Abstract
Background: Quantification of T-cell-receptor-excision circles (TRECs) and kappa-deleting-recombination-excision circles (KRECs) from dried blood spots (DBS) allows detection of neonates with severe T-cell and/or B-cell lymphopenia that are potentially affected by severe combined immunodeficiency (SCID), as well as X-linked agammaglobulinemia (XLA). Methods: Determination of TRECs and KRECs using a triplex RT-PCR (TRECS-KRECS-β-actin) assay from prospectively collected DBS between February 2014 and December 2016 in three hospitals in Seville, Spain. Cut-off levels were TRECs < 6/punch, KRECs < 4/punch and b-actin > 700/punch. Internal (SCID, XLA, ataxia telangiectasia) and external controls (CDC) were included. Results: A total of 8943 DBS samples obtained from 8814 neonates were analysed. Re-punching was necessary in 124 samples (1.4%) due to insufficient β-actin values (<700 copies/punch). Preterm neonates (GA < 37 weeks) and neonates with a BW < 2500 g showed significantly lower TRECs and KRECs levels (p < 0.001). Due to repeated pathological results, ten neonates were re-sampled (0.11%), of which five neonates (0.055%) confirmed the pathological results: one case was a fatal chromosomopathy (TRECs 1/KRECs 4); two were extreme premature newborns (TRECs 0/KRECs 0 and TRECs 1/KRECs 20 copies/punch); and 2 neonates were born to mothers receiving azathioprine during pregnancy (TRECs 92/KRECs 1 and TRECs 154/KRECs 3 copies/punch). All controls were correctly identified. Conclusions: Severe T- and B-cell lymphopenias were correctly identified by the TRECS-KRECS-β-actin assay. Prematurity and low BW are associated with lower TREC and KREC levels. Extreme prematurity and maternal immune suppressive therapy can cause false positive results of TRECs and KRECs values.
1. Introduction
In Spain, there is no universal neonatal screening for potentially lethal diseases of the immune system, although it has been demonstrated that these pathologies meet most of the established screening criteria, including the existence of relatively non-invasive screening methods, the proven benefit of an early diagnosis, the presence of an initial asymptomatic period, high morbidity and mortality, the availability of curative or effective therapeutic options, and a favourable cost-benefit analysis [1,2,3,4,5]. The prevalence of severe primary immunodeficiencies (PID) like X-linked agammaglobulinemia (XLA) and severe combined immunodeficiencies (SCID) in Spain is unknown due to the absence of systematic epidemiological studies. A recent survey regarding the diagnosis of SCID cases in Andalusia and Catalonia between 2010 and 2014 revealed 14 and 7 proven cases, respectively, resulting in an estimated incidence of 1:35,000–1:50,000 (personal communication Dr O Neth, Dr P Soler). The common lack of a suggestive family history and the absence of specific clinical symptoms in the first weeks of life often lead to a delayed diagnosis, associated with prolonged intensive care treatment, high morbidity and mortality [6,7,8].
Andalusia leads in the number of births in Spain, with about 80,000 per year (https://www.juntadeandalucia.es). In February 2014, our group initiated a prospective, observational and longitudinal pilot study aiming to determine T-cell-receptor-excision circle (TREC) and kappa-deleting-recombination-excision circle (KREC) levels in dried blood spots (DBS) obtained from neonates born in the three major public hospitals in Seville, Spain (Hospitales Universitarios Virgen del Rocío, Hospital Universitario Virgen Macarena and the Hospital de Especialidades Virgen del Valme). Preliminary results of this project have been published in national as well as international journals, leading to positive feedback from patients’ organisations, local and national media, and scientific societies (AEDIP, http://www.aedipe.es) [9,10]. Here, we present the most recent data of this study. In addition, the current situation of PID newborn screening in Spain is discussed.
2. Materials and Methods
Neonates born in three hospitals in Seville (Hospital Universitario Virgen del Rocío, Hospital Universitario Virgen de Macarena and Hospital Virgen de Valme) were prospectively enrolled in this ongoing, observational and longitudinal study to determine TREC and KREC levels in DBS between February 2014 and December 2016. DBS were included once the legal guardians signed informed consent forms. The local ethics committees approved this study.
