1. Introduction
Critical congenital heart disease (CCHD) screening using pulse oximetry is a point of care newborn screen that relies on the detection of low blood oxygen levels to identify infants who may have CCHD or other life threatening neonatal conditions. Particularly useful for identifying asymptomatic infants with CCHD in well-baby nurseries, the importance of this screen is in its ability to allow for the detection of CCHD prior to when the infant is discharged from the birth hospital. The late detection of CCHD, after hospital discharge, has been shown to increase morbidity and mortality [
1].
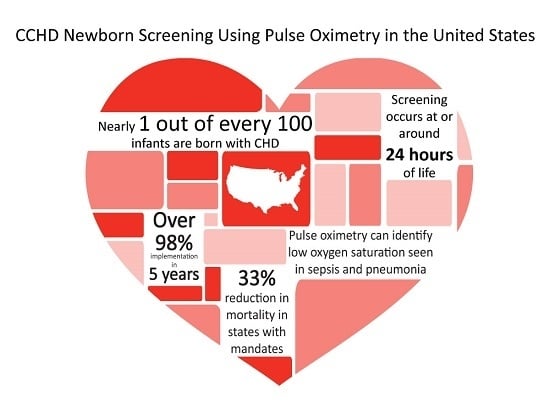
Congenital heart disease (CHD) is the most common birth defect. In the U.S., approximately 40,000 infants are born with CHD, with 25% of those having CCHD [
2,
3,
4]. The primary targets for CCHD screening were identified through expert consensus in 2011. The list included those seven lesions most likely to be identified using pulse oximetry: hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, transposition of the great arteries, tricuspid atresia, and truncus arteriosus [
5]. This list of core conditions was expanded in 2016, this time by an expert panel convened by the Centers for Disease Control (CDC) and the American Academy of Pediatrics (AAP) to include coarctation of the aorta, double-outlet right ventricle, Ebstein’s anomaly, interrupted aortic arch, single ventricle, and other critical cyanotic lesions not specified. The expert panel also acknowledged the added benefit of identifying secondary targets, including hemoglobinopathy, hypothermia, infection (including sepsis), lung disease, noncritical CHD, persistent pulmonary hypertension, and other hypoxemic conditions as important public health targets of CCHD screening in the U.S. [
6].
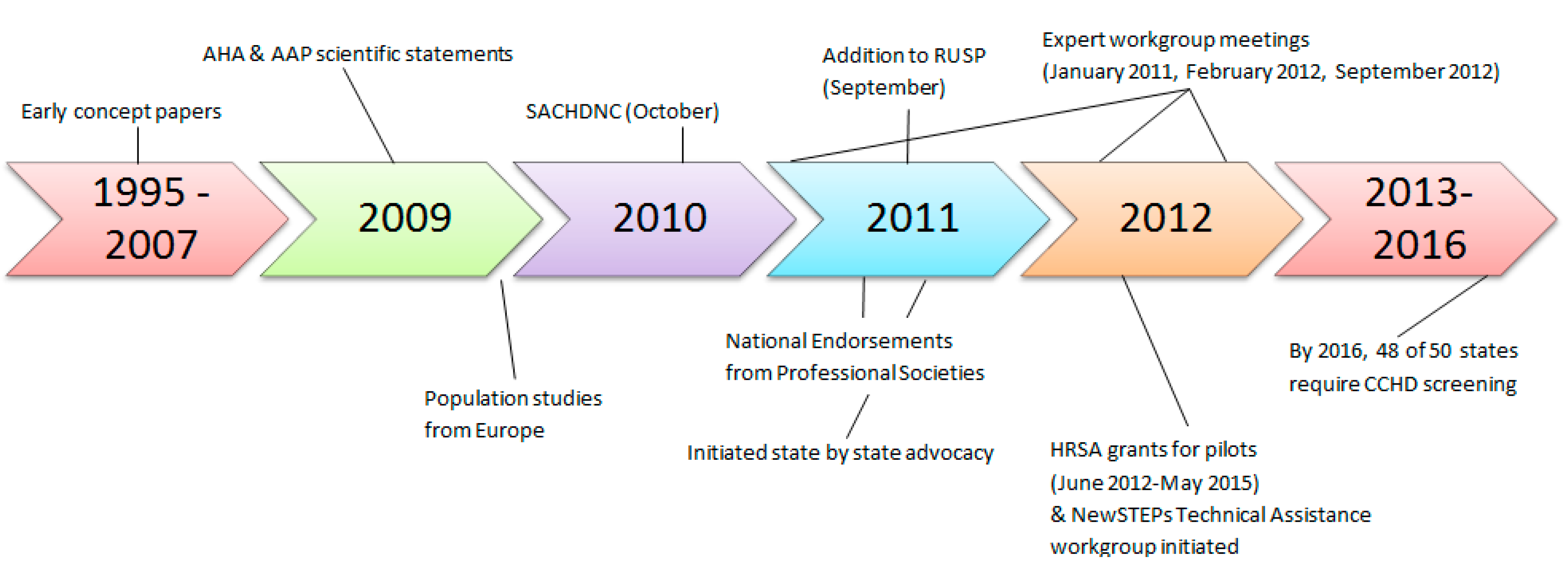
The goal of this article is to give an overview and insight into how the U.S. was able to achieve systematic implementation of CCHD screening using pulse oximetry including a nationally endorsed screening algorithm, centralized resources coordinated at the state and federal government levels, shared educational strategies, and toolkits; thus, moving within five years from screening in only a few hospitals, mainly associated with research studies with no state requirements, to nearly universal implementation in all but two states.
2. Early Studies and 2009 Scientific Statement
The need for additional methods to identify infants with CCHD early and prior to circulatory collapse was made very clear in one research study that investigated missed diagnosis of CCHD in California. More than 50% of CCHD deaths (up to 30 infants a year) could be attributed to late or missed diagnosis in the neonatal period in the state of California alone [
7]. Evidence presented in the Chang study and others [
