Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program
Abstract
:1. Introduction
2. Newborn Screening Recommendations by the Health Council of the Netherlands
3. The Response of the Dutch Ministry of Health, Welfare and Sport
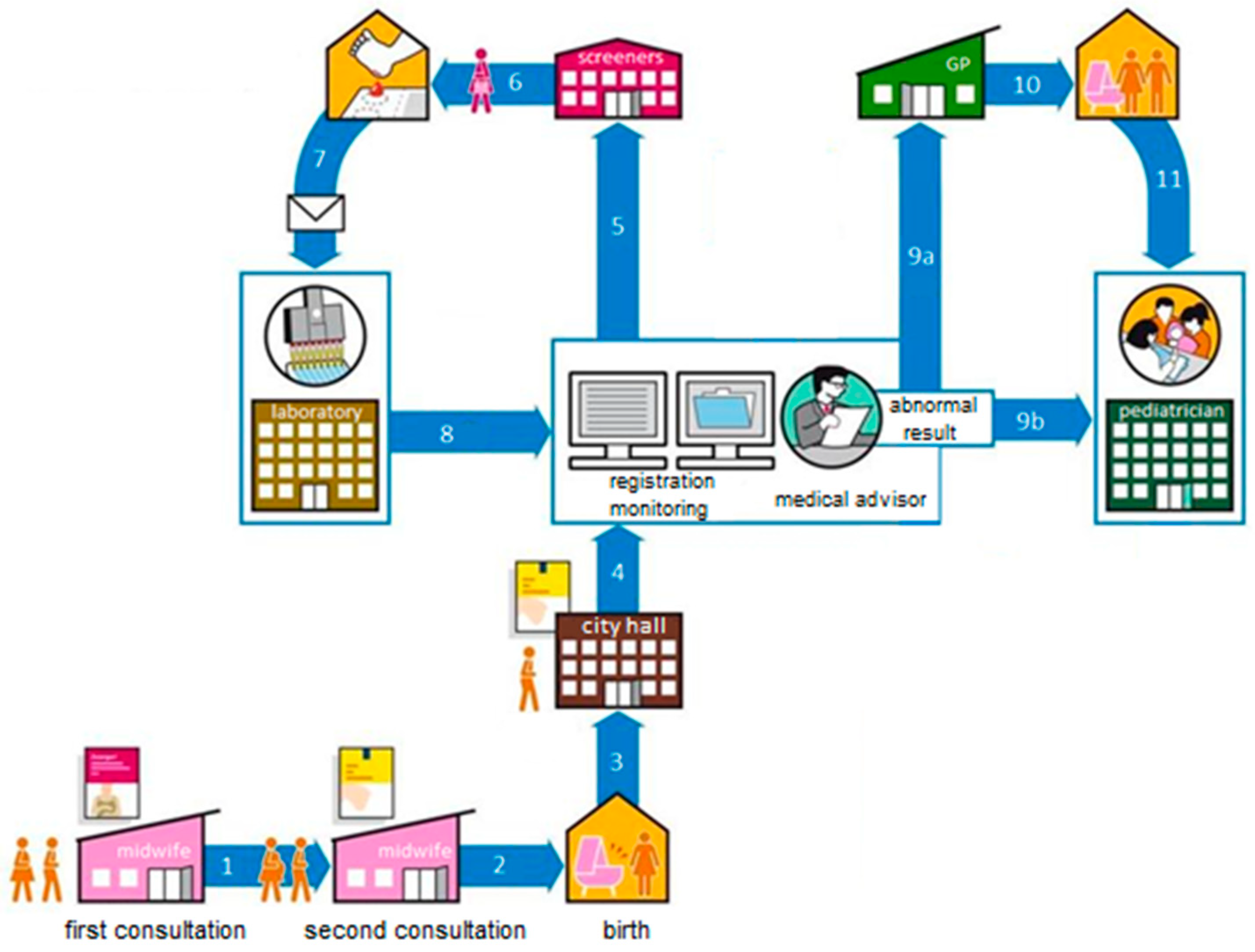
4. Feasibility Study by the Centre for Population Screening
5. Cost-Effectiveness Analysis of Newborn Screening for SCID
6. SCID Screening Assays Comparison Study
7. SCID Screening Prospective Implementation Pilot Study
8. International Collaboration
Author Contributions
Acknowledgments
Conflicts of Interest
References
- van der Ploeg, C.P.B.; Wins, S.; Olthof, R.; Eekhout, I.; Verkerk, P.H. The Newborn Blood Spot Screening in the Netherlands—Monitor 2017; National Institute of Public Health and the Environment: Bilthoven, Dutch, 2018. [Google Scholar]
- Almannai, M.; Marom, R.; Sutton, V.R. Newborn screening: A review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr. Opin. Pediatr. 2016, 28, 694–699. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Almannai, M.; Sutton, V.R. Newborn screening: History, Current Status, and Future Directions. Pediatr. Clin. N. Am. 2018, 65, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.W.; Grossman, W.J.; Laessig, R.H.; Hoffman, G.L.; Brokopp, C.D.; Kurtycz, D.F.; Cogley, M.F.; Litsheim, T.J.; Katcher, M.L.; Routes, J.M. Development of a routine newborn screening protocol for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2009, 124, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Gerstel-Thompson, J.L.; Wilkey, J.F.; Baptiste, J.C.; Navas, J.S.; Pai, S.Y.; Pass, K.A.; Eaton, R.B.; Comeau, A.M. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem. 2010, 56, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonagura, V.R.; et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014, 20, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, E.; Lev, A.; Simon, A.J.; Stauber, T.; Daas, S.; Saraf-Levy, T.; Broides, A.; Nahum, A.; Marcus, N.; Hanna, S.; et al. First year of Israeli Newborn Screening for Severe Combined Immunodeficiency-Clinical Achievements and Insights. Front. Immunol. 