1. Introduction
The goal of newborn screening (NBS) is to identify genetic and metabolic disorders that might cause significant health problems if not recognized in the first 24–48 h of life when they are asymptomatic and preventive treatment is available [
1,
2]. Tandem mass spectrometry (MS/MS), an advanced technology, is used to identify many disorders at one test [
3,
4]. NBS programs have been conducted in the United States since the 1960s. In the past two decades, numerous countries have expanded NBS to include tens of conditions. One of these countries is Israel, which has used an expanded NBS panel since 2008. The panel includes a total of 11 conditions, to supplement the two conditions that have been screened for over the last forty years. Around the time the program was expanded, one study reported that 91% of 277 educated women (ages 25–34, medium to high socio-economic status) who had recently delivered in Israeli hospitals stated that they were not aware that NBS had been performed [
5]. This high percentage of unaware mothers spurred this investigation of the educational and informed consent issues surrounding the expanded NBS program in Israel.
While the knowledge regarding parental understanding, preferences, and satisfaction toward NBS education during the prenatal period is growing [
6,
7,
8,
9,
10] and, in many cases, the lack thereof is explicitly criticized [
11], the attitudes and reflections of health professionals on parental education and informed consent for NBS remains under-investigated [
12]. This paper presents the findings of an empirical study exploring the views of officials and practicing physicians involved in the NBS process in Israel regarding issues of informed consent and parental education.
The Israeli program was worth exploring for several other reasons. First, it demonstrates the expansion of NBS on a national level, unlike many other programs in the United States and Europe. Second, the NBS process in Israel is conducted under governmental regulations, which are frequently revised. The most recent publications of the Ministry of Health, Department of Community Genetics are summary documents from May 2012 [
13] and the end of 2014 [
14], which provide updated results about program operations. The 2015 regulation [
15] added Severe Combined Immunodeficiency (SCID) to the guidelines. Thus, decision-making and regulation became more dynamic and flexible. Finally, and most importantly, the recent implementation of the expanded panel coincided with data collection for this study. Therefore, the ethical, legal, and social issues raised throughout the process were still fresh in respondents’ minds, making the findings and conclusions of the study particularly timely.
2. Education and Informed Consent for NBS
In 2001, the American Academy of Pediatrics (AAP) recommended that states evaluate the informed consent process for NBS tests in order to improve parental education and informed response to test results [
16]. The AAP stated that tests with established value do not require a signed consent form, but it necessitates provision of basic information to parents regarding the purpose of screening. Along these lines, the Committee on Genetics of the American College of Gynecologists and Obstetricians (ACOG) recommended that obstetricians provide counseling about NBS to pregnant women and their partners [
17]. Almost a decade later, experts in the field welcomed the ACOG recommendations, especially in light of the increasing complexity of test panels and treatments [
18]. At the same time, the limited practicality of the recommendations, given obstetricians’ other responsibilities, had been acknowledged by practitioners [
19].
The inherent tension between the practice of NBS and the principle of informed consent was acknowledged in a thorough report by the American President’s Council of Bioethics [
6]. According to the report, this tension increased following the recommendation of the American College of Medical Genetics in 2005 to expand the panel to include more conditions, in particular “untreatable” conditions for which the benefit for the neonate from screening is uncertain (e.g., Fragile X Syndrome) [
20], as well as cases in which there is a risk of inadvertent disclosure of carrier status. In these instances, parental informed decisions and the questionable justification for mandatory screening became crucial ethical issues [
21,
22]. Scholars argued that if screening panels are expanded to include diseases that do not meet the classic Wilson and Jungner criteria [
23], such as Duchene Muscular Dystrophy, screening should be voluntary and require informed consent [
24,
25]. Furthermore, empirical investigation revealed that perinatologists believe that parental education is important. At the same time, they admit that they do not discuss it with parents because they believe hospital staff and pediatricians will do so and because their knowledge about expanded screening is limited [
26].
In Israel, NBS for phenylketonuria (PKU) was initiated as early as 1964, only a few years after it had been established in the United States [
27,
28]. Screening for congenital hypothyroidism began in 1978 and proved to be efficient in terms of early detection and treatment [
29]. The program was significantly expanded in 2008, following the recommendation of an advisory committee appointed by the Ministry of Health (for a comprehensive review of the evolution of the expanded Israeli NBS program see the author’s dissertation [
30]). In the past decade, three guideline documents for the regulation and operation of the expanded NBS program in Israel were published by the Ministry of Health [
15,
31,
32]. The earlier document referred only to screening for phenylketonuria (PKU) and congenital hypothyroidism. The second document referred to the expanded screening panel, which includes 11 conditions [
33]. This guideline document (in clause 2.1) advises the responsible physician or another member of the medical team who follows the pregnancy, to explain the significance and meaning of NBS to the prospective mother or parents as delivery approaches. The provision of this general NBS information should be documented in the pregnancy follow-up record. While the 2007 document did not mention the issue of parental consent to screening, in the 2009 document an opt-out option for all conditions in the expanded panel was established [
32] (clause 4).
In order to improve parental NBS education, a website was launched in 2009. It provides general information about NBS to the public (Israel Ministry of Health: the Department of Community Genetics website at URL:
https://www.old.health.gov.il/yelod/, in Hebrew, obtaining results requires both the mother’s and baby’s ID). In addition, the website enables access to NBS results of infants born in 2009 or later, whose screening results were negative (e.g., in the normal range). Information about the website is included in the governmental regulation [
32] (clause 2.1). According to the program directors, the website is also referred to in a brochure distributed by hospitals to pregnant women registered to deliver at its facilities, as well as in a letter the mother receives before she is discharged from the hospital after delivery. In case of a home delivery, the pregnant woman should be asked to sign a special form which informs her about the significance of NBS and her responsibility to perform it in time [
32] (clause 2.2). In the case of a hospital delivery, prior to drawing blood for NBS, the obstetrician or midwife should ascertain that the mother knows about the screening and its significance. The educational process and the opt-out option as they are actually practiced will be discussed at length in the following sections.
3. Methods
Data were collected in two phases. First, twenty-one semi-structured, in-depth interviews with three groups of health care professionals were conducted between July 2007 and March 2008. Purposive sampling was used and sample size was determined by thematic saturation, a standard qualitative methodology concept that describes the point at which themes are fully accounted for and no new concepts emerged from successive interviews.
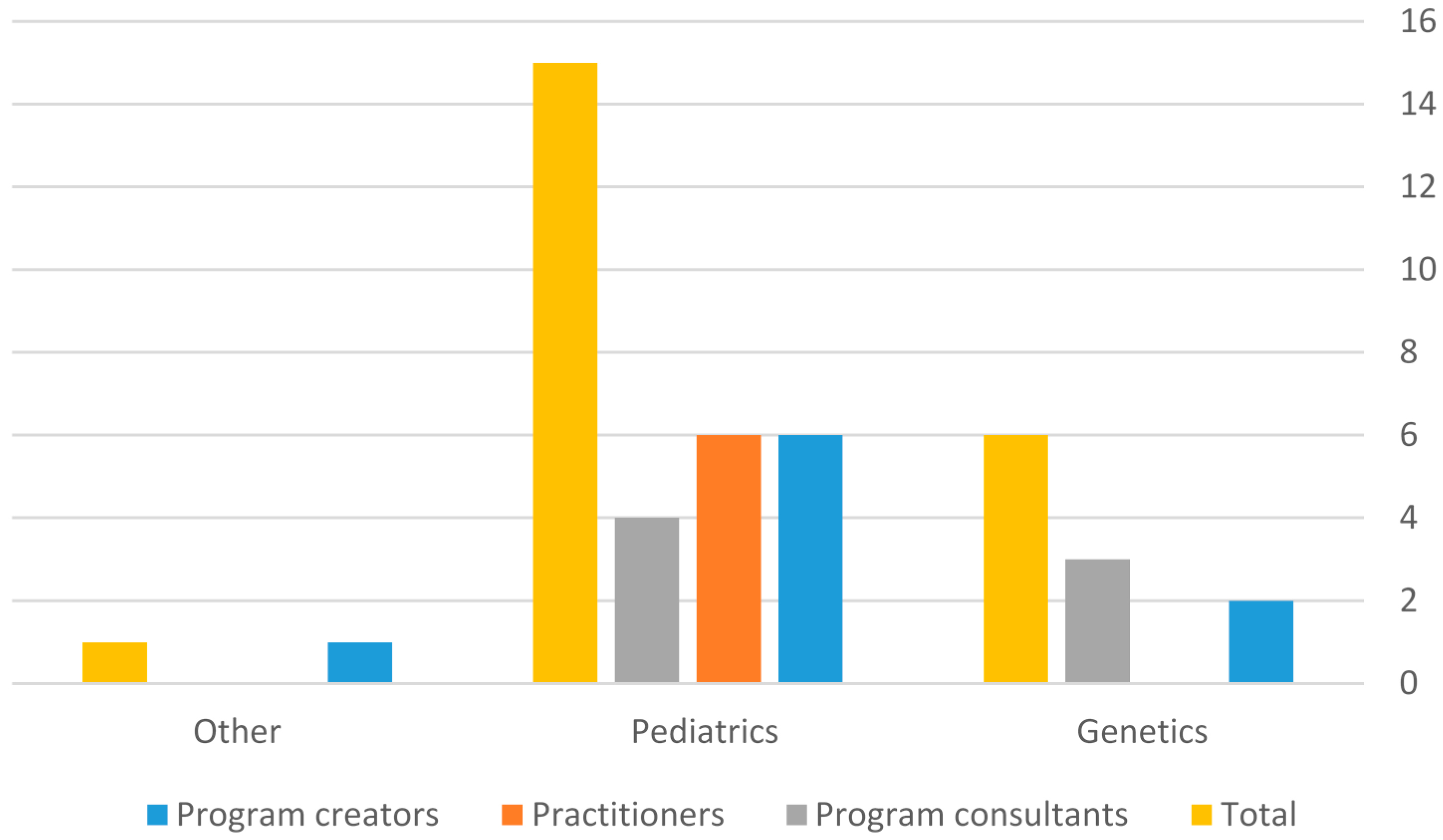
In terms of their roles, three distinct groups of health professionals were interviewed. The first group included Israeli physicians and officials who were substantially involved in the creation, design, implementation, and delivery of the expanded NBS program (program creators—PC); three were key informants. The second group consisted of Israeli physicians involved in the delivery of “screen positive” results to parents or in the follow-up of newborns (practitioners—PR). In the third group, six informants provided the context and the background for the study or self-identified as an external program consultant (CON). The consultants were physicians or NBS program officials whose programs served as a model for the emerging program in Israel. Four were American and two were European. They assisted in the selection of the other interviewees who belonged to the group of program creators or practitioners. The interviews were conducted in two phases. The first phase was conducted during the design and implementation of the emerging program. The second phase was performed eight years later (in the summer of 2016) and included three additional interviews: a practitioner who had not been interviewed previously and two key informants who were interviewed in the first phase and were still involved in the NBS program. The relatively long interval between the two phases allowed the participants in the second phase to reflect on the development of the new program from both prospective and retrospective perspectives, thus enriching the depth of the information. It is noteworthy, that among the interviewees, six of the nine program creators were pediatricians (with various subspecialties) and only two were geneticists, one specialized in internal medicine. In the Practitioners group, all six interviewees were pediatricians. In the consultants group, four of the seven were pediatricians, three were geneticists. The pediatricians in the three groups specialized in endocrinology, metabolism, neurology, neonatology, clinical immunology, or gastroenterology.
The interviewees’ general characteristics and primary medical specialties are summarized in
Table 1 and
Figure 1, respectively.
In the interview (which covered a broad range of topics) the respondents were asked about their background, ethical, legal, and cultural concerns in the decision-making process about the expansion of the panel and reporting of results. Among other questions, interviewees were asked to discuss the current education provided to prospective parents or parents of newborns, as well as the informed consent process in the expanded NBS program. In addition, they were asked if they think the existing process should be modified. Selected questions from the interview guide are presented in
Figure 2.
The interview guides were pretested with three interviewees (key informants) during March and April 2007. Based on these pilot interviews, the questions were revised before the interview process was continued. In addition, the interview guide was adjusted before each interview was conducted based on the interviewee’s background, specialty, and/or the role he/she played or was supposed to play in the emerging NBS program.
Written and verbal informed consent was obtained from all interviewees for the interview itself and for recording it. All interviewees were provided with a description of the goals of the study prior to data collection. An application for human subject research was submitted to the Case Western Reserve University Institutional Review Board and an exemption was granted in June 2007, given that no risk of harm was associated with the in-depth interview. Respondents disclosed only professional information as part of their routine job and no personal health (or other) information. Privacy of interviewees and data confidentiality were protected. Interview data were entered into the computer using ATLAS.ti 5.0 (Scientific Software Development GmbH, Berlin, Germany), a software program for coding and analysis of textual ethnographic and interview data.
Data analysis for this project was exclusively qualitative and data were analyzed in a circular manner, using an interpretive cycle. Data were described and organized using an editing approach. In addition, an emersion-crystallization approach which consists of coding at the completion of analysis was used [
34]. Interviews were coded and analyzed using the
Grounded Theory method. Increased attention was given to the voice of the respondents, in order to achieve rigorous comprehension of their experiences. Open coding was performed by reading through the interviews and identifying important repeating themes. A code guide was created using
Atlas-ti. Repeating statements and views were described as codes. Similar codes were grouped into code families.
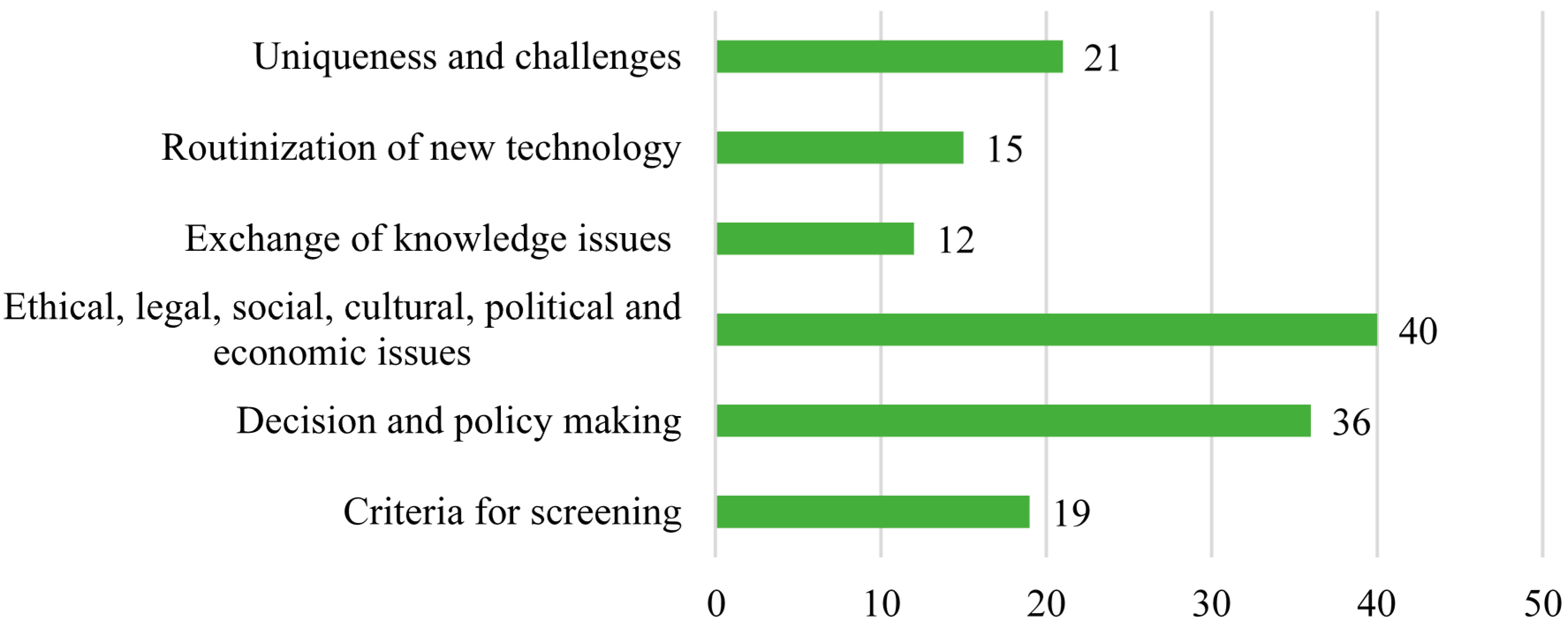
Codes were linked into theories of the social construct of medical knowledge, routinization of new technologies, evidence-based medicine, research paradigms for introducing technological advances, the unique social and cultural context in Israel, and the influence of American and European experiences on the creation and implementation of the NBS program. Code notes and theory notes were recorded to describe relationships among themes. Verbatim quotes from informants were used to exemplify the concepts and theories. Six code families were identified, including criteria for screening; decision- and policy-making issues; ethical, legal, social, cultural, political, and economic issues; and the exchange of knowledge, routinization of a new technology, and uniqueness and challenges of the program. The code families (and number of codes in each family) are described in
Figure 3.
The detailed coding guide, including the number of quotations creating each code, is available in
Supplementary Materials, which appears at the end of the manuscript. The following sections refer to the two major code families (consisting of 53% of the total number of codes): 1. Decision- and policy-making issues and 2. Ethical, legal, social, cultural, political, and economic issues.
4. Results
A clear distinction emerged from the data between views of program creators and practitioners. As noted earlier (
Figure 1) among the interviewees, six of the nine program creators were pediatricians (with various subspecialties) and only two were geneticists. In the practitioners group, all six interviewees were pediatricians. In the consultants group, four of the seven were pediatricians. Therefore, the differing views of the two groups are more likely related to their position or role in the program rather than with their medical specialty.
The two groups diverged regarding two major themes: First, program creators described “the indifferent parental attitude to the conduct of NBS”, which in their view was particularly surprising given the widespread use and high motivation to perform prenatal screening in Israel. Practitioners, on the other hand, were not concerned about this parental attitude or articulate that it is an obstacle to successful program implementation. Second, when asked about the content and timing of education for NBS, its dissemination, and the warranted standard of consent for NBS, only program creators discussed the need for a better mechanism for NBS education and unanimously advocated for higher standards of informed consent than the opt-out option currently in use. In the practitioners group, on the other hand, a continuum of opinions emerged regarding two issues: (a) how consent for screening should be obtained from parents, and (b) what should be the content of the information conveyed. While some practitioners argued that informed consent is impossible to achieve, others suggested that implied consent (by arriving at the hospital and staying post-delivery) is sufficient. For yet another group, general consent for newborn medical care seemed sufficient. Several respondents opined that the existing opt-out option for screening is appropriate and, finally, some agreed with program creators that written informed consent should be obtained. The themes and group differences regarding it are discussed in detail in the following section.
4.1. Program Creators Are Concerned by “Indifferent” Parental Attitude to NBS
The first theme revealed by data analysis was the opinion shared by program creators, regarding what they described as an “indifferent” attitude of parents of newborns to the practice of NBS, in general, and to the expansion of the panel of disorders in particular. These respondents found the “indifferent” approach particularly surprising because, in their view, it stood in sharp contrast to the overarching acceptance and extensive use of prenatal testing in Israeli society. The gap in parental attitudes towards the two procedures is reflected in the comment of a public official who was a key player in initiating and implementing the program: “
I cannot understand it…had we been in a country characterized by no awareness of the public to medicine I would say ‘they just do not know about it’ but…anytime there is something about prenatal screening in the New York Times people ask me ‘How come you are not doing it in Israel?’… However,
when they talked about the recommendations for expansion of NBS authored by the American College of Medical Genetics in 2005 [
35],
there were no questions”.