As part of the routine neonatal screening process heel prick blood samples were collected on Schleicher & Schuell #912 filter paper between the 3rd and 5th day post-partum. Heel pricks in preterm neonates (gestational age (GA) < 37 weeks) were repeated every two weeks until week 37 of corrected GA, or birth weight (BW) ≥ 2500 g, or normality in the assay. Two 3.2 mm discs were punched for each sample, and stored at 4 °C until use. Data collection included selected demographic and clinical information, such as gender, GA, BW, pathological results from routine neonatal screening, and TREC, KREC, β-actin copy numbers.
DNA extraction from DBS samples and DNA purification (DNA Elution and Purification Solution, Qiagen, Germantown, MD, USA) TRECs, KRECs, and ACTB (β-actin) copy numbers were determined as previously described [9]. Efficacy of DNA extraction from DBS samples and their quality was reflected by the amount of ACTB copy numbers.
Internal (designed and obtained by plasmid cloning) and external controls were provided by the NBS Quality Assurance Program of the Centers for Disease Control and Prevention (CDC), USA (pathologic TRECS, n = 4; normal TRECS, n = 9; DNA free samples, n = 7). Furthermore, 7 clinical samples obtained from previously diagnosed patients were included.
Based on our previous experience, the cut-off scores for an estimated 99.8% sensitivity in detecting severe T- and/or B-cell lymphopenias were defined as: TRECs < 6/punch, ACTB > 700/punch and KRECs < 4/punch [9]. False-positive results were defined as values below the established cut-offs for TRECs or KRECs in absence of SCID or inherited agammaglobulinemia, respectively. Abnormal or inconclusive results required a new punch from the same DBS and a repeat PCR-assay (re-test) was performed. Subsequently, a pathological result in the re-test required a new heel prick sample (re-sample), and the confirmation of a result below the established cut-off resulted in a physical assessment of the neonate in the immunology clinic (Hospital Virgen del Rocio). If the material of the 1st DBS was insufficient, re-punching was performed (re-call) with extraction of a 2nd DBS (re-sample).
Quantitative variables were tested (Kolmogorov–Smirnov test, or Shapiro–Wilk test) for normal distribution, and the results expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR), as appropriate. Rates for abnormal, inconclusive, and normal results in the TREC/KREC-assay were calculated (IC 95%). To assess the assay reliability, the proportion of false-positive results was calculated. For qualitative variables, the chi-squared or the Fisher’s exact test were applied to estimate variable associations with T- and B-cell lymphopenias. For quantitative variables, the Student’s t-test for independent samples or the Mann–Whitney U test were performed. Correlations were assessed by means of the Pearson tests. p-Values of < 0.05 were considered as statistically significant. All statistical operations were performed using the IBM software SPSS Statistics version 24.
3. Results
3.1. Clinical Data
8943 samples obtained from 8814 neonates were analysed (approximately 10% of life births) in the area of Occidental Andalusia in this time period. Of these neonates 50.7% were males; 73.2% were born at term (GA ≥ 37 weeks) and 78.0% had a BW ≥ 2500 g. The mean GA was 38.9 weeks (±2.4 weeks) and the mean BW was 3140 g (±623 g) (Table 1).

Table 1.
Results of TREC/KREC PCR-assay of DBS in relation to gestational age (GA) and birth weight (BW).
3.2. TREC/KREC/ACTB Assay Performance
A total of 8943 DBS from 8814 neonates were analysed. Whilst 8809 (98.50%) samples were normal, 124 (1.39%) showed abnormal results requiring re-punching. Of those, 10 neonates required re-sampling (0.11%) due to persistent pathological results; five subsequently had normal results, and the remaining five (0.055%) were confirmed to be pathological, as summarized in Table 2. In addition, 65 DBS samples could not be included in the study due to insufficient material.

Table 2.
Summary of pathological results from prospective DBS [10].
