Abstract
Severe combined immunodeficiency (SCID) and spinal muscular atrophy (SMA) are being added to the Newborn Bloodspot Screening (NBS) programme in the Republic of Ireland. To support this expansion, we conducted a scoping review to identify reported timeframes for implementing national, regional or state-wide expanded NBS programmes. We performed a scoping review of the literature published between 2015 and 2025. Eligible articles described the timeframes for implementation of expanded NBS programmes for SCID, SMA or additional metabolic conditions. Sources included PubMed, Embase, citation searching, the International Journal of Neonatal Screening and grey literature. A narrative synthesis was undertaken. Fourteen articles met the inclusion criteria, describing the addition of new conditions—SCID (N = 7), SMA (N = 4), or multiple conditions (N = 3) to expanded NBS programmes in the United States (US), Europe (Belgium, Catalonia, the Czech Republic, Estonia, Germany, Norway, Poland, Portugal, Slovakia, Slovenia, Sweden, and Tuscany), Hong Kong and New Zealand. In most jurisdictions, the implementation of NBS programmes for new conditions took two to six years. The implementation of NBS for new conditions requires considerable time and coordinated efforts. Further research providing greater detail on the specific implementation steps, along with associated timelines, would provide valuable guidance for jurisdictions aiming to expand NBS programmes globally.
1. Introduction
As of June 2025, nine conditions are screened for through the National Newborn Bloodspot Screening (NBS) Programme in the Republic of Ireland. These are phenylