Reflections on 50 Years of Cystic Fibrosis Newborn Screening Experience with Critical Perspectives, Assessment of Current Status, and Predictions for Future Improvements
Abstract
1. Introduction
Purpose of This Review and Commentary
2. CF Newborn Screening Explored with Meconium Analyses
3. CF NBS Gains Traction with IRT
4. Resistance and Skepticism Prevail for a Decade
5. The Wisconsin RCT—Design and Planning
5.1. Unique Challenges and an Ethical Dilemma
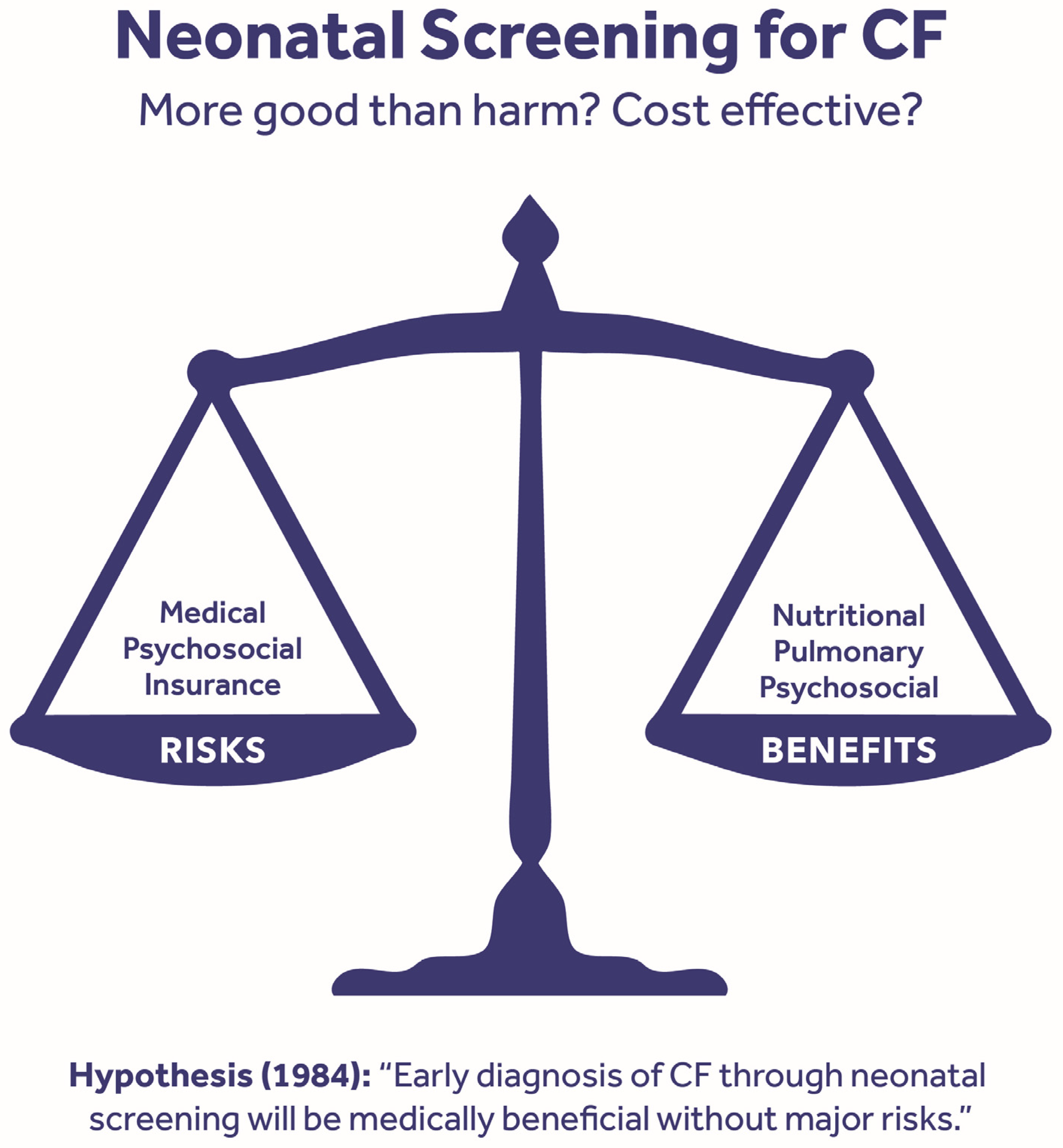
5.2. Hypothesis and Organization
5.3. Outcomes of Interest
5.4. Accrual of Patients and Observations on Birth Prevalence and IRT Flaws
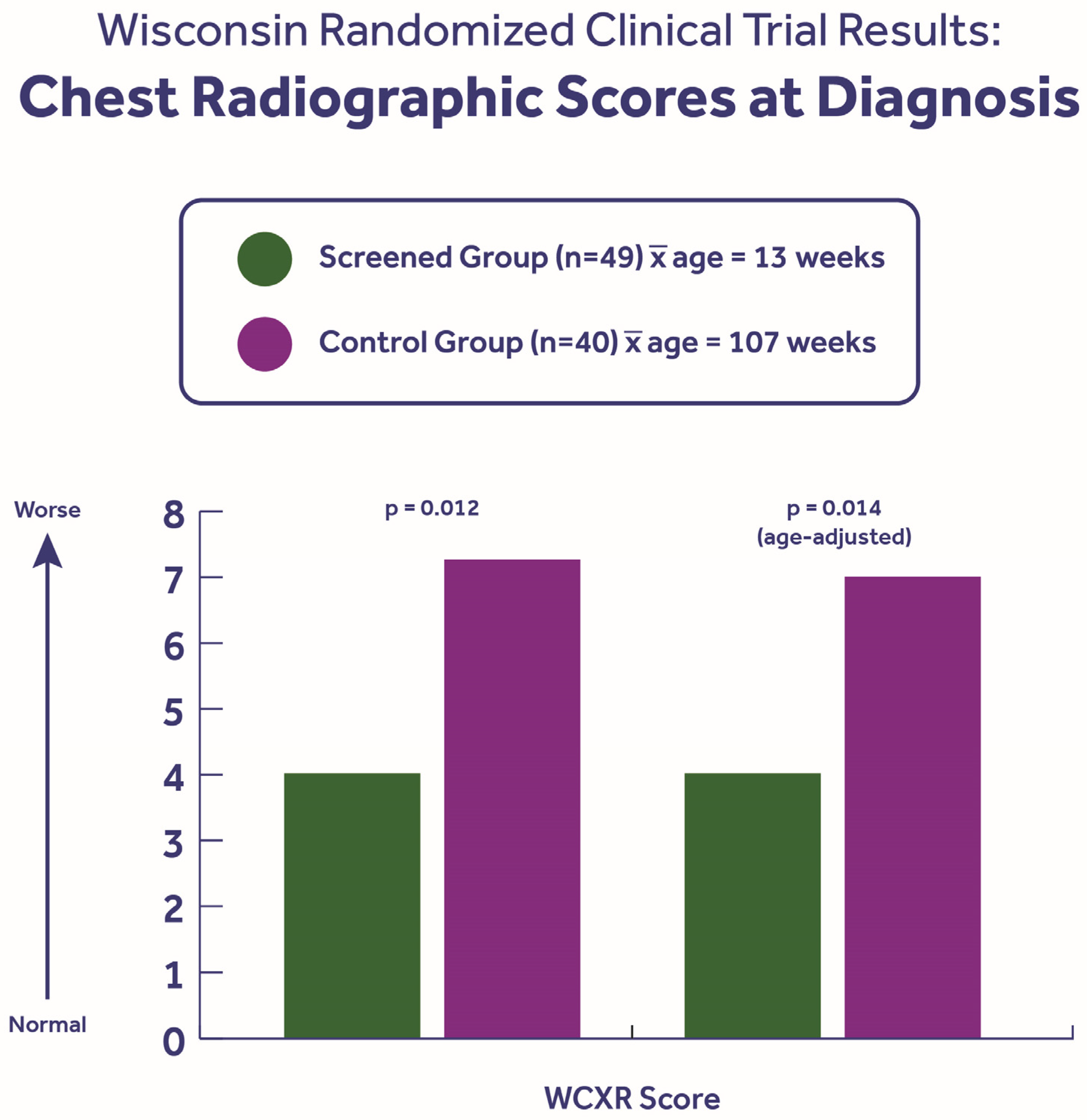
5.5. Benefits of Early Diagnosis
5.6. Risks of CF NBS—Real Potential for Harm
5.7. Wisconsin RCT Impact
- On the basis of a preponderance of evidence, the health benefits to children with CF outweigh the risk of harm and justify screening for CF.
- Newborn screening for CF should be accompanied by rigorous infection control practices.
- The net balance of benefits and risks is contingent on how newborn screening for CF is implemented.
- Newborn screening systems should ensure parental and provider education.
6. CFTR Gene Discovery and F508del Frequency—Discoveries That Rescued CF NBS
“With advances in technology and the recent identification of one of the cystic fibrosis mutations and the identification of other mutations to soon follow, we believe that the strategy for cystic fibrosis newborn screening will need to evolve into a true two-tier screening test. The first tier would be the IRT assay; if the IRT assay is positive, the second tier would be performed on the same original blood spot, and it would be a probe for the cystic fibrosis mutations. The implementation of cystic fibrosis screening, however, should be delayed until a clear benefit of newborn screening has been identified.”
6.1. Expansion of DNA/CFTR Panels
6.2. CFTR Gene Sequencing to Expand Panels Further
6.3. The CFTR2 Project’s Important Role in NBS
6.4. Next-Generation Sequencing
7. Equity and Timeliness
8. Current Situation
8.1. Successes to Appreciate and Celebrate
8.2. Shortcomings—Needs/Opportunities
9. Predictions Without a Timeframe
- CF NBS will evolve into an equitable, more sensitive, and specific NGS-based primary DNA (genetic) test if the ethical, legal, and social issues of detecting CFTR variant carriers are resolved and public acceptance occurs—both of which are likely, in my opinion. This prediction was actually made by my CDC mentor, Harry Hannon, as analytical molecular biology techniques made whole-genome sequencing feasible. I believe that some NBS labs will bundle variants for a variety of genetic disorders, as some programs are now doing on a research basis [87,88], and that this will become increasingly desirable and affordable for NBS labs. The inadvertent detection of some disorders with no therapy currently available as well as single variant cases of diseases like CF can be managed by informatics filters, as we have done in whole-genome sequencing studies [31]. And carrier detection may become desirable if/when it is accepted that carriers have disease risks, as has become true for CFTR variant carriers [89]. But NGS will need to be augmented by methods to detect genomic structural abnormalities like duplications and deletions as well as significant intronic variants.
- CRMS/CFSPID will become much less significant if the CFTR panels are “refined,” as Rock et al. [73] recommended, or if IRT is no longer used an analyte. In fact, IRT is a flawed biomarker [85,90,91] and may eventually be abandoned. If the lessons of history apply, IRT will indeed be supplanted by better technology like NGS.
- CF diagnoses will be made routinely (genetically) by 1–2 weeks of age based on identifying two pathogenic variants, thus facilitating earlier/better care, including breastfeeding [45,86,92] and the early administration of nutritional supplements to avoid malnutrition and its consequences, as well as the initiation of infant-approved CFTR modulator drugs like ivacaftor. There will be many advantages of beginning care by 1–2 weeks of age, when babies are more likely to be breastfed successfully with support by CF centers and primary care physicians [86,92].
- Improved therapy for infants will occur through better CFTR defect management drugs, as they are being improved continuously and will become more affordable with potential injectable administration to overcome non-adherence. Although drugs like ivacaftor and trikafta can be very effective, not all patients benefit, and gene editing may become more appealing.
- After the diagnosis and initiation of treatment, the increasingly routine care of many, if not most, children with CF will eventually be conducted predominantly by pediatricians in association with primary care delivery [80] and in close collaboration with CF center specialists for decisions about highly specialized therapies like CFTR-directed drugs. Pediatric pulmonologists are likely to be needed less, with lung disease being prevented, but a role for CF subspecialists in confirming diagnoses and managing complex interventions will remain.
Funding
Acknowledgments
Conflicts of Interest
References
- Shwachman, H.; Kulczycki, L.L. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J. Dis. Child. 1958, 96, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.W.; Doershuk, C.F.; Wise, M.; Eddy, G.; Nudelman, H.; Spector, S. therapeutic regimen for patients with cystic fibrosis. J. Pediatr. 1964, 65, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Shwachman, H.; Redmond, A.; Khaw, K.T. Studies in cystic fibrosis. Report of 130 patients diagnosed under 3 months of age over a 20-year period. Pediatrics 1970, 46, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Shwachman, H.; Stamm, S.J.; Docter, J.M.; Murphy, T.F.; Mahmoodian, A.; Kennedy, J.L. Screening test for newborns for cystic fibrosis using the presence of albumin in meconium as an indicator. In Fundamental Problems of Cystic Fibrosis and Related Diseases; Mangos, J.A., Talamo, R.C., Eds.; Intercontinental Medical Book Corp: New York, NY, USA, 1973; p. 277. [Google Scholar]
- Howell, D.A.; Lederberg, S.; Brusilow, S.W.; Childs, B.; Cook, C.D.; Heath, E.C.; Murray, R.F.; Waring, W.W. Evaluation of testing for cystic fibrosis. J. Pediatr. 1975, 88, 711–750. [Google Scholar]
- di Sant’Agnese, P.A.; Darling, R.C.; Perera, C.A.; Shea, E. Sweat electrolyte disturbances associated with childhood pancreatic disease. Am. J. Med. 1953, 15, 777. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Vogt, R.F.; Mei, J.V. William harry hannon—A life well lived. Int. J. Neonatal Screen. 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Travert, G.; Heeley, M.; Heeley, A. History of newborn screening for cystic fibrosis-the early years. Int. J. Neonatal Screen. 2020, 6, 8. [Google Scholar] [CrossRef]
- Stephan, U.; Busch, E.W.; Kollberg, H.; Hellsing, K. Cystic fibrosis detection by means of a test-strip. Pediatrics 1975, 55, 35–38. [Google Scholar] [CrossRef]
- Bruns, W.T.; Connell, T.R.; Lacey, J.A.; Whisler, K.E. Test strip meconium screening for cystic fibrosis. Am. J. Dis. Child. 1977, 131, 71–73. [Google Scholar] [CrossRef]
- Pederzini, F.; Faraguna, D.; Giglio, L.; Pedrotti, D.; Perobelli, L.; Mastella, G. Development of a screening system for cystic fibrosis: Meconium or blood spot trypsin assay or both? Acta Paediatr. Scand 1990, 79, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Shwachman, H. Presumptive tests for cystic fibrosis based on serum protein in meconium. Pediatrics 1968, 41, 989. [Google Scholar] [CrossRef]
- Kollberg, H.; Heilsing, K. Screening for cystic fibrosis. Arch. Dis. Child. 1972, 47, 836. [Google Scholar] [CrossRef][Green Version]
- Guthrie, R.; Susi, A.A. simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef]
- Grob, F.; Lain, S.; Olivieri, A. Newborn screening for primary congenital hypothyroidism: Past, present and future. Eur. Thyroid. J. 2025, 14, e240358. [Google Scholar] [CrossRef]
- Crossley, J.R.; Elliott, R.B.; Smith, P.A. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet 1979, 1, 472–474. [Google Scholar] [CrossRef]
- Elias, E.; Redshaw, M.; Wood, T. Diagnostic importance of changes in circulating concentrations of immunoreactive trypsin. Lancet 1977, 2, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Brown, A.R.; Urwin, R.; Brown, D.A. Cystic fibrosis screening by dried blood spot trypsin assay: Results in 75,000 newborn infants. J. Pediatr. 1983, 102, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V.; Sherry, G.; Bayliss, U. Neonatal screening for cystic fibrosis: A comparison of two strategies for case detection in 1.2 million babies. J. Pediatr. 1995, 127, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.B.; Abman, S.H.; Sokol, R.J.; Accurso, F.J. Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations. N. Engl. J. Med. 1991, 325, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Reardon, M.C.; Accurso, F.J.; Hammond, K.B.; Sokol, R.J. Hypoalbuminemia at diagnosis as a marker for severe respiratory course in infants with cystic fibrosis identified by newborn screening. J. Pediatr. 1985, 107, 933–935. [Google Scholar] [CrossRef]
- Taussig, L.M.; Boat, T.F.; Dayton, D.; Fost, N.; Hammond, K.; Holtzman, N.; Johnson, W.; Kaback, M.M.; Kennel, J.; Rosenstein, B.J.; et al. Neonatal screening for cystic fibrosis: Position paper. Pediatrics 1983, 72, 741–745. [Google Scholar] [CrossRef]
- Holtzman, N.A. Routine screening of newborns for cystic fibrosis: Not yet. Pediatrics 1984, 73, 98–99. [Google Scholar] [CrossRef]
- Fost, N.; Farrell, P.M. A prospective randomized trial of early diagnosis and treatments of cystic fibrosis: A unique ethical dilemma. Clin. Res. 1989, 37, 495–500. [Google Scholar]
- Farrell, P.M. Improving the health of patients with cystic fibrosis through newborn screening. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Adv. Pediatr. 2000, 47, 79–115. [Google Scholar] [CrossRef]
- Farrell, P.M.; Li, Z.; Kosorok, M.R.; Laxova, A.; Green, C.G.; Collins, J.; Lai, H.C.; Makholm, L.M.; Rock, M.J.; Splaingard, M.L. Longitudinal evaluation of bronchopulmonary disease in children with cystic fibrosis. Pediatr. Pulmonol. 2003, 36, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ryley, H.C.; Deam, S.M.; Williams, J.; Alfaham, M.; Weller, P.H.; Goodchild, M.C.; Carter, R.A.; Bradley, D.; Dodge, J.A. Neonatal screening for cystic fibrosis in Wales and the West Midlands: 1. Evaluation of immunoreactive trypsin. J. Clin. Pathol. 1988, 41, 726–729. [Google Scholar] [CrossRef]
- Koscik, R.E.; Kosorok, M.R.; Farrell, P.M.; Collins, J.; Peters, M.E.; Laxova, A.; Green, C.G.; Zen, L.; Rusakow, L.S.; Hardie, R.C.; et al. Wisconsin cystic fibrosis chest radiograph scoring system: Validation and standardization for application to longitudinal studies. Pediatr. Pulmonol. 2000, 29, 457–467. [Google Scholar] [CrossRef]
- Farrell, P.M.; Li, Z.; Kosorok, M.R.; Laxova, A.; Green, C.G.; Collins, J.; Lai, H.C.; Rock, M.J.; Splaingard, M.L. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am. J. Respir. Crit. Care Med. 2003, 168, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Li, Z.; Laxova, A.; Rock, M.J.; Levy, H.; Collins, J.; Ferec, C.; Farrell, P.M. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann. Am. Thorac. Soc. 2014, 11, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lai, H.J.; Song, J.; Zhao, Z.; Lu, Q.; Murali, S.G.; Brown, D.M.; Worthey, E.A.; Farrell, P.M. Impact of intrinsic and extrinsic risk factors on early-onset lung disease in cystic fibrosis. Pediatr. Pulmonol. 2023, 58, 3071–3082. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Shen, G.; Splaingard, M.; Colby, C.E.; Laxova, A.; Kosorok, M.R.; Rock, M.J.; Mischler, E.H. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics 1997, 100, E2. [Google Scholar] [CrossRef]
- Kosorok, M.R.; Jalaluddin, M.; Farrell, P.M.; Shen, G.; Colby, C.E.; Laxova, A.; Rock, M.J.; Splaingard, M. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr. Pulmonol. 1998, 26, 81–88. [Google Scholar] [CrossRef]
- Farrell, P.M.; Collins, J.; Broderick, L.S.; Rock, M.J.; Li, Z.; Kosorok, M.R. Association between mucoid Pseudomonas infection and bronchiectasis in children with cystic fibrosis. Radiology 2009, 252, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Kosorok, M.R.; Wei, W.; Farrell, P.M. The incidence of cystic fibrosis. Stat. Med. 1996, 15, 449–462. [Google Scholar] [CrossRef]
- Rock, M.J.; Mischler, E.H.; Farrell, P.M.; Wei, L.J.; Bruns, W.T.; Hassemer, D.J.; Laessig, R.H. Newborn screening for cystic fibrosis is complicated by age-related decline in immunoreactive trypsinogen levels. Pediatrics 1990, 85, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.J.; Mischler, E.H.; Farrell, P.M.; Bruns, W.T.; Hassemer, D.J.; Laessig, R.H. Immunoreactive trypsinogen screening for cystic fibrosis: Characterization of infants with a false-positive screening test. Pediatr. Pulmonol. 1989, 6, 42–48. [Google Scholar] [CrossRef]
- Dhondt, J.L.; Farriaux, J.P.; Briard, M.L.; Boschetti, R.; Frézal, J. Results of pilot screening activities in the French neonatal screening program cystic fibrosis, congenital adrenal hyperplasia and sickle cell disease. Screening 1993, 2, 87–89. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.; Laxova, A.; Shen, G.; Koscik, R.E.; Bruns, W.T.; Splaingard, M.; Mischler, E.H. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N. Engl. J. Med. 1997, 337, 963–969. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.R.; Rock, M.J.; Laxova, A.; Zeng, L.; Lai, H.C.; Hoffman, G.; Laessig, R.H.; Splaingard, M.L. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koscik, R.L.; Lai, H.J.; Laxova, A.; Zaremba, K.M.; Kosorok, M.R.; Douglas, J.A.; Rock, M.J. Preventing early, prolonged vitamin E deficiency: An opportunity for better cognitive outcomes via early diagnosis through neonatal screening. J. Pediatr. 2005, 147, S51–S56. [Google Scholar] [CrossRef]
- Koscik, R.L.; Farrell, P.M.; Kosorok, M.R.; Zaremba, K.M.; Laxova, A.; Lai, H.C.; Douglas, J.A.; Rock, M.J.; Splaingard, M.L. Cognitive function of children with cystic fibrosis: Deleterious effect of early malnutrition. Pediatrics 2004, 113, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Lai, H.J.; Li, Z.; Kosorok, M.R.; Laxova, A.; Green, C.G.; Collins, J.; Hoffman, G.; Laessig, R.; Rock, M.J.; et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J. Pediatr. 2005, 147, S30–S36. [Google Scholar] [CrossRef]
- Zhang, Z.; Lindstrom, M.J.; Farrell, P.M.; Lai, H.J.; Wisconsin Cystic Fibrosis Neonatal Screening Group. Pubertal height growth and adult height in cystic fibrosis after nwborn screening. Pediatrics 2016, 137, e20152907. [Google Scholar] [CrossRef] [PubMed]
- Jadin, S.A.; Wu, G.S.; Zhang, Z.; Shoff, S.M.; Tippets, B.M.; Farrell, P.M.; Miller, T.; Rock, M.J.; Levy, H.; Lai, H.C. Growth and pulmonary outcomes during the first 2 y of life of breastfed and formula-fed infants diagnosed with cystic fibrosis through the Wisconsin Routine Newborn Screening Program. Am. J. Clin. Nutr. 2011, 93, 1038–1047. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Ostrenga, J.; Cromwell, E.A.; Magaret, A.; Szczesniak, R.; Fink, A.; Schechter, M.S.; Faro, A.; Ren, C.L.; Morgan, W.; et al. Real-world associations of US Cystic Fibrosis Newborn Screening Programs with nutritional and pulmonary outcomes. JAMA Pediatr. 2022, 176, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.; Emerson, J.; McNamara, S.; Joubran, K.; Retsch-Bogart, G.; Graff, G.R.; Gutierrez, H.H.; Kanga, J.F.; Lahiri, T.; Noyes, B.; et al. Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis epic observational cohort. Pediatr. Pulmonol. 2010, 45, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Ersig, A.L.; Lee, S. Psychosocial issues related to newborn screening: A systematic review and synthesis. Int. J. Neonatal Screen. 2022, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.S.; Farrell, P.M.; Corey, M. A debate on why my state (province) should or should not conduct newborn screening for cystic fibrosis (14th Annual North American Cystic Fibrosis Conference). Pediatr. Pulmonol. 2001, 32, 385–396. [Google Scholar] [CrossRef]
- Khendek, L. Shining new light on newborn screening of cystic fibrosis in the province of Quebec. Can. J. Public Health. 2017, 108, e335–e337. [Google Scholar] [CrossRef] [PubMed]
- Cono, J.; Qualis, N.; Khoury, M.J.; Hannon, W.H. Newborn screening for cystic fibrosis: A paradigm for public health genetics policy development: Proceedings of a 1997 workshop. MMWR 1997, 46, 405. [Google Scholar]
- Holtzman, N. Is public health ready for genetics? Arch. Pediatr. Adolesc. Med. 2001, 155, 117–118. [Google Scholar] [CrossRef]
- Grosse, S.D.; Boyle, C.A.; Botkin, J.R.; Comeau, A.M.; Kharrazi, M.; Rosenfeld, M.; Wilfond, B.S. Newborn screening for cystic fibrosis: Evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm. Rep. 2004, 53, 1–36. [Google Scholar] [PubMed]
- Campbell, P.W., 3rd; White, T.B. Newborn screening for cystic fibrosis: An opportunity to improve care and outcomes. J. Pediatr. 2005, 147, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.W.; Grossman, W.J.; Laessig, R.H.; Hoffman, G.L.; Brokopp, C.D.; Kurtycz, D.F.; Cogley, M.F.; Litsheim, T.J.; Katcher, M.L.; Routes, J.M. Development of a routine newborn screening protocol for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2009, 124, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Sontag, M.K.; Wright, D.; Beebe, J.; Accurso, F.J.; Sagel, S.D. A new cystic fibrosis newborn screening algorithm: IRT/IRT1 upward arrow/DNA. J. Pediatr. 2009, 155, 618–622. [Google Scholar] [CrossRef]
- Kerem, B.S.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef]
- Farrell, P.M.; Mischler, E.H.; Fost, N.C.; Wilfond, B.S.; Tluczek, A.; Gregg, R.G.; Bruns, W.T.; Hassemer, D.J.; Laessig, R.H. Current issues in neonatal screening for cystic fibrosis and implications of the CF gene discovery. Pediatr. Pulmonol. 1991, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.G.; Wilfond, B.S.; Farrell, P.M.; Laxova, A.; Hassemer, D.; Mischler, E.H. Application of DNA analysis in a population-screening program for neonatal diagnosis of cystic fibrosis (CF): Comparison of screening protocols. Am. J. Hum. Genet. 1993, 52, 616–626. [Google Scholar]
- Rommens, J.; Kerem, B.S.; Greer, W.; Chang, P.; Tsui, L.C.; Ray, P. Rapid nonradioactive detection of the major cystic fibrosis mutation. Am. J. Hum. Genet. 1990, 46, 395–396. [Google Scholar]
- Ranieri, E.; Ryall, R.G.; Morris, C.P.; Nelson, P.V.; Carey, W.F.; Pollard, A.C.; Robertson, E.F. Neonatal screening strategy for cystic fibrosis using immunoreactive trypsinogen and direct gene analysis. Br. Med. J. 1991, 302, 1237–1240. [Google Scholar] [CrossRef][Green Version]
- Férec, C.; Verlingue, C.; Parent, P.; Morin, J.F.; Codet, J.P.; Rault, G.; Dagorne, M.; Lemoigne, A.; Journel, H.; Roussey, M.; et al. Neonatal screening for cystic fibrosis: Result of a pilot study using both immunoreactive trypsinogen and cystic fibrosis gene mutation analyses. Hum. Genet. 1995, 96, 542–548. [Google Scholar] [CrossRef]
- Comeau, A.M.; Accurso, F.J.; White, T.B.; Campbell, P.W., 3rd; Hoffman, G.; Parad, R.B.; Wilfond, B.; Rosenfeld, M.; Sontag, M.K.; Massie, J.; et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics. 2007, 119, e495–e518. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.S.; Cutting, G.R.; Desnick, R.J.; Driscoll, D.A.; Klinger, K.; Mennuti, M.; Palomaki, G.E.; Popovich, B.W.; Pratt, V.M.; Rohlfs, E.M.; et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet. Med. 2004, 6, 387–391. [Google Scholar] [CrossRef]
- Audrézet, M.P.; Munck, A. Newborn screening for CF in France: An exemplary national experience. Arch. Pediatr. 2020, 27, eS35–eS40. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, M.; Yang, J.; Bishop, T.; Lessing, S.; Young, S.; Graham, S.; Pearl, M.; Chow, H.; Ho, T.; Currier, R.; et al. Newborn screening for cystic fibrosis in California. Pediatrics. 2015, 136, 1062–1072. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation; Borowitz, D.; Parad, R.B.; Sharp, J.K.; Sabadosa, K.A.; Robinson, K.A.; Rock, M.J.; Farrell, P.M.; Sontag, M.K.; Rosenfeld, M.; et al. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J. Pediatr. 2009, 155, S106–S116. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Munck, A.; Davies, J.C.; de Winter-de Groot, K.M.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.J.; McColley, S.; et al. Updated guidance on the management of children with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen positive, inconclusive diagnosis (CRMS/CFSPID). J. Cyst. Fibros. 2021, 20, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.L.; Borowitz, D.S.; Gonska, T.; Howenstine, M.S.; Levy, H.; Massie, J.; Milla, C.; Munck, A.; Southern, K. Cystic fibrosis transmembrane conductance regulator-related metabolic syndrome and cystic fibrosis screen positive, inconclusive diagnosis. J. Pediatr. 2017, 181, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Sosnay, P.R.; Salinas, D.B.; White, T.B.; Ren, C.L.; Farrell, P.M.; Raraigh, K.S.; Girodon, E.; Castellani, C. Applying cystic fibrosis transmembrane conductance regulator genetics and CFTR2 data to facilitate diagnoses. J. Pediatr. 2017, 181, S27–S32.e1. [Google Scholar] [CrossRef] [PubMed]