3.3. TREC and KREC Levels According to GA and BW
Table 1 summarizes the TREC and KREC values according to GA and BW. TREC and KREC copy numbers were lower in preterm neonates than in term neonates (p < 0.001). Likewise, newborns with a BW < 2500 g showed lower TREC and KREC copy numbers than those with a BW ≥ 2500 g (p < 0.001). A positive correlation between GA and BW with TRECs (r = 0.117, p ≤ 0.001; r = 0.118, p ≤ 0.001) as well as with KRECs (r = 0.05, p ≤ 0.001; r = 0.001, p ≤ 0.001) was observed. For both variables, this association was more pronounced for the TRECs. ACTB copy numbers were not affected by GA and/or BW (data not shown).
3.4. Positive Results
Five of the 8814 prospectively tested newborns showed abnormal results in the assay (Figure 1 and Table 2). Patient 1 (P1) was a preterm female diagnosed with a complex chromosomopathy. In this case, confirmation of the initially abnormal results with a second sample, or with lymphocyte subset (LSS) determination, could not be performed due to the unfortunately rapid fatal clinical course. Two other individuals (P2, P3) with positive results in the 1st test run were identified as blood product-dependent extreme preterm neonates. In both individuals, ACTB and TRECs were found to be low. In addition, P3 also showed reduced KREC copy numbers. All three patients had a low-for-age lymphocyte count. However, the CD4, CD8, CD19 and NK cell distributions of patients 2 and 3 were normal. A 2nd sample of the latter demonstrated normal KREC and ACTB levels whilst TRECs remained reduced. Repeated LSS were subsequently normal for age in P3, but were not available for P2. Low KREC numbers were detected in P4 and P5. Interestingly, further evaluation revealed that both mothers had received immunosuppressive treatment (including azathioprine) for systemic lupus erythematosus (SLE) and Crohn’s disease during pregnancy. While the family of P4 refused to perform a second heel prick, P5 was tested and showed persistent low KREC levels until one month of age. A blood sample taken from the mother of P5 revealed B-cell lymphopenia (39 cells/μL) and pathological TRECs and KRECs copy numbers being 0 and 1, respectively, whilst receiving azathioprine treatment. Subsequently, a further blood analysis of P5 4 weeks after stopping breast-feeding revealed normal KREC levels in the infant (Table 2). Both patients remained clinically asymptomatic.
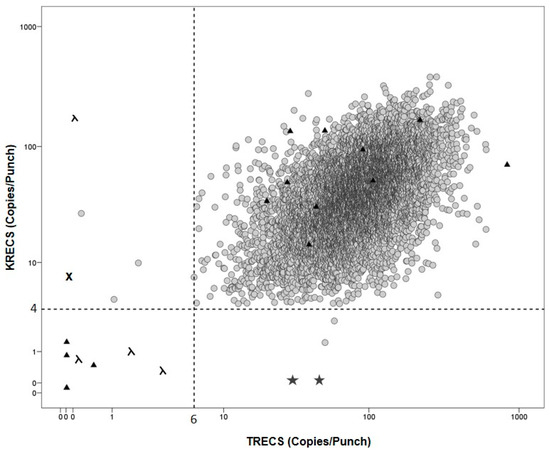
Figure 1.
TRECS and KRECS values from DBS and controls grey: prospectively enrolled newborns; ▲: CDC controls; λ: SCID controls; ★: XLA controls; ✖: AT control; grey dotted line: cut-offs for TRECs < 6 and KRECs < 4 copies/punch.
3.5. External Control Samples
A total of 20 blinded samples (n = 20) were provided for quality assessment from the CDC. All positive (low TREC copy numbers, β-actin above cut-off) and negative samples (TREC and β-actin above cut-off) were correctly identified as shown in Figure 1.
3.6. Internal Control Samples
As previously shown [10], seven heel-prick samples from patients with known underlying PID were included (Table 3). TREC levels in four patients with a SCID diagnosis (age range 0.2–9.6 months) were below the cut-off (range 0–6 copies/punch). Furthermore, KRECs were abnormal (range 0–4 copies/punch) in two subjects with a T-B-SCID phenotype. KREC levels were correctly absent in two patients with XLA and one patient with ataxia telangiectasia had absent TREC and low KREC levels. ACTB levels in all samples tested were normal.