8] demonstrated that additional methods of detection for CCHD, aside from prenatal ultrasound and physical examination of the neonate, were needed. If infants with CCHD could be identified, diagnosed, and receive an intervention (cardiovascular surgery or cardiac catheterization), survival and morbidity outcomes could be improved.
Although the concept of using pulse oximetry as a screening mechanism was explored in research articles both in the U.S. and Europe as early as 1993 [
9,
10,
11,
12], screening had not yet been implemented in U.S. newborn nurseries or required in any states. In 2005, Mississippi proposed legislation suggesting that pulse oximetry screening be used as a strategy to identify additional instances of newborns with CCHD [
13]. Tennessee also considered early pulse oximetry screening legislation, but at that time, cardiologists, concerned about false positive studies, were hesitant to support the concept of CCHD screening as a state mandate [
14]. Pulse oximetry screening was gaining significant attention as a potential strategy to improve the timely recognition of CCHD; the scientific community responded by reviewing the state of evidence related to the use of pulse oximetry in newborns to detect CCHD [
15].
On behalf of the AAP Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn and the American Heart Association (AHA) Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research, an expert writing group was tasked with evaluating the state of evidence on the routine use of pulse oximetry to detect CCHD. In 2009, they released a scientific statement concluding, based on an analysis of papers from 1966 to 2008, the following: CCHD was not being detected in some newborns prior to discharge from their birth hospital, resulting in significant morbidity and occasional mortality; if routine pulse oximetry is performed on asymptomatic newborns after 24 h of life but prior to discharge, additional CCHD could be detected, particularly in hospitals where on-site pediatric cardiologists and pediatric cardiovascular services were available; and that screening could be conducted at very low cost and risk of harm [
15]. However, the expert group went on to emphasize that further studies in “larger populations and across a broad range of newborn delivery systems” was required to determine whether pulse oximetry testing should become the standard of care in the routine assessment of neonates [
15].
3. Evidence from Europe
While the AHA/AAP expert writing group was performing their review of the evidence and grappling with the need for population level data to validate using pulse oximetry as a CCHD screening tool, researchers in Europe were poised to publish several important studies that would provide the precise evidence needed. Studies from Sweden, the United Kingdom, and Germany demonstrated that screening for CCHD at the population level had the required sensitivity and specificity to meet the criteria for newborn screening.