2017, 8, 1448. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Yu, H.; Lee, N.; Ho, H.; Kao, S.; Lu, M.; Jaing, T.; Lee, W.; Chang, K.; Shieh, C.; et al. Newborn Screening for Severe Combined Immunodeficiency in Taiwan. Int. J. Neonatal. Screen. 2017, 3, 16. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Neonatal Screening; Health Council of the Netherlands: The Hague, The Netherlands, 2005.
- Health Council of the Netherlands. Neonatal Screening for Cystic Fibrosis; Health Council of the Netherlands: The Hague, The Netherlands, 2010.
- Health Council of the Netherlands. Neonatal Screening: New Recommendations; Health Council of the Netherlands: The Hague, The Netherlands, 2015.
- Letter of the Dutch Minister for Health (mw. drs. E.I. Schippers) to the Chairman of the House of Representatives of the Netherlands on the Expansion of the Newborn Screening Program. Available online: http://artsenjgz.nl/nieuwsbericht/reactie-minister-van-vws-op-advies-gezondheidsraad-over-14-nieuwe-aandoeningen-in-de-hielprikscreening/> (accessed on 28 May 2018).
- Dekkers, E.H.B.M.; Klein, A.W.; Lock, A.J.J.; Vermeulen, H.M. Uitvoeringstoets uitbreiding neonatale hielprikscreening. Rijksinstituut voor Volksgezondheid en Milieu 2017. [Google Scholar] [CrossRef]
- Ding, Y.; Thompson, J.D.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S.D. Cost-Effectiveness/Cost-Benefit Analysis of Newborn Screening for Severe Combined Immune Deficiency in Washington State. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Davis, J.; Pai, S.Y.; Bonilla, F.A.; Puck, J.M.; Apkon, M. A Markov model to analyze costeffectiveness of screening for severe combined immunodeficiency (SCID). Mol. Genet. Metab. 2011, 104, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Health Partners Consulting Group. Cost-Effectiveness of Newborn Screening for Severe Combined Immune Deficiency. A Report Prepared for the National Screening Unit. Available online: https://www.nsu.govt.nz/system/files/resources/cost-effectiveness-newborn-screening-severe-combined-immune-deficiency.pdf (accessed on 20 November 2018).