When asked to explain the gap in knowledge between prenatal and postnatal screening, program creators mentioned several possible reasons. First, respondents acknowledged that a major contributing factor to the lack of public interest in NBS is insufficient parental education about the process. A program creator clarified that throughout the planning phase of the expansion process, the program directorship decided that in its early phase the current mechanism of opting out would be left intact. In the near future, he explained, the efforts of the program directors will be focused on the plan to have a real opting-out mechanism by providing education about the purpose and broader social and ethical implications of NBS before consent is obtained. He acknowledged that, in the meantime, since the mother is not aware of the option to refuse screening, this is in fact “…the worst kind of opting out because the mother does not have a real choice…”
A public official who was very involved in initiating the program justified the rationale of not providing relevant information: “I thought we would start with public education (for NBS—S.Z.) but we could not afford it because it would create panic...” Another program creator supported this opinion: “…maybe it is better not to bring it up (the issue of informed consent for screening—S.Z.) Once you start asking, various organizations will start saying they are opposing this and that…why bring it up? In this case, it is quiet so far. In the U.S. there was a heated debate… there still is”.
The second explanation offered by program creators to the “indifferent” parental attitude to NBS is psychological and socio-cultural in nature. In contrast to prenatal screening, NBS is performed after the baby is born when there is no longer an option of terminating the pregnancy. In a previous study, Rachel Grob [
36] showed that early detection of cystic fibrosis (CF) through NBS, as opposed to diagnosis after symptoms appeared, affected parents’ feeling of competence to care for their newborn and their sense of who the child is. Rather than “falling in love with the new baby”, the disease becomes the center of attention.
In the current study, one program creator articulated this psychological effect of NBS on parents, as opposed to prenatal screening:
“…Why is NBS less attractive than prenatal screening? The prenatal phase is unknown. People can’t live with the unknown. However, once the mother gives birth, once she affirms that the baby has ten toes and ten fingers, and in general looks “normal”, the anxiety level lessens drastically. At this point, it is extremely difficult to get the mother interested in asymptomatic future disorders that may or may not affect her baby…approaching a mother immediately after she has had a baby, while she holds this charming thing in her arms…people are not interested in talking about diseases once the baby is born”.
In short, the program official argues that by the time the newborn’s blood is collected and tested, the process of psychological bonding between the infant and parents is strengthened and at this point it is too difficult to think about a possible serious disease. Parents prefer to overlook the possibility of early identification of a disorder or a disease that may only become symptomatic in the future. (This comment relates to the fact that some of the disorders included in the expanded screening panel such as medium chain acyl-CoA dehydrogenase (MCAD) may manifest clinically in some individuals identified as “screen positive” but not in others, despite the similar genetic makeup of the two individuals-S.Z.).
As for the future, a program creator explicitly made a distinction between different goals of screening and explained that obtaining consent in an improved manner would be essential if the scope of screening is extended or the future use of bloodspots for research is permitted. He admitted that “…the idea is to get women’s signatures during pregnancy but, not too close to delivery…this is a very complicated process…but if we include borderline and problematic conditions…or use (residual NBS bloodspots—S.Z.) for research, we must obtain consent for screening…”
4.2. Practitioners Expressed Diverse Opinions Regarding Informed Consent and Education for NBS
The second theme that emerged from the interviews focused on the diverse reflections of practitioners on the timing and the content of education, its dissemination, and the standard of informed consent for NBS. One practitioner emphasized the limited time physicians can devote to patients. These time constraints, he explained, do not allow them to provide detailed information about the procedure to each prospective mother in her own language. As an alternative, he suggested, “…it should be publicized in the media that there is going to be NBS, and the purpose of identifying severe diseases for which treatment exists”. Another Israeli physician from the practitioners group thought that only general NBS information should be disseminated to parents. He stated that, “I think the general concept of identifying conditions before symptoms arrive should be better explained, rather than the provision of details and horrifying information on serious diseases and how bad it can get”.
Regarding the required standard of consent, the same respondent suggested that: “She (the mother—S.Z.) should know it (NBS) is performed, but there is no need to ask for her consent, not now and not with the expansion. People can’t grasp it…parents shouldn’t be signed on consent forms all day long…not even in the prenatal period and if she (the mother—S.Z.) did not agree, there is a legal cause of action”. Other practitioners thought that implied consent for NBS would be sufficient. An Israeli practitioner argued that “…by arriving to give birth at an Israeli hospital where the service (of NBS—S.Z.) is offered, the woman implies that she agrees to receive all examinations and services included in the “health basket” (the medications, services, and other medical benefits every Israeli resident is entitled to according to the National Health Insurance Law, 1994). Obviously, if parents object to screening, it is their responsibility to inform the hospital about it”.
Keeping the existing opt-out option was recommended by several other practitioners.
Other interviewees from the practitioners group criticized the existing informed consent mechanism. An Israeli practitioner said: “People know nothing. This whole informed consent mechanism is fake. A person gets in the hospital and signs…forms, among them those things (NBS consent forms). He has no clue what it is. There is no informed consent de facto. De jure there is, de facto there isn’t”. An international practitioner described a similar situation in his own country: “There is ‘consent’, there is no ‘informed’. I think it (the informed consent mechanism—S.Z.) should have been modified. Not now, a long time ago. I have done blood work and got abnormal results for hypothyroidism. While for most of the kids confirmatory testing would be normal, if the parent doesn’t know about it (the NBS process) he can suffer a heart attack when we ask him to come in because there was a problem with the heel prick. We are responsible for this heart attack. We should have said from the beginning that ‘we are doing screening…sometimes we’ll call you back for second testing. It doesn’t say the kid is sick’…”
Finally, another practitioner was pessimistic regarding the possibility of achieving meaningful informed consent in the near future: “If you want a signature that is one thing. If you want… informed consent… that is not possible, even with physicians”. One international expert strengthened this discouraging view by posing the rhetorical question "How are you going to educate a person to the level that they can make a truly informed consent about things they can’t even pronounce?”
The major themes that emerged from the interviews are discussed in the next section.
5. Discussion
Data analysis revealed two major themes. The first is what program creators exclusively described as an “indifferent” parental attitude to the process of NBS in contrast to prenatal screening. The second is the diverse opinions articulated by practitioners regarding the desired content and timing of NBS education, the mechanism by which it should be disseminated, and the warranted standard of consent for its conduct.
The immediate and trivial explanation given by program creators for parental indifference parents towards the process of NBS was the lack of education they receive (p. 7 par. 2–3). The second argument, which was widely discussed by program creators to explain this unexpected parental approach, is the ongoing psychological bonding with the baby which begins just after birth. At this time, respondents from this group claimed, parents find it hard to deal or even think about the possibility of early identification of a disorder or disease that may only become symptomatic in the future, therefore they choose to ignore it (p. 7 par. 4–p. 8 par. 1). This line of thinking did not emerge from the interviews with practitioners.
It is noteworthy that this parental behavior does not agree with the literature regarding current attitudes to prenatal screening in Israel. Israeli society is characterized by quasi-universal utilization of ultrasound, among both Jewish and Arab women [
37]. Among the key motivational factors for pregnant women in Israel to perform prenatal testing is the fear of giving birth to a severely sick or disabled child [
38,
39]. However, abortions are frequently performed when fetuses have mild to moderate congenital abnormalities without serious discussion of its moral appropriateness [
40]. These studies portray high awareness and willingness of prospective parents to make difficult decisions to prevent any possibility of a disability in their child in the prenatal period.
Indeed, in the case of NBS, the baby is “here to stay” and there is no longer an option for abortion. However, one would anticipate that the emerging bonding of parents with their baby is more likely to encourage, rather than discourage, parents to do everything to improve the child’s health and well-being, including identifying rare genetic and metabolic disorders that may manifest symptoms later in life. In other words, in this cultural and social environment, one would expect that parents of newborns, who are willing to go a long way to avoid a disabled baby, would proactively seek expanded NBS, which might allow them to significantly improve the baby’s health in case a genetic or metabolic disorder is identified by NBS and diagnostically confirmed. However, the results of this study show that this is not the case.
The question evolving from this theme is why program creators, in contrast to practitioners, emphasized the seeming indifference of parents towards the process of NBS. Understandably, given their position as program creators, the officials and physicians who were very involved in the design and implementation of the new, expanded program, were eager to know what parents thought about it. Admittedly, program creators were better suited than practitioners were to identify and acknowledge their contribution to the indifferent attitude of parents. (p. 7 par. 2–3). I suggest that because program creators are responsible for all programmatic aspects, including obtaining consent and dissemination of information, they focused on parental indifference towards the program, were motivated to understand the reasons for it, and planned to confront and resolve it in the near future.
The second theme that emerged from the data concerned the varied opinions articulated by practitioners regarding the desired content and timing of education for parents, the mechanism for its dissemination, and the current standards of parental education and informed consent for NBS. Indeed, the opinions of practitioners regarding what information should be conveyed to parents about NBS, when it should be conveyed, how it should be disseminated, and what is the warranted standard for obtaining consent for screening were substantially diverse.
Once again, it should be noted that program creators explicitly voiced their deep concerns, perhaps even self-criticized the lack of education, the insufficient mechanism for its dissemination, and the current opt-out mechanism, which one of them referred to as “the worst kind of opting-out” (p. 7 par. 2). To remedy this situation, they discussed the plans to improve both the dissemination of NBS information to parents and the opt-out mechanism in the future.
As opposed to this approach, practitioners expressed diverse views including that informing parents about the general concept of identifying conditions before symptoms arise rather than providing detailed information about the diseases would suffice, in order not to frighten people (p. 8 par. 3–4 and p. 9 par. 1–2) or similarly-supported publicizing NBS through the media (p. 15 par. 2), instead of using the traditional route of communicating information to parents personally by the medical team throughout advanced pregnancy or post-delivery. Others articulated the position that parents ought to inform the medical team if they objection to screening (p. 9 par. 4). Yet two other interviewees, one international practitioner and a local one, argued that the current informed consent process is basically non-existent (p. 9 par. 1) .In other words, practitioners did not discuss the creators’ plan for improving mechanisms for education and consent for screening.