Table 3.
Summary from internal positive controls [10].
4. Discussion
Within Spain, this is the largest study using the triplex TRECs/KRECs/ACTB-assay for NBS of severe T- and B-cell lymphopenias. In this ongoing study, we prospectively collected and analysed 8943 samples from 8814 newborns. Values of TRECs and KRECs were found to be similar to other previously studied populations [11,12]. The initially chosen cut-off levels for TRECs (<15 copies/punch) and KRECs (<10 copies/punch) were progressively adjusted in order to avoid unnecessary sample drawing and analysis for reasons of unjustified harm to the newborn and additional costs [9,10]. The re-punch rate was 1.4% meanwhile the re-sample rate was low, at 0.11%, complying with previous studies [11,12,13,14,15]. Poor pre-analytic sample quality, mainly due to inappropriately prepared DBS, resulted in ACTB below the cut-off, and was by far the most frequently identified reason for re-punching. Assuming the birth rate of our regional health care area (25,000 births/year) this would result in the repetition of 345 samples and re-sampling of 25 neonates, half of which would require a medical assessment. We are in the process of starting training programs with the hospitals and primary care centres’ nurseries, aiming to improve routine NBS performance and subsequently the re-punch and re-sample rates. The close collaboration with the regional screening centre enabled us to avoid additional harm to newborns with insufficient samples in most cases. Only two newborns were re-sampled, with the remaining 8 newborns being previously contacted by the metabolic screening centre, as additional samples were required in order to complete the routine NBS repetition.
The aim of the study was to detect severe T and B cell lymphopenias including cases suffering from SCID, and agammaglobulinemia cases. An important variability has been observed in the used algorithms and cut-off values in previous studies [3]. TRECs cut-off values have ranged from 7 to 252 TRECs/μL across different states in the USA, while in Europe, different cut-off values have been recommended by different countries [3,10,12]. Nonetheless, the majority of typical SCID cases seem to be detected using 25 TRECs/μL as cut-off value, despite variability in the assays [3].
We further confirmed an association between TREC and KREC levels with the variables GA and BW. Lower TREC values have been previously described in preterm neonates [9,10,11,12,15] and a recent study from Sweden, together with results published by our group, demonstrated the influence of GA and BW on the KREC levels [9,10,12]. Two extreme preterm neonates admitted to the neonatal intensive care unit showed TREC levels below the established cut-off, and none had pathological KREC levels, suggesting that the addition of KRECs to the assay will likely not increase the number of false-positive results even in the preterm population. The impact of GA and BW on TRECs was more pronounced compared to the KRECs. This finding might be explained by the fact that B-cell development precedes T-cell maturation in human foetuses [16].
As previously described [10], two term neonates had KRECs below the cut-off with normal TREC values. Both neonates were born to mothers receiving immunosuppressive therapy during pregnancy in the form of azathioprine for SLE and Crohn’s disease, highlighting the importance of a complete clinical anamnesis. The potential association of immunosuppressive therapy and abnormal KRECs but normal TRECs appears to be transitory; however, it may contribute to higher rates of false-positive results. In addition, this observation potentially expands our knowledge in immune maturation and the impact of immunosuppressive treatment in this vulnerable period [12,16].
The technique used in this study has proven to be highly reliable, relatively non-invasive and cost-effective, making the nationwide implementation feasible for PID newborn screening in Spain and other European countries. A recently performed study from Catalonia by La Agencia de Calidad y Evaluación Sanitarias de Cataluña (AQuAS) proposed NBS for SCID at a cost of up to 7.72 €/sample as being cost effective (personal communication Dr P. Soler). The technical cost of the in-house TREC/KREC/ACTB assay is 1.96 €/sample (the commercialised TRECs/KRECs/ACTB assay being 4.70 €/sample) compared with 4.67 €/sample of the commercial TREC assays used in the USA [3,15]. The potential to detect and further characterise a broader spectrum of severe PID in the light of a marginal increase in costs may support the use of the TREC/KREC/ACTB assay [1,3,9,12].