Perhaps most influential was a study from Sweden by Granelli et al. It was complete but not published in time to be considered in the analysis by the 2009 AAP/AHA writing group. This study analyzed 39,821 newborns who were screened using pulse oximetry and compared the strategy of physical exam alone with pulse oximetry screening alone, and in combination physical exam and pulse oximetry screening. The results were compelling, in addition to having an acceptable sensitivity (82.8% when combined with physical assessment) and specificity (97.8%); many of the false positives of pulse oximetry screening were not CCHD, but true positives for other important pathologies including persistent pulmonary hypertension of the newborn (PPHN), pneumonia, and infections, adding to the value of the screen outside of identifying unknown infants with CCHD. Although not all forms of CCHD can be detected by using pulse oximetry, this study concluded that 92% of ductal dependent cases could be identified if screening was performed in newborn delivery hospitals prior to discharge [
16].
An additional study was published in 2010. It involved 34 institutions in Germany in which 42,240 infants were screened (sensitivity 77.78%, specificity 99.90%, and negative predictive value 99.99%). Based on the analysis of those screens, the study team concluded that the addition of pulse oximetry screening could substantially close the postnatal diagnostic gap (those cases of CCHD not identified through prenatal ultrasound or physical assessment) to 4.4% [
17].
A meta-analysis conducted by researchers in the United Kingdom, which identified 13 high quality primary studies involving 229,421 infants screened using pulse oximetry, provided additional key support. The calculated sensitivity (76.5%) was similar to the Granelli study and the false positive rate overall was 0.14%. Interestingly, when the data was further broken down, the study found that the false positive rate, if the screening was conducted after 24 h, was significantly lower than if the screening was conducted prior to 24 h of life (false positive rate <24 h 0.5% versus >24 h 0.05%) [
18]. This distinction would later factor heavily into the development of the U.S. nationally endorsed protocol. However, it may not have properly acknowledged that additional secondary conditions make up the majority of those false positives.
These studies from Europe provided valuable evidence that would help to inform the development of the U.S. recommended strategies. In fact, two of the U.S. CCHD stakeholder meetings included expert representation from among the authors of the Swedish and UK studies.
4. Call to Action as CCHD Screening Is Added to the RUSP
In October 2010, following the availability of new evidence from Europe and a formal scientific evidence review process, the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC), whose responsibility it is to identify, evaluate, and make recommendations on which newborn screens should be added to the Recommended Uniform Screening Panel (RUSP), evaluated the research, heard the testimony of experts and families, and agreed that CCHD screening using pulse oximetry be recommended at the national level as part of the standard of care for newborn screening in the US. An additional review to propose a plan of action and to address the evidence gaps by the Interagency Coordinating Committee (ICC) was also completed prior to endorsement by the Secretary of Health and Human Services [
19].
A group of experts and stakeholders came together for a two-day meeting sponsored by SACHDNC and hosted by the American College of Cardiology (ACC) at the Heart House in January of 2011. Participants included physicians, nurses, scientists, representatives from Health Resources & Services Administration (HRSA), ACC, AAP, AHA, the American College of Medical Genetics, March of Dimes, the Association of Maternal and Child Health Programs, The Association of Public Health Laboratories (APHL), the National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), parent advocacy groups, industry partners, state public health program, and healthcare organizations [
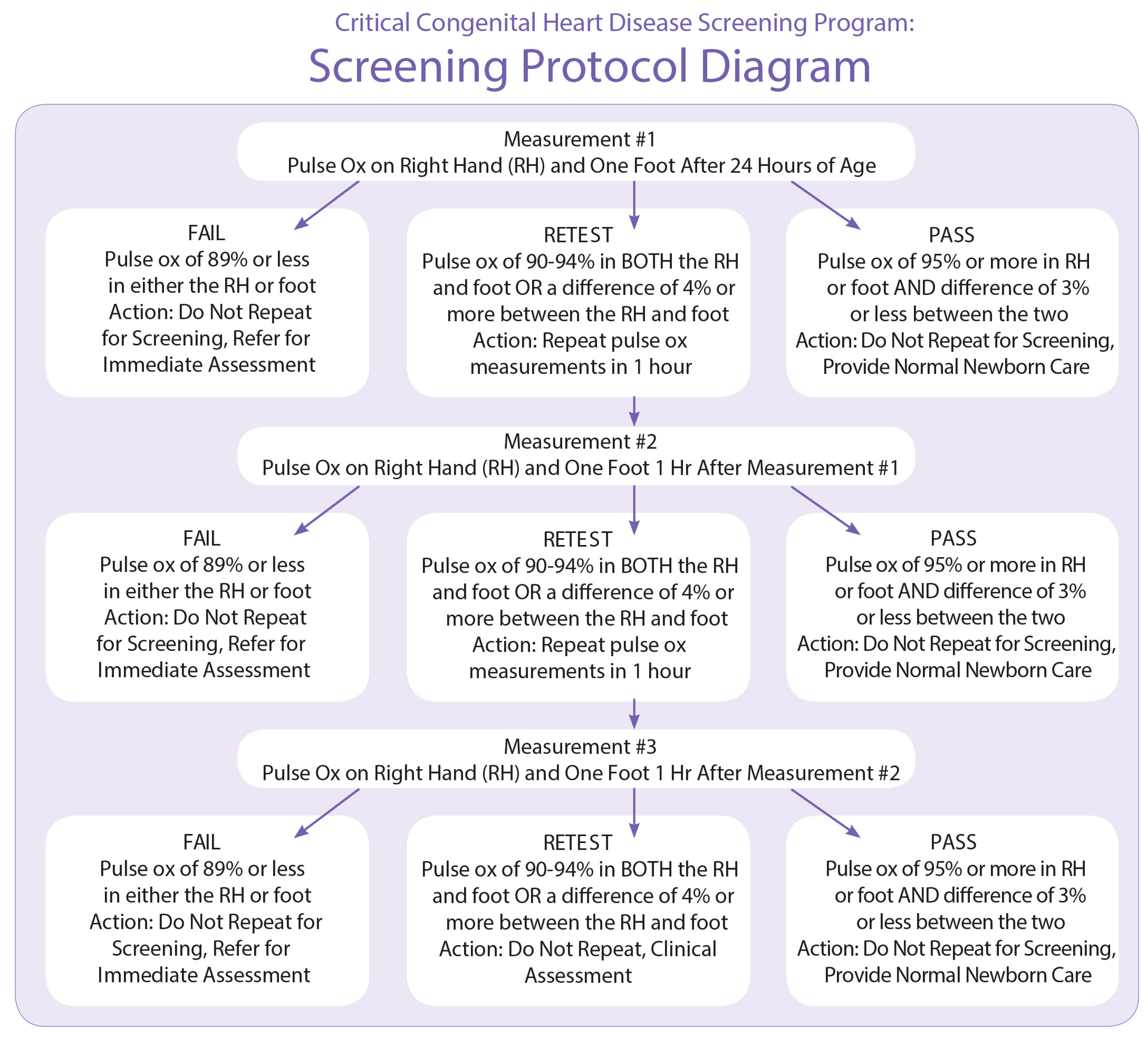
19]. Initial recommendations and a screening algorithm (see
Appendix A) based in large part on the Swedish protocol, specifying that screening be conducted using two limbs (the right hand and either foot) [
16] were developed [
5]. This algorithm was chosen mainly for its acceptable sensitivity (82.76%) and high specificity (97.88%) when paired with physical assessment. The expert group also recommended screening take place “at or around 24 h or prior to discharge” to maximize sensitivity while minimizing the number of false positives [
5].
CCHD screening was added to the RUSP by Secretary Kathleen Sebelius in September of 2011 [
20]. This recommendation was a first major step toward systematic implementation and represented buy-in at the federal or national level. The AAP [
21], AHA, ACC, and March of Dimes also quickly endorsed CCHD screening.