I suggest that the divergent opinions regarding education and informed consent among practitioners, as opposed to program creators, evolved from the different roles of the two groups in the program design and implementation. Program creators are oriented from the outset towards defining and measuring a successful operation of the program in various aspects, including education and informed consent. As clinicians who have direct responsibility on the program, they also set up the criteria for evaluation of the program. Therefore, they are in a better position to appreciate the difficulties in obtaining meaningful consent and provision of better education. Practitioners, on the other hand, are oriented towards the medical care and the well-being of the newborn as a whole, rather than on the specific program objectives, as important and essential as it may be. This point of view leads to the differing standards by which practitioners evaluate the process of education and informed consent in the expanded program. Data reveal two additional statements of practitioners, which strengthens the view regarding their holistic approach to NBS. First, practitioners are skeptical about the possibility of achieving “genuine informed consent” for NBS regardless of the mechanism used in practice (p. 9 par. 1–2). Second, practitioners suggested that achieving higher standards of informed consent and education are not realistic because of the direct effect on their day-to-day workload (p. 8 par. 3). Both statements, I argue, are commensurate with the argument that practitioners indeed focus on the flow of care rather than on achieving the programmatic goals of parental education and informed consent.
6. The Current State of NBS in Israel
As noted, in order to evaluate the current state regarding informed consent and education for NBS, three short interviews were conducted in July–August 2016. A second interview with two program officials did not reveal a clear picture. One of them argued that nothing has really changed since the implementation of the expanded program in 2008, and parents were “still unaware of the NBS process” as discussed in the previous sections. The other respondent, however, was adamant that parents were significantly more aware, because in practice hospitals refer the mother to the NBS website in the discharge letter. He noted that “the higher awareness is shown in the increased visits to the NBS website (thousands as opposed to hundreds in the past) and parental inquiries regarding the procedure”. As for refusals, he noted that they are still relatively rare. Around 200 refusals were reported among the 170,000 newborns screened for each year, less than half of the “insistent refusals” arose from home deliveries who never reached the community clinics (Tipat Chalav, in Hebrew) where NBS is performed after home deliveries. At the same time, he noted that the indifferent parental approach to NBS is still evident. “Once parents enter the NBS website and view the list of conditions, you would expect that they ask about confirmation of screening or what else they should do to make sure their child is healthy, but they never do. Rather, they find the initial negative screening results sufficient and in fact, the whole issue seems more of a hassle to them”. As for the opt-out option, he explains that the possibility of obtaining informed consent from parents on the newborn discharge form was discussed at a Knesset (the Israeli Parliament—S.Z.) committee and was acknowledged as “an important, yet problematic issue” which was left to be determined in the future, once the screening process would include molecular (in addition to metabolic) conditions, according to the requirements of the Genetic Information Law (2000) [
41].
An interview with a neonatologist, practicing in a large hospital in the center of Israel, strengthened the holistic view of NBS education and consent process from the perspective of practitioners. The interviewee described the NBS program as “outstanding”. She explained that parents receive information from a nurse at the neonatal unit about the procedure at the time of the heel prick. In case they refuse screening, a brochure about the process is offered [
42] and the opt-out option is explained. The practitioner reported that she had never experienced parental refusal for NBS.
In addition to the interviews, the different perspectives of program creators and practitioners regarding NBS were reflected in a professional panel on the ethical issues in Cystic Fibrosis the author participated in, in June 2016. In Israel, the inclusion of cystic fibrosis (CF) in the NBS panel became a new topic for debate between program creators and practitioners as the final version of this paper was written in June 2016. In the professional panel, program creators and Ministry of Health officials resented NBS for CF because, in their view, prenatal screening for this condition eliminates the need for mass screening and the right not to know genetic information, which is violated if a carrier is identified through molecular testing, as opposed to the “Dor Yeshorim” program which allows for matching couples before conception without disclosing their genetic information (program creator, personal communication, August 2016). Practitioners, on the other hand, have justified NBS for at least some CF mutations as an alternative for prenatal carrier screening, given the cultural barrier which prevents many pregnant women from performing prenatal screening for CF and the importance of early identification of the disease for prognosis [
43]. Ultimately, NBS for CF was not included in the 2016 health basket (i.e., a procedure whose expenses are covered by the government). A possible explanation given by a program official in the Ministry of Health (personal communication, June 2016), is that it is a genetic, rather than a metabolic condition.
This debate demonstrates the different perspectives of program creators and practitioners. The NBS program creators’ point of view stems from their perceived role and sense of responsibility to obtain consent for screening, inform parents about “screen positive” results and ensure future follow-up of the identified neonate. For them, as program officials, if the condition is included in the prenatal screening panel, it is redundant to re-screen for it postnatally. In the eyes of practitioners, however, the NBS program does not stand alone, but rather as part of the newborn medical care. In other words, given their holistic perspective, their goals are the overall health and well-being of the baby and providing the best possible medical care. Therefore, they are tolerant towards “repeat” NBS for some CF mutations in order to identify sick babies that may have been missed by prenatal screening.