The most appropriate strategies for managing infants with SCID or infants with other T or B cell lymphopenias detected in the asymptomatic phase by NBS, and the protocols for pretransplantation care or transplantation to use remains to be determined [17,18]. In contrast, it has been clearly shown that early diagnosis of SCID patients using NBS program and subsequent performance of HSCT increases the overall survival of these patients >90% [15].
The data presented here further demonstrates the TREC/KREC/ACTB-assay for NBS to be effective, although no classical SCID was detected so far, likely due to the low sample size, a limitation of this ongoing study. Within the Southern Spanish population, retesting was mainly necessary due to insufficient DBS quality; therefore, improvement of DBS preparation is important. TREC and KREC levels correlated with GA and BW. As none of the preterm neonates showed isolated abnormal KRECs the addition of KREC levels to TREC determination might not increase re-punching and re-sampling rates. The impact of immunosuppressive therapy during pregnancy on the results of KRECs is an interesting finding, highlighting the importance of an accurate maternal history and warranting further investigation.
In recent years, the national patients’ organisation (AEPID), the scientific organisations for immunology, paediatrics and paediatric infectious diseases (SEI, AEP, SEIP), as well as initiatives with local pilot screening projects (Seville, Madrid) have substantially contributed to raising awareness for the implementation of PID newborn screening systematically in our country.
It is of note that, during the period of this pilot study (February 2014–December 2016) a total of 7 SCID (including 2 ADA-SCID) and 3 XLA patients were diagnosed in Andalusia (personal communication Dr M. Santamaria, Cordoba; Dr D. Moreno, Malaga; Dr J.L. Santos, Granada; and Dr F. Lendinez, Almeria). Diagnosis of all patients was delayed, as they were identified in the context of infections and/or failure to thrive, and two patients subsequently died prior to HSCT. Assuming 80,000 births/year, the incidence of SCID and XLA in Andalusia, would be at least 1:34,000 and 1:120,000, respectively, as it does not include infants or children deceasing without diagnosis.
In this regard, it is highly encouraging that Catalonia, as the first out of 17 autonomous states in Spain, started to screen systematically for PIDs in 2017, determining TRECs in DBS; preliminary results are awaited. This first step is of outstanding importance in achieving the implementation of PID screening on a national level, as this would allow for a prompt diagnosis and effective treatment strategies for patients with severe immunodeficiencies.
5. Conclusions
The TREC/KREC/ACTB-assay for NBS of SCID and agammaglobulinemia is effective and reliable. In our setting, retesting is mainly related to insufficient DBS quality. Gestational age and birthweight consistently correlate with TREC and KREC levels. We observed that maternal immunosuppressive therapy during pregnancy might influence KREC but not TREC levels; a finding that warrants further validation and investigation.
Acknowledgments
This work was funded by Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III (PI13/01104), and Ayudas para contratos de formación en Investigación Rio Hortega. Instituto de Salud Carlos III.
Author Contributions
Beatriz de Felipe performed the TRECS and KRECS assay. Beatriz de Felipe, Peter Olbrich, Carmen Delgado-Pecellin and Olaf Neth designed the database and the experimental design. Berta Sánchez and José Manuel Lucena performed the corresponding immunology testing, Peter Olbrich, Walter Goycochea-Valdivia, Paula Sanchez-Moreno and Olaf Neth were responsible for the clinical evaluation of patients in clinics. Beatriz de Felipe, Peter Olbrich, Walter Goycochea-Valdivia, Paula Sanchez-Moreno and Olaf Neth drafted the manuscript. Araceli Ferrari-Cortes, Joséfa Salguero Martin de Soto, Josefina Marquez, Carmen Salamanca and Carlos Jimenez Contreras were involved in the consent and recruitment of patients. All authors have reviewed and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borte, S.; von Döbeln, U.; Hammarström, L. Guidelines for newborn screening of primary immunodeficiency diseases. Curr. Opin. Hematol. 