The need for an additional workgroup and stakeholders meeting arose to address challenges such as the selection of screening equipment, the standards for reporting screening outcomes, the training and education of health care providers and families whose infants were being screened, payment for screening, appropriate follow-up diagnostic testing, public health involvement, and oversight and to identify areas for future research [
22]. This additional work group meeting also took place at the Heart House in Washington, D.C. in February, 2012. Importantly, the recommendations from this work group included a minimum data set for both hospital level and state public health level reporting [
22].
Secretary Sebelius, as a part of her 2011 adoption of CCHD screening to the RUSP, included a Federal Agency Plan of Action focused on the key areas of (1) research, (2) surveillance, (3) screening standards and infrastructure, and (4) education and training [
20]. NIH was tasked with determining the impact of CCHD screening on the health outcomes of infants as well as the development of registries to help address research questions related to screening. The CDC’s main area of focus would be surveillance, including the evaluation of cost-effectiveness analysis and the monitoring of CCHD mortality and its link to other health outcomes. The Health Resources and Services Administration (HRSA) was charged with completing a thorough evaluation in collaboration with SACHDNC to evaluate the potential public health impact of universal screening for CCHD and to develop screening standards, and support the development of education tools and the infrastructure required for a public health approach to this point of care screen [
20].
HRSA responded by funding six CCHD state demonstration projects over a three year period, specifically to support the validation and dissemination of CCHD screening protocols and for the development of infrastructure around point-of-care screening for CCHD [
23]. The state programs chosen were: Michigan, New Jersey, Utah, Virginia, Wisconsin and a consortium of five New England states (Maine, New Hampshire, Vermont, Rhode Island, and Connecticut) [
23]. Shortly after the grants to the states were awarded, a third stakeholders meeting took place in Washington, D.C. in September of 2012 to kick-off and coordinate state efforts. Initial lessons learned following the completion of the grant period were published by the grantees in 2017 [
23].
The need for building public health infrastructure and sharing best practices and lessons learned with other states was particularly important as CCHD screening was only the second point of care newborn screen to be implemented nationally. The first was newborn hearing screening. HRSA also provided limited funding for another CCHD specific initiative, the Newborn Screening Technical assistance and Evaluation Program (NewSTEPs). NewSTEPs partnered with federal agencies in examining CCHD screening as well as state public health newborn screening programs to provide a central platform for CCHD screening resources. It also brought together a technical assistance CCHD work group and monthly webinars specifically related to implementation, education, and the spread of CCHD best practices [
24].
Since the 2011 addition to the RUSP, federal agencies continue to work towards addressing the needs identified in Secretary Sebelius’ recommendations. Researchers at the CDC have published studies examining the potential impact of screening implementation on the detection of CCHD and lives saved [
25], and cost-effectiveness [
26].
5. State-by-State Advocacy
The addition of CCHD screening to the RUSP at the federal level is non-binding on the states. To become required by law, each state would individually need to mandate CCHD screening, which, to date, all of the states, except for three, have done, either by statute, regulation, or executive order. Indiana, New Jersey and Maryland were the first three states to require CCHD screening in 2011, prior to the addition of CCHD screening to the RUSP. Parent groups, nurses, physicians, and professional societies worked together to go state-by-state advocating that CCHD screening be adopted as law. Peak advocacy efforts within the U.S. occurred in 2013 when 25 states adopted CCHD screening [
27]. By early 2015, 43 states and the District of Columbia required CCHD screening.
There are nuances in how states selected to implement, with varying levels of public health involvement. Differences in state CCHD screening laws include whether the mandate would be funded, whether all of the infants would be screened or whether exceptions to screening were permissible (special care nurseries, premature infants, out of hospital births, screening at altitude) and whether any aggregate or individual CCHD screening data would be reported. Most states choose to implement the algorithm recommended by the AAP with New Jersey, Tennessee, and Minnesota being among the few exceptions [
6]. Data collection, the extent to which education was provided, and the monitoring of implementation by state public health departments also vary greatly.