An indication of the holistic view of practitioners is demonstrated in a refusal form which is currently used by a general hospital in Israel. The form informs parents about screening for 12 inherited diseases (including PKU and congenital hypothyroidism), which are of major importance for the future development of the newborn, and the significance of the early diagnosis. In addition, the form notes that “the process involves screening; thus, the diagnosis rate is not 100%, but rather close to it. The Ministry of Health oversees the follow-up”. Interestingly, the same brochure informs the parent about eight more procedures the newborn is about to go through, in addition to NBS, including cord blood treatment, eye antibiotics, intravenous vitamin K injection, Hepatitis B vaccination, oral sucrose as a pain killer, hearing screening and “other blood tests and treatments as required/recommended by the Ministry of Health”. For each of these conditions, including NBS, there is an opt-out option on the form. Gathering all those conditions and procedures in one form strengthens, I argue, the holistic view of practitioners.
7. Conclusions
This study examined reflections of physicians and officials in Israel, and internationally, regarding informed consent and education of parents about the expanded NBS program in Israel. Semi-structured interviews revealed two major themes. First, program creators who were involved in the creation, design, implementation, and delivery of the expanded NBS program were concerned about an “indifferent” parental attitude toward NBS. They explained this attitude by the psychological bonding of parents with the baby, which takes place around the time of the heel prick, and the uncertainty regarding future manifestation of symptoms of conditions identified by NBS. Practitioners, on the other hand, who were involved in only the delivery and follow-up of NBS results, did not discuss this parental attitude. The second theme is with respect to the divergence between program creators and practitioners regarding how to evaluate parental education about NBS and the informed consent process. The evaluation includes the content and timing of NBS education and the desired standard of consent to the process. Program creators were concerned about the lack of parental education and the existing opt-out mechanism. Practitioners, on the other hand, advocated for providing general information about the concept of NBS, in person or through public media, rather than disseminating detailed information about the process. In addition, they indicated that parents are responsible for seeking out information about NBS, if they so wish. Others were simply content with the current process.
I suggest that program creators, due to their position, assess NBS as an independent, stand-alone process about which parents should be informed and educated. This perspective leads them to focus on the indifference of parents to NBS as opposed to prenatal screening as a non-optimal achievement of one programmatic aspect, education and informed consent. Practitioners, on the other hand, perceive the medical care of the newborn holistically, focusing on the overall well-being of the baby. Therefore, they would be satisfied if the best possible medical care is provided to the newborn, by screening, confirmatory diagnosis, and follow up, even if parents are less informed about the process. For practitioners, given their holistic approach, the provision of general information about NBS to parents before the heel prick, or even in the public media, might suffice. Some are satisfied with implied parental informed consent by their arrival at the hospital for delivery. As for informed consent, most practitioners agree with the current opt-out option. Finally, the findings that practitioners were skeptical about the possibility of achieving genuine informed consent for NBS, their prospects and worries regarding an increased workload (which, in turn, may harm the quality of care of screen positive babies) if requirements for parental education and consent are intensified, strengthen the argument regarding the holistic view of practitioners.
7.1. Limitations
There is a small group of program creators and practitioners who, according to the sampling strategy, were involved in the creation, design, implementation, and operation of NBS in Israel from the outset of the program expansion. Consequently, data analysis is solely qualitative and the breadth of the data is limited. Given the context of NBS, which is done mostly at one central laboratory, using mixed methodology was not practical and analysis is based on semi-structured interviews only. Being a dissertation work, the data was collected and analyzed by only one researcher; thus, it may be biased to some extent.
7.2. The Present and Future of Expanded NBS in Israel and Internationally
For historical, political, and practical reasons, similar to the situation in the NBS program at hand, decision-making in the field of NBS in many countries is still performed using a non-inclusive model; that is, by a relatively small group of public officials and practicing physicians [
21]. Therefore, the observations of this study are relevant to health care professionals internationally. Notably, the data collected in this study shows that the education and informed consent process for expanded NBS in Israel were planned from the outset (2007) to be upgraded in the near future. Revising the program after a decade may not improve the indifference of parents towards the process, given the psychological reasons discussed above; yet, considering the holistic point of view to the decision-making process regarding education and informed consent of parents may well contribute to a balanced evaluation of successful education and consent mechanisms in the NBS program.
It is, therefore recommended, that prior to further expansion of NBS programs, issues of parental education, communicating results, and the process of informed consent for screening, be thoroughly discussed among an expanded forum of practitioners. This forum could include obstetricians, medical geneticists, metabolic disease specialists, neonatologists, pediatricians, endocrinologists, pediatric neurologists, and other relevant clinical specialists who are likely to be involved in the delivery of NBS results and in the follow-up of “screen positive” newborns. The different reflections of program creators and practitioners about the NBS process and its interpretation, as discussed here, might serve as a good starting point for a fruitful discussion.