- Claustres, M.; Thèze, C.; des Georges, M.; Baux, D.; Girodon, E.; Bienvenu, T.; Audrezet, M.P.; Dugueperoux, I.; Ferec, C.; Lalau, G.; et al. CFTR-France, a national relational patient database for sharing genetic and phenotypic data associated with rare CFTR variants. Hum. Mutat. 2017, 38, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.W.; Atkins, A.E.; Cordovado, S.; Hendrix, M.; Earley, M.C.; Farrell, P.M. Improving newborn screening for cystic fibrosis using next-generation sequencing technology: A technical feasibility study. Genet. Med. 2016, 18, 231–238. [Google Scholar] [CrossRef]
- Rock, M.J.; Baker, M.; Antos, N.; Farrell, P.M. Refinement of newborn screening for cystic fibrosis with next generation sequencing. Pediatr. Pulmonol. 2023, 58, 778–787. [Google Scholar] [CrossRef]
- Sicko, R.J.; Stevens, C.F.; Hughes, E.E.; Leisner, M.; Ling, H.; Saavedra-Matiz, C.A.; Caggana, M.; Kay, D.M. Validation of a custom next-generation sequencing assay for cystic fibrosis newborn screening. Int. J. Neonatal Screen. 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- McColley, S.A.; Martiniano, S.L.; Ren, C.L.; Sontag, M.K.; Rychlik, K.; Balmert, L.; Elbert, A.; Wu, R.; Farrell, P.M. Disparities in first evaluation of infants with cystic fibrosis since implementation of newborn screening. J. Cyst. Fibros. 2023, 22, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Martiniano, S.L.; Elbert, A.A.; Farrell, P.M.; Ren, C.L.; Sontag, M.K.; Wu, R.; McColley, S.A. Outcomes of infants born during the first 9 years of CF newborn screening in the United States: A retrospective Cystic Fibrosis Foundation patient registry cohort study. Pediatr. Pulmonol. 2021, 56, 3758–3767. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Wu, R.; Farrell, P.M.; Ren, C.L.; Sontag, M.K.; Elbert, A.; McColley, S.A. Late diagnosis in the era of universal newborn screening negatively affects short- and long-term growth and health outcomes in infants with cystic fibrosis. J. Pediatr. 2023, 262, 113595. [Google Scholar] [CrossRef]
- Smyth, A.R.; Bell, S.C.; Bojcin, S.; Bryon, M.; Duff, A.; Flume, P.; Kasshirskaya, N.; Munck, A.; Ratjen, F.; Schwarzenberg, S.J. European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J. Cyst. Fibros. 2014, 13, S23–S42. [Google Scholar] [CrossRef]
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn screening for CF across the globe—Where is it worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Farrell, P.M. Update on advances in cystic fibrosis towards a cure and implications for primary care clinicians. Curr. Probl. Pediatr. Adolesc. Health Care 2024, 54, 101637. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Zaleski, C.; Stachiw-Hietpas, D.; Modaff, P.; Adamski, C.R.; Nelson, M.R.; Reise, C.A.; Ghate, S.; Josephson, K.D. A tailored approach to family-centered genetic counseling for cystic fibrosis newborn screening: The Wisconsin model. J. Genet. Couns. 2011, 20, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Langfelder-Schwind, E.; Raraigh, K.S.; CF Newborn Screening Genetic Counseling Workgroup; Parad, R.B. Genetic counseling access for parents of newborns who screen positive for cystic fibrosis: Consensus guidelines. Pediatr. Pulmonol. 2022, 57, 894–902. [Google Scholar] [CrossRef]
- Zhang, Z.; Shoff, S.M.; Lai, H.J. Incorporating genetic potential when evaluating stature in children with cystic fibrosis. J. Cyst. Fibros. 2010, 9, 135–142. [Google Scholar] [CrossRef] [PubMed]
- McGarry, M.E.; Raraigh, K.S.; Farrell, P.M.; Shropshire, F.; Padding, K.; White, C.; Dorley, M.C.; Hicks, S.; Ren, C.L.; Tullis, K.; et al. Cystic fibrosis newborn screening; a systematic review-driven consensus guideline from the United States Cystic Fibrosis Founation. Int. J. Neonatal Screen. 2025, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, M.; Sacramento, C.; Comeau, A.M.; Hale, J.E.; Caggana, M.; Kay, D.M.; Lee, R.; Reilley, B.; Thompson, J.D.; Nash, S.Z.; et al. Missed cystic fibrosis newborn screening cases due to immunoreactive trypsinogen levels below program cutoffs: A national survey of risk factors. Int. J. Neonatal Screen. 2022, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Bach, T.R.; Miller, T.; McDonald, C.M.; Maguiness, K.M.; Seffrood, E.E.; Leonard, J.B.; Farrell, P.M. Breastfeeding, growth, and lung disease in the first 3 years of life in children with cystic fibrosis. J. Cyst. Fibros. 2025, 24, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Veldman, A.; Kiewiet, M.B.G.; Heiner-Fokkema, M.R.; Nelen, M.R.; Sinke, R.J.; Sikkema-Raddatz, B.; Voerhoeve, E.; Westra, D.; Dolle, M.E.T.; Schielen, P.C.J.I.; et al. Towards ext-Generation Sequencing (NGS)-Based Newborn Screening: A technical study to prepare for the challenges ahead. Int. J. Neonatal Screen. 2022, 8, 17. [Google Scholar] [CrossRef]