By the end of 2016, only two states, Idaho and Wyoming, were not screening. One state, Kansas, implemented at all hospital newborn nurseries without a state mandate. As of September 2017, the last two states that do not require screening, Idaho and Wyoming, have proposed regulations pending that would require CCHD screening. Rapid adoption by the states can largely be contributed to the alignment of several key forces, the validation of pulse oximetry as an effective screening method at the federal level, the endorsement of screening by national professional medical societies, and the support in the form of initial financial resources by federal and state public health agencies. Parents and clinical experts also played key roles as advocates [
28], testifying at both the state and federal levels in support of CCHD screening and its ability to save lives through early identification.
6. Systematic Implementation
Once required by state law, there was still considerable work to be done to implement CCHD screening in hospitals with newborn nurseries. Several different strategies were employed to ensure an efficient approach. These included the use of CCHD implementation toolkits, the sharing of educational videos for providers and families, a train-the-trainer approach, and the dissemination of resources, best practices, and solutions to common challenges through the aforementioned NewSTEPs webinars.
Systematic implementation was aided by the publication of a feasibility study conducted in Maryland, demonstrating that CCHD screening could be successfully implemented at a community hospital without the need for additional staff members, taking an average of only 3.5 min to screen and with few barriers [
29]. Showing that CCHD screening was feasible in a community hospital was important. Prior to this study, most screening implementation was conducted at large, often urban centers and most often associated with research.
In 2013, NewSTEPs, which continues to function as a part of a partnership between the Association of Public Health Laboratories (APHL) and the Colorado School of Public Health, began hosting monthly CCHD technical assistance webinars to assist in the dissemination of best practices and working solutions to commonly identified challenges to screening [
23]. Early topics included: educational resources available for parents and screeners, data collection including electronic reporting resources, defining roles and resources from a public health perspective, special populations, and how to address cost/equipment issues [
30]. NewSTEPs, in partnership with the Pediatric Congenital Heart Association, also brought together the HRSA grantees and other leaders in CCHD for an in-person meeting in February 2014. The purpose of this meeting was to discuss the current status of CCHD screening in the U.S. and to share ideas and provide guidance for state screening programs involved in all stages of implementation [
23].
Toolkits containing materials used to implement in a hospital newborn nursery made it possible to implement screening in a new hospital without having to gather and develop all of the necessary components for implementation each time. These toolkits contained important background evidence on CCHD screening, information on the screening protocol, education for providers, nurses, and parents, as well as competencies and forms to facilitate the documentation of screening results. Children’s National Medical Center developed one such CCHD screening implementation toolkit that was shared nationally and adapted to be state specific in Alaska, Missouri, Utah, and Colorado. Other states, including Rhode Island, Texas, and Wisconsin also created and extensively used CCHD screening implementation toolkits within their states.
Educational videos and training modules, incorporating evidence based content, were created and shared to provide those staff tasked with implementing CCHD screening with information on the importance, benefits and limitation of screening, technical assistance in how to perform the screen as well as information for parents on understanding the screens purpose and results [
31,
32]. Donations from families, federal and state funds all helped support the development of these freely available resources.
7. Lessons Learned
An early concern discussed extensively in stakeholder meetings and prior to the addition to the RUSP was that CCHD screening would result in too many false positives. This initial concern was not valid; early adopters did not find that the number of false positives overwhelmed the care delivery system in the way of unnecessary referrals to specialists or unnecessary echocardiograms [
33,
34]. Screening with pulse oximetry is not diagnostic for CCHD, it simply identifies an infant for follow-up to determine the cause for hypoxia. Referral and assessment by a pediatric cardiologist and an echocardiogram are required for a diagnosis of CCHD. Other causes of low blood oxygen levels should also be considered, such as assessment and laboratory work for infectious or respiratory causes. This distinction was particularly important in places that were geographically isolated or remote, where an infant would have to be transported over great distances for follow-up to occur. If another cause for a failed screen could be identified first, a referral and echocardiogram may not be required. Initial U.S. recommendations for follow-up stated that a comprehensive evaluation for the causes of hypoxemia be conducted and if the hypoxemia is not explained, a diagnostic echocardiogram with interpretation by a pediatric cardiologist is needed [
5]. However, subsequent research from Europe has shown that echocardiography is not always needed if another condition is found to be causing the low blood oxygen saturation, and that only 29% of those that fail the screen require echocardiograms [
35]. The number of false positives in the U.S. did not result in a large number of unnecessary echocardiograms once CCHD screening implementation was underway [
29,
34].
