Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
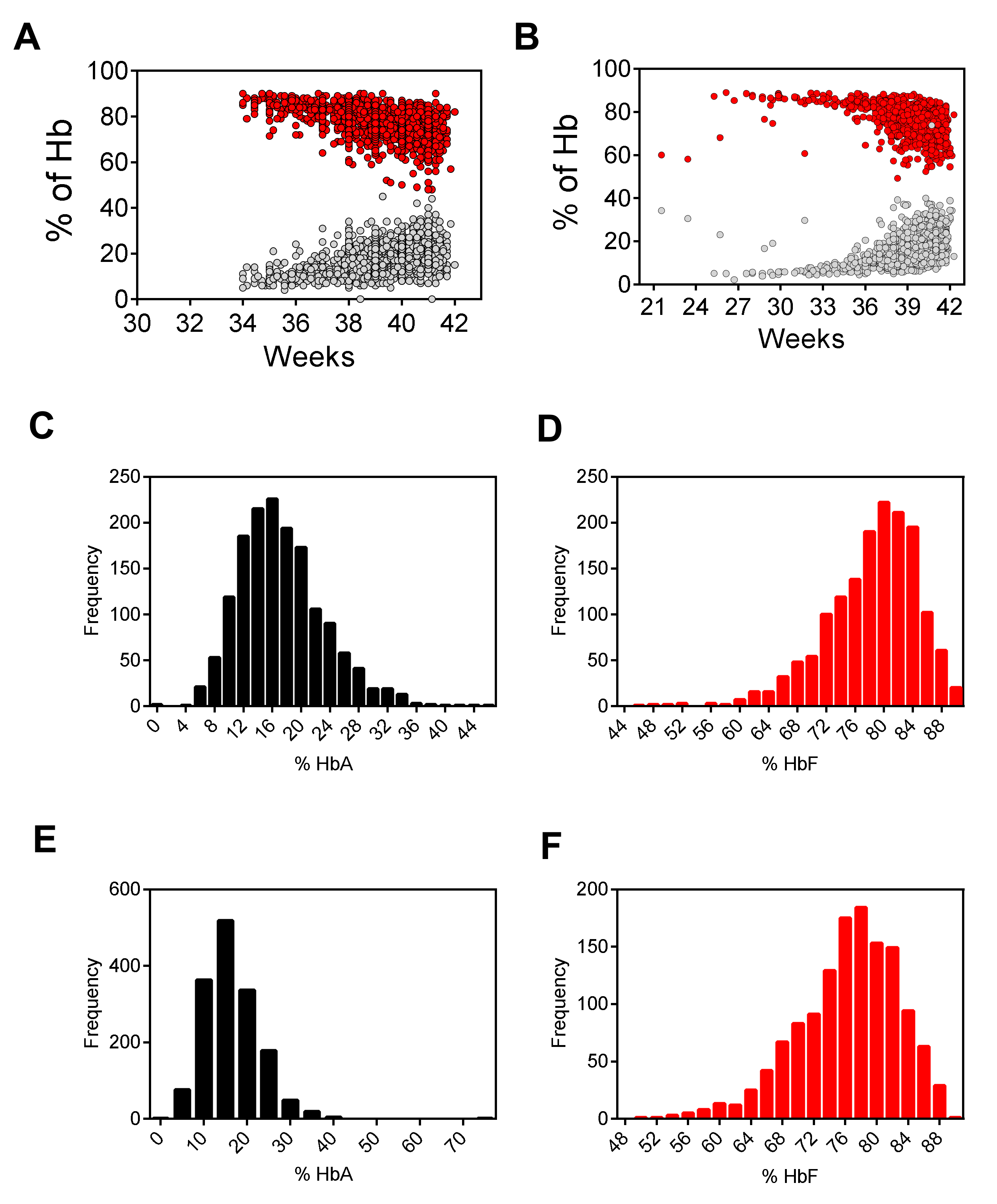
2.2. High Performance Liquid Chromatography (HPLC)
2.3. Molecular Analysis of the β-globin Gene
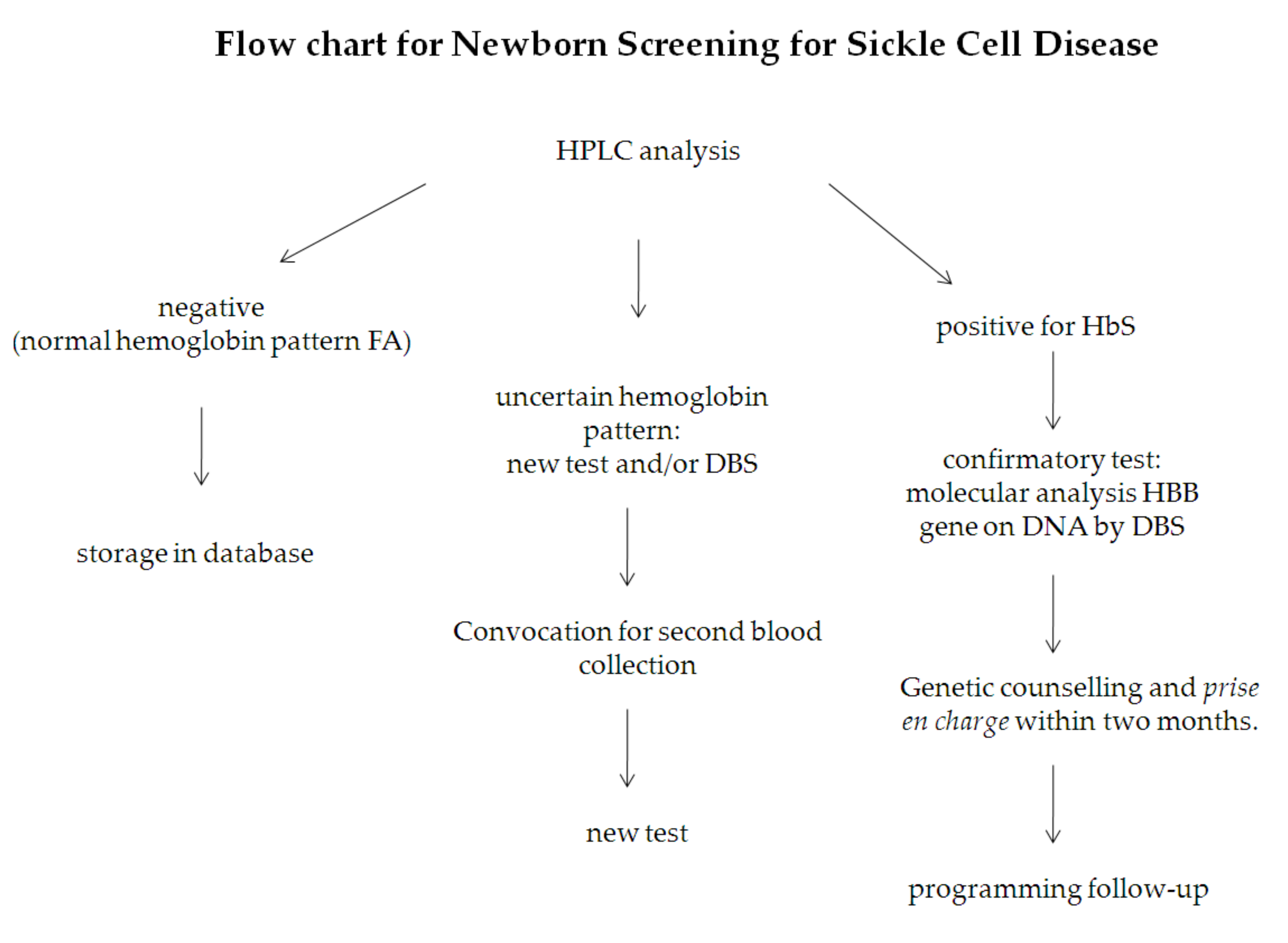
2.4. Management of Results
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- National Health System (NHS). Sicke Cell Disease in Childhood. Standard and Guidelines for clinical Care. 2nd Edition 2010. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/408961/1332-SC-Clinical-Standards-WEB.pdf (accessed on 20 December 2018).
- De Montalembert, M.; Ferster, A.; Colombatti, R.; Rees, D.C.; Gulbis, B. European Network for Rare and Congenital Anemias. ENERCA clinical recommendations for disease management and prevention of complications of sickle cell disease in children. Am J. Hematol. 2011, 86, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Perrotta, S.; Samperi, P.; Casale, M.; Masera, N.; Palazzi, G.; Sainati, L.; Russo, G.; Italian Association of Pediatric Hematology-Oncology (AIEOP) Sickle Cell Disease Working Group. Organizing national responses for rare blood disorders: The Italian experience with sickle cell disease in childhood. Orphanet J. Rare Dis. 2013, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.R.G.; Sainati, L.; Venturelli, D. SITE-AIEOP Recommendations for Sickle Cell Disease Neonatal Screening. Collana Scientifica Site n°5. Available online: http://www.site-italia.org/collana_scientifica.php (accessed on 20 December 2018).
- Lobitz, S.; Telfer, P.; Cela, E.; Allaf, B.; Angastiniotis, M.; Backman, J.C.; Badens, C.; Bento, C.; Bouva, M.J.; Canatan, D.; et al. Newborn screening for sickle cell disease in Europe: Recommendations from a Pan-European Consensus Conference. Br. J. Haematol. 2018, 183, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.aismme.org (accessed on 20 December 2018).
- De Zen, L.; Dall’Amico, R.; Sainati, L.; Colombatti, R.; Testa, E.R.; Catapano, R.; Zanolli, F. Screening neonatale per le emoglobinopatie su Dried Blood Spot. In Proceedings of the XXXVI Congresso Nazionale Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP), Pisa, Italy, 6–8 June 2010. [Google Scholar]
- Rolla, R.; Castagno, M.; Zaffaroni, M.; Grigollo, B.; Colombo, S.; Piccotti, S.; Dellora, C.; Bona, G.; Bellomo, G. Neonatal screening for sickle cell disease and other hemoglobinopathies in “the changing Europe”. Clin. Lab. 2014, 60, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, D.; Lodi, M.; Palazzi, G.; Bergonzini, G.; Doretto, G.; Zini, A.; Monica, C.; Cano, M.C.; Ilaria, M.; Montagnani, G.; et al. Sickle cell disease in areas of immigration of high-risk populations: A low cost and reproducible method of screening in northern Italy. Blood Transfus. 2014, 12, 346–351. [Google Scholar] [PubMed]
- Ballardini, E.; Tarocco, A.; Marsella, M.; Bernardoni, R.; Carandina, G.; Melandri, C.; Guerra, G.; Patella, A.; Zucchelli, M.; Ferlini, A.; et al. Universal neonatal screening for sickle cell disease and other haemoglobinopathies in Ferrara, Italy. Blood Transfus. 2013, 11, 245–249. [Google Scholar] [PubMed]
- RapportoAnnuale ISTAT. 2017. Available online: https://www.istat.it/it/files//2017/05/RapportoAnnuale 2017.pdf (accessed on 20 December 2018).
- Martella, M.; Cattaneo, L.; Viola, G.; Azzena, S.; Cappellari, A.; Baraldi, E.; Zorloni, C.; Masera, N.; Biondi, A.; Basso, G.; et al. Universal Newborn Screening for Sickle Cell Disease: Preliminary Results of the First Year of a Multicentric Italian Project. In Proceedings of the 22nd Annual Congress of the European Hematology Association, Madrid, Spain, 22–25 June 2017. [Google Scholar]
- Lobitz, S.; Klein, J.; Brose, A.; Blankenstein, O.; Frömmel, C. Newborn screening by tandem mass spectrometry confirms the high prevalence of sickle cell disease among German newborns. Ann. Hematol. 2017, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Detemmerman, L.; Olivier, S.; Bours, V.; Boemer, F. Innovative PCR without DNA extraction for African sickle cell disease diagnosis. Hematology 2017, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.B.; Awad, S.; Happich, M.; Muckenthaler, L.; Lindner, M.; Gramer, G.; Okun, J.G.; Hoffmann, G.F.; Bruckner, T.; Muckenthaler, M.U.; et al. Significant prevalence of sickle cell disease in Southwest Germany: Results from a birth cohort study indicate the necessity for newborn screening. Ann. Hematol. 2016, 95, 397–402. [Google Scholar] [CrossRef] [PubMed]
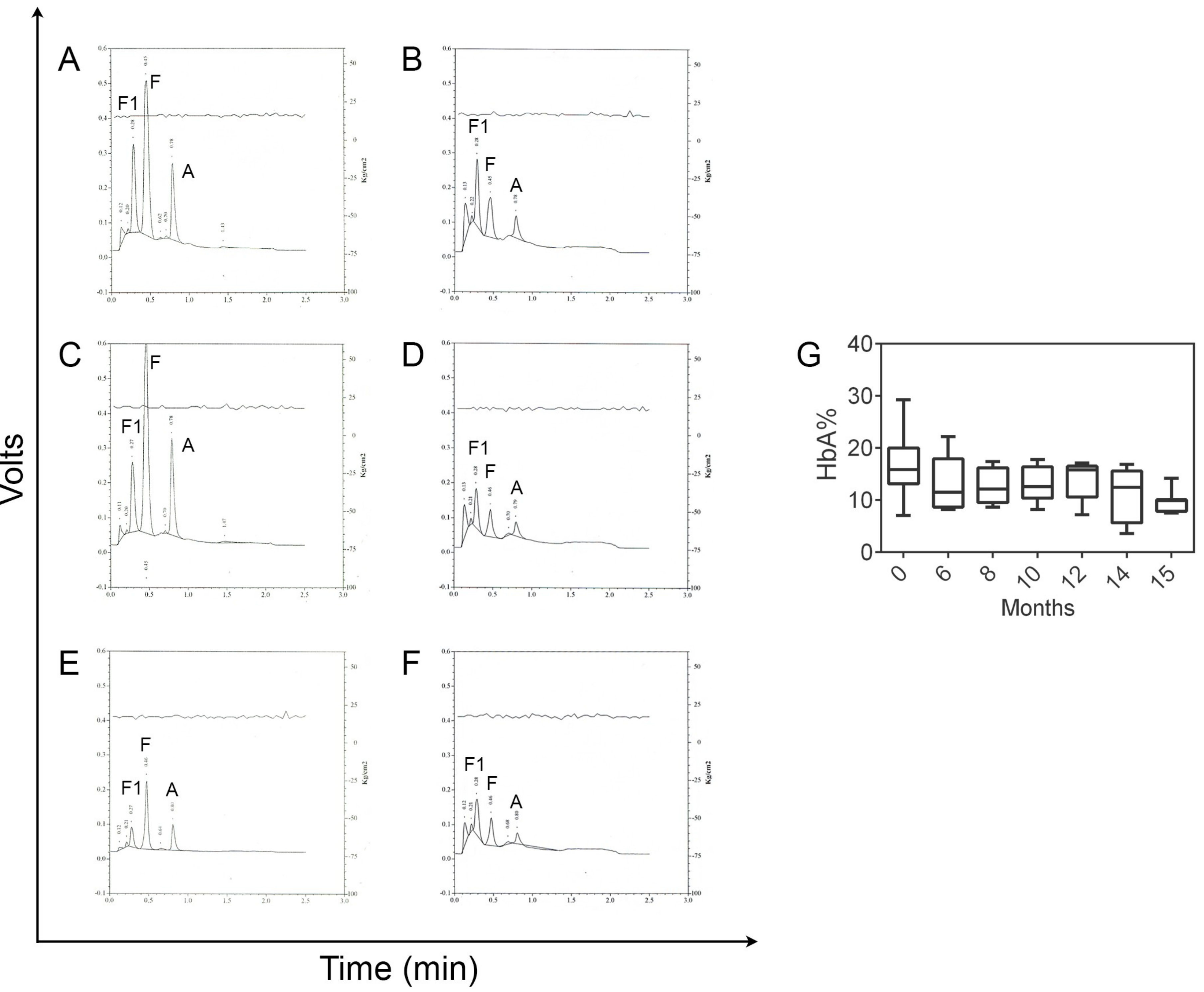
- Martella, M.; Viola, G.; Università di Padova, Padova, Italy. Chromatograms derived from HPLC analysis. Unpublish work. 2018. [Google Scholar]
- Frömmel, C.; Brose, A.; Klein, J.; Blankenstein, O.; Lobitz, S. Newborn Screening for Sickle Cell Disease: Technical and Legal Aspects of a German Pilot Study with 38,220 Participants. BioMed Res. Int. 2014, 2014, 695828. [Google Scholar] [CrossRef] [PubMed]
- Bouva, M.J.; Mohrmann, K.; Brinkman, H.B.J.M.; Kemper-Proper, E.A.; Elvers, B.; Loeber, J.G.; Verheul, F.E.A.M.; Giordano, P.C. Implementing neonatal screening for haemoglobinopathies in the Netherlands. J. Med. Screen. 2010, 17, 58–65. [Google Scholar] [CrossRef] [PubMed]
- CorteÂs-Castell, E.; PalazoÂn-Bru, A.; Pla, C.; Goicoechea, M.; Rizo-Baeza, M.M.; Juste, M.; Gil-Guillen, V.F. Impact of prematurity and immigration on neonatal screening for sickle cell disease. PLoS ONE 2017, 12, e0171604. [Google Scholar] [CrossRef] [PubMed]
- Allaf, B.; Patin, F.; Elion, J.; Couque, N. New approach to accurate interpretation of sickle cell disease newborn screening by applying multiple of median cutoffs and ratios. Pediatr. Blood Cancer 2018, 65, e27230. [Google Scholar] [CrossRef] [PubMed]
| Hb Pattern (HPLC) | HBB Genotype | Newborns (n.) |
|---|---|---|
| FAS | HBB: c.20A>T/wt | 37 (0.68%) |
| FS | HBB: c.20A>T/c.20A>T | 3 (0.055%) |
| FSC | HBB: c.20A>T/c.19A>T | 1 (0.02%) |
| FAC | HBB: c.19A>T/wt | 9 (0.16%) |
| FAD | HBB: c.364G>C/wt | 8 (0.15%) |
| FAE | HBB: c.79C>T/wt | 4 (0.07%) |
| Total | 62 (1.14%) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martella, M.; Viola, G.; Azzena, S.; Schiavon, S.; Biondi, A.; Basso, G.; Corti, P.; Colombatti, R.; Masera, N.; Sainati, L. Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. Int. J. Neonatal Screen. 2019, 5, 2. https://doi.org/10.3390/ijns5010002
Martella M, Viola G, Azzena S, Schiavon S, Biondi A, Basso G, Corti P, Colombatti R, Masera N, Sainati L. Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. International Journal of Neonatal Screening. 2019; 5(1):2. https://doi.org/10.3390/ijns5010002
Chicago/Turabian StyleMartella, Maddalena, Giampietro Viola, Silvia Azzena, Sara Schiavon, Andrea Biondi, Giuseppe Basso, Paola Corti, Raffaella Colombatti, Nicoletta Masera, and Laura Sainati. 2019. "Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease" International Journal of Neonatal Screening 5, no. 1: 2. https://doi.org/10.3390/ijns5010002
APA StyleMartella, M., Viola, G., Azzena, S., Schiavon, S., Biondi, A., Basso, G., Corti, P., Colombatti, R., Masera, N., & Sainati, L. (2019). Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. International Journal of Neonatal Screening, 5(1), 2. https://doi.org/10.3390/ijns5010002






