033