Misinterpretation of the screening algorithm and protocol violations were also reported as early implementation was undertaken. These issues could be addressed or minimized by implementing quality metrics and through the use of electronic decision support tools [
33,
36]. Best practices are still being developed with regards to screening special populations, particularly home births, births at altitude, and evaluating whether screening of infants in neonatal intensive care units is effective [
37]. Colorado requires CCHD screening of infants born at moderate altitude, accepting a higher false positive rate when compared to infants born at sea level [
38]. Physicians, midwives, and nurses in Wisconsin and Pennsylvania have tailored the AAP recommended screening protocol for use in out of hospital births [
30]. Best practices related to screening these special populations continue to be studied [
23,
24].
Although a few early adopter states have published data on their initial implementation of CCHD screening [
34], states that received grants from HRSA cited the lack of sustained funding for data collection activities to be the most common and important challenge identified [
23]. Initial federal and state funds were limited and not renewed. Data collection activities vary greatly by state. In some states, the public health department is not permitted to collect newborn screening data; whereas in others, such as Maryland, Minnesota, Virginia, Florida, and New Jersey results are reported centrally to the state department of health sometimes using electronic birth certificates or rely on the dried blood spot cards as the reporting mechanism. Other states have developed automated systems and have the ability to extract data directly from the pulse oximeter devices and electronically transmit and report results [
23]. One survey conducted in 2015 reported that 74% of states collected CCHD screening data or had plans to do so, however, that the amount of data collected varied from aggregate results on whether infants passed or failed, to all individual screening results [
27].
The dramatic variation in the amount of CCHD screening data collected and the differences in how the data is collected (electronic, paper, aggregate data vs. individual screening results) has made it difficult to analyze the data to accurately assess and inform the impact that screening has on reducing CCHD morbidity and mortality through early detection and intervention. In particular, the need for an “assessment of the certainty of diagnosis using standardized public health surveillance case definitions” is needed to be able to allow for consistent comparisons across state screening programs and over time [
24]. Although a national web-based repository to collect data on CCHD screening outcomes was created through NewSTEPs, without a requirement to collect CCHD screening data, which many of the CCHD screening mandates lack, the data is not available or submitted, to date, for systematic review and analysis nationally.
8. Conclusions
During a follow-up Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) meeting in August of 2017, committee members discussed whether CCHD screening may be one of the most successful and impactful additions to the RUSP, particularly in its ability to gain the attention and buy-in of the general public, and publicity in the form of newspaper articles and news coverage. Outcomes analysis to assess the national impact of requiring CCHD screening in the U.S. is currently underway and may rely heavily on administrative data, birth defect and death registry data in the absence of population level CCHD outcomes reporting, since no such robust U.S. clinical dataset currently exists. Data from Europe that was crucial in informing initial recommendations and strategies related to screening may continue to be informative for future U.S. algorithm refinements particularly around the question of ideal timing of the screen [
39].
A preliminary report, examining state death registry data from 2007–2013, was presented to ACHDNC in the August 2017 meeting. This report focused on the impact of state policies requiring CCHD screening, and found a 33% reduction in CCHD infant deaths in those states requiring screening and a calculated potential reduction of approximately 120 infant deaths due to CCHD per year in the U.S. as a whole [
40]. This reduction in infant deaths is the primary outcome hoped for by the many individuals, government entities, researchers, physicians, nurses, public health staff, industry partners, parents, and others that worked to ensure that CCHD screening using pulse oximetry would be implemented in the U.S. as a national policy. As research studies, federal and state programs continue to focus on CCHD screening, we will learn more about the granular impact of public health involvement and the implementation of screening on decreasing the late identification of infants with CCHD, and the subsequent impact on morbidity and neurodevelopmental outcomes as well as evidence based recommendations for special settings [
24].