2013, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Modell, V.; Knaus, M.; Modell, F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol. Res. 2014, 60, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Van der Spek, J.; Groenwold, R.H.; van der Burg, M.; van Montfrans, J.M. TREC based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review. J. Clin. Immunol. 2015, 35, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.C.; Mahlaoui, N.; Mignot, C.; Le Bihan, C.; Rabetrano, H.; Hoang, L.; Neven, B.; Moshous, D.; Cavazzana, M.; Blanche, S.; et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data. J. Allergy Clin. Immunol. 2015, 135, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Thompson, J.D.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S.D. Cost-Effectiveness/Cost-Benefit Analysis of Newborn Screening for Severe Combined Immune Deficiency in Washington State. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, A.; Colarusso, G.; Hughes, S.M.; Gennery, A.R.; Slatter, M.; Cant, A.J. Clinical features that identify children with primary immunodeficiency. Pediatrics 2011, 127, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Xu-Bayford, J.; Allwood, Z.; Slatter, M.; Cant, A.; Davies, E.G. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: The case of newborn screening. Blood 2011, 17, 2443–2446. [Google Scholar]
- Chan, A.; Scalchunes, C.; Boyle, M.; Puck, J.M. Early vs. delayed diagnosis of severe combined immunodeficiency: A family perspective survey. Clin. Immunol. 2011, 138, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, P.; de Felipe, B.; Delgado-Pecellin, C.; Rodero, R.; Rojas, P.; Aguayo, J.; Marquez, J.; Casanovas, J.; Sanchez, B.; Lucena, J.M.; et al. A first pilot study on the neonatal screening of primary immunodeficiencies in Spain: TRECS and KRECS identify severe T- and B-cell lymphopenia. An. Pediatr. 2014, 81, 310–317. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, B.; Olbrich, P.; Lucenas, J.M.; Delgado-Pecellin, C.; Pavon-Delgado, A.; Marquez, J.; Salamanca, C.; Soler-Palacin, P.; Gonzalez-Granado, L.I.; Antolin, L.F.; et al. Prospective neonatal screening for severe T- and B-lymphocyte deficiencies in Seville. Pediatr. Allergy Immunol. 2016, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Borte, S.; von Döbeln, U.; Fasth, A.; Wang, N.; Janzi, M.; Winiarski, J.; Sack, U.; Pan-Hammarström, Q.; Borte, M.; Hammarström, L. Neonatal screening for severe primary immunodeficiency diseases using high-throughput triplex real-time PCR. Blood 2012, 119, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.; Ohlsson, A.; Borte, S.; Jonsson, S.; Zetterström, R.H.; King, J.; Winiarski, J.; von Döbeln, U.; Hammarström, L. Newborn Screening for Severe Primary Immunodeficiency Diseases in Sweden-a 2-Year Pilot TREC and KREC Screening Study. J. Clin. Immunol. 2017, 37, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Audrain, M.; Thomas, C.; Mirallie, S.; Bourgeois, N.; Sebille, V.; Rabetrano, H.; Durand-Zaleski, I.; Boisson, R.; Persyn, M.; Pierres, C.; et al. Evaluation of the T-cell receptor excision circle assay performances for severe combined immunodeficiency neonatal screening on Guthrie cards in a French single centre study. Clin. Immunol. 2014, 150, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Rashid, S.; Premachandra, T.; Harvey, K.; Ifederu, A.; Wilson, M.C.; Gaspar, H.B. Screening of neonatal UK dried blood spots using a duplex TREC screening assay. J. Clin. Immunol. 2014, 34, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonilla, F.A.; et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, E.; Lev, A.; Lee, Y.N.; Simon, A.J.; Yinon, Y.; Lipitz, S.; Amariglio, N.; Weisz, B.; Notarangelo, L.D.; Somech, R. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci. Transl. Med. 2015, 7, 276ra25. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, M.J.; Dvorak, C.C.; Cowan, M.J.; Puck, J.M. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J. Allergy Clin. Immunol. 2017, 139, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Inborn Errors Working Party (IEWP). Available online: https://esid.org/layout/set/print/Working-Parties/Inborn-Errors-Working-Party-IEWP/Resources/UPDATED!-EBMT-ESID-GUIDELINES-FOR-HAEMATOPOIETIC-STEM-CELL-TRANSPLANTATION-FOR-PI (accessed on 25 September 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).