- Smith, H.S.; Zettler, B.; Genetti, C.A.; Hickingbotham, M.R.; Coleman, T.F.; Lebo, M.; Nagy, A.; Zoul, H.; Mahanta, L.; Christensen, K.D.; et al. The BabySeq Project: A clinical trial of genome sequencing in a diverse cohort of infants. Am. J. Hum. Genet. 2024, 111, 2094–2106. [Google Scholar] [CrossRef]
- Farrell, P.M.; Langfelder-Schwind, E.; Farrell, M.H. Challenging the dogma of the healthy heterozygote: Implications for newborn screening policies and practices. Mol. Genet. Metab. 2021, 134, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kloosterboer, M.; Hoffman, G.; Rock, M.; Gershan, W.; Laxova, A.; Li, Z.; Farrell, P.M. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics 2009, 123, e338–e346. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L., Jr.; Hannon, W.H.; Hoffman, G.; Ojodu, J.; Farrell, P.M. Immunoreactive Trypsinogen (IRT) as a biomarker for cystic fibrosis: Challenges in newborn dried blood spot screening. Mol. Genet. Metab. 2012, 106, 1–6. [Google Scholar] [CrossRef]
- Miller, T.; Antos, N.J.; Brock, L.A.; Wade, T.; Goday, P.S. Lactation consultation sustains milk intake in infants with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 358–362. [Google Scholar] [CrossRef] [PubMed]


| Intrinsic Determinants |
| • Genotype |
| • Modifier genes |
| • Pancreatic status |
| • Meconium ileus |
| • Nutritional status |
| Extrinsic Determinants |
| • Age in weeks at diagnosis |
| • Environmental exposures |
| • Parental education, especially the level of maternal education |
| • Socioeconomic status |
| • Daycare |
| • Integrated clinic |
| • Pseudomonas aeruginosa |
| • Staphylococcus aureus |
| • Height percentile < 10th |
| • Hospitalizations |
| • Pulmonary exacerbations |
| Accomplishments |
| • Earlier diagnoses routinely for most patients |
| • Avoiding the “diagnostic odyssey” |
| • Concurrent genotyping with IRT/DNA |
| • Prompt access to CF specialist care |
| • Early GI/nutrition Rx (PERT and nutrient supplements) |
| • Preempting lung disease |
| • CFTR modulator therapy for infants (e.g., ivacaftor) |
| • Opportunity for genetic counseling |
| • Fewer hospitalizations |
| • Much improved quality of life |
| • Optimism among parents/children |
| • Partnerships of CF specialists with NBS lab leaders |
| • Better organization of care centers |
| • Increased understanding of the disease |
| Needs/Opportunities |
| • Overcome 5–20% false negative NBS results |
| • Reduce false positive results |
| • Achieve equity everywhere (especially eliminate racial/ethnic disparities) |
| • Improve timeliness (diagnose by 1–2 w/o) |
| • Improve nutrition-related outcomes through breastfeeding and supplements |
| • Reduce sweat-testing failures |
| • Avoid excessive medications |
| • Overcome variations in follow-up efficiency and geo-barriers |
| • Extend globally where worthwhile |
| • Reduce CRMS/CFSPID |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, P.M. Reflections on 50 Years of Cystic Fibrosis Newborn Screening Experience with Critical Perspectives, Assessment of Current Status, and Predictions for Future Improvements. Int. J. Neonatal Screen. 2025, 11, 88. https://doi.org/10.3390/ijns11040088
Farrell PM. Reflections on 50 Years of Cystic Fibrosis Newborn Screening Experience with Critical Perspectives, Assessment of Current Status, and Predictions for Future Improvements. International Journal of Neonatal Screening. 2025; 11(4):88. https://doi.org/10.3390/ijns11040088
Chicago/Turabian StyleFarrell, Philip M. 2025. "Reflections on 50 Years of Cystic Fibrosis Newborn Screening Experience with Critical Perspectives, Assessment of Current Status, and Predictions for Future Improvements" International Journal of Neonatal Screening 11, no. 4: 88. https://doi.org/10.3390/ijns11040088
APA StyleFarrell, P. M. (2025). Reflections on 50 Years of Cystic Fibrosis Newborn Screening Experience with Critical Perspectives, Assessment of Current Status, and Predictions for Future Improvements. International Journal of Neonatal Screening, 11(4), 88. https://doi.org/10.3390/ijns11040088