- van der Ploeg, C.P.B.; van den Akker-van Marle, E.; Bredius, R.G.M.; Staal, F.; van den Burg, M.; Verkerk, P. Kosteneffectiviteits- en Kostenbatenanalyse (KEA/KBA) Voor Het screenen op SCID Binnen de Nederlandse Hielprikscreening; TNO: Leiden, The Netherlands, 2017. [Google Scholar]
- Van der Ploeg, C.P.B.; Blom, M.; Bredius, R.G.M.; van der Burg, M.; Schielen, P.C.J.I.; Verkerk, P.H.; van den Akker-van Marle, M.E. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur. J. Pediatr. 2018. resubmitted. [Google Scholar]
- Clément, M.C.; Mahlaoui, N.; Mignot, C.; le Bihan, C.; Rabetrano, H.; Hoang, L.; Neven, B.; Moshous, D.; Cavazzana, M.; Blanche, S.; et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data. J. Allergy Clin. Immunol. 2015, 135, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Pico-Knijnenburg, I.; Sijne-van Veen, M.; Boelen, A.; Bredius, R.G.M.; van der Brug, M.; Schielen, P.J.C.I. An evaluation of the TREC assay with regard to the integration of SCID screening into the Dutch newbon screening program. Clin. Immunol. 2017, 180, 106–110. [Google Scholar] [CrossRef] [PubMed]
- PerkinElmer. EnLite Neonatal TREC Kit. Available online: https://newbornscreening.perkinelmer.com/products/enlite_neonatal_trec_instrument/enlite_neonatal_trec_kit (accessed on 25 May 2018).
- ImmunoIVD. SCREEN-ID Neonatal Screening Kit. Available online: http://immunoivd.com/technology.html (accessed on 24 May 2018).
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; WHO: Geneva, Switzerland, 1968; Available online: http://www.who.int/bulletin/volumes/86/4/07-050112BP.pdf (accessed on 18 May 2018).
- De Pagter, A.P.J.; Bredius, R.G.M.; Kuijpers, T.W.; Tramper, J.; van der Burg, M.; van Montfrans, J.; Driessen, G.J. Overview of 15-year severe combined immunodeficiency in the Netherlands: Towards newborn blood spot screening. Eur. J. Pediatr. 2015, 174, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
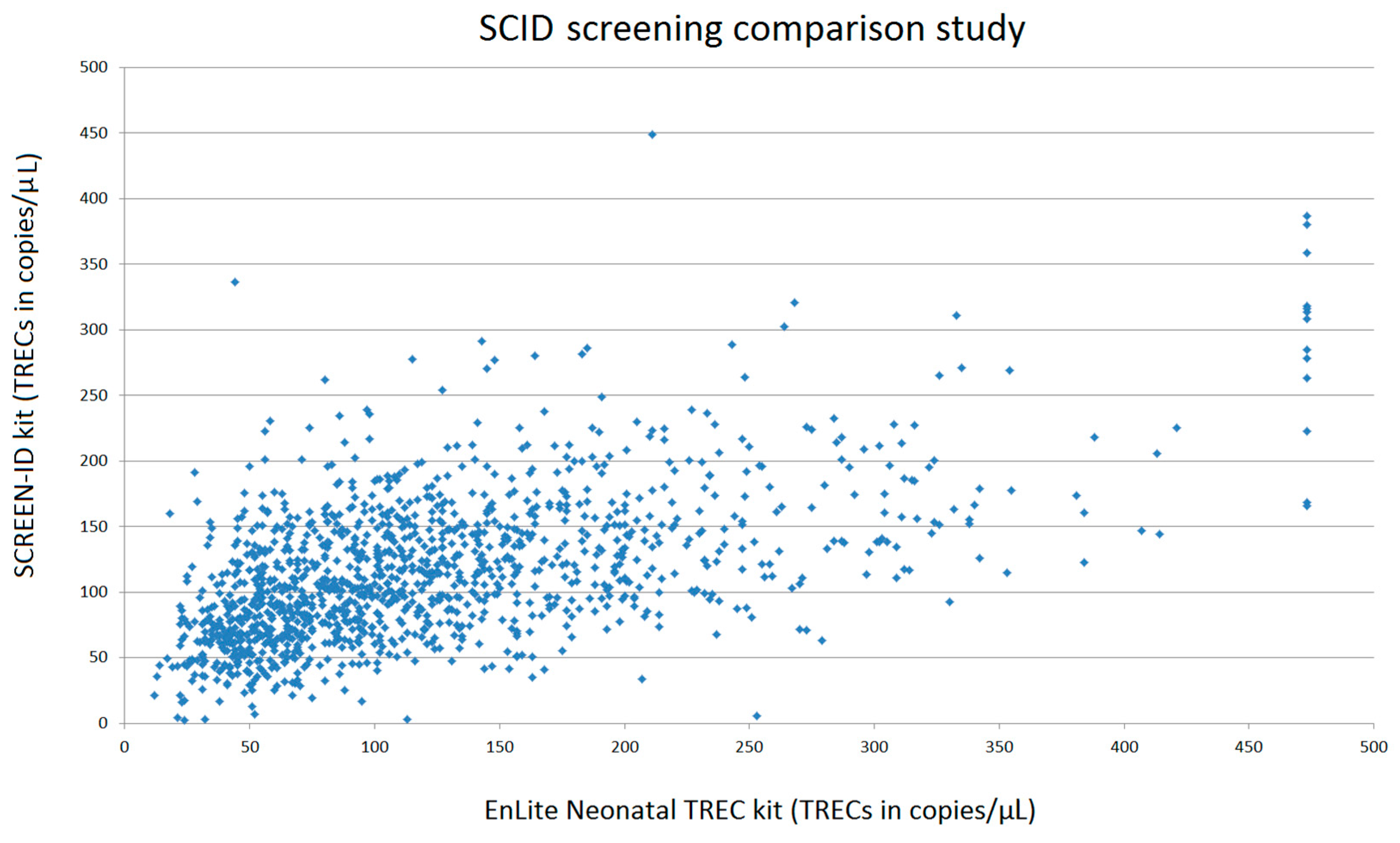

| EnLite Neonatal TREC Assay | SCREEN-ID Assay | |
|---|---|---|
| Average (TREC copies/μL) | 123 | 116 |
| Median (TREC copies/μL) | 102 | 109 |
| 2.5 percentile (TREC copies/μL) | 28 | 33 |
| Number of heel prick cards below 2.5 percentile | 32 | 32 |
| Number of heel prick cards below manufacturer’s cut-off | 38 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blom, M.; Bredius, R.G.M.; Weijman, G.; Dekkers, E.H.B.M.; Kemper, E.A.; Van den Akker-van Marle, M.E.; Van der Ploeg, C.P.B.; Van der Burg, M.; Schielen, P.C.J.I. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. Int. J. Neonatal Screen. 2018, 4, 40. https://doi.org/10.3390/ijns4040040
Blom M, Bredius RGM, Weijman G, Dekkers EHBM, Kemper EA, Van den Akker-van Marle ME, Van der Ploeg CPB, Van der Burg M, Schielen PCJI. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. International Journal of Neonatal Screening. 2018; 4(4):40. https://doi.org/10.3390/ijns4040040
Chicago/Turabian StyleBlom, Maartje, Robbert G.M. Bredius, Gert Weijman, Eugènie H.B.M. Dekkers, Evelien A. Kemper, M. Elske Van den Akker-van Marle, Catharina P.B. Van der Ploeg, Mirjam Van der Burg, and Peter C.J.I. Schielen. 2018. "Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program" International Journal of Neonatal Screening 4, no. 4: 40. https://doi.org/10.3390/ijns4040040
APA StyleBlom, M., Bredius, R. G. M., Weijman, G., Dekkers, E. H. B. M., Kemper, E. A., Van den Akker-van Marle, M. E., Van der Ploeg, C. P. B., Van der Burg, M., & Schielen, P. C. J. I. (2018). Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. International Journal of Neonatal Screening, 4(4), 40. https://doi.org/10.3390/ijns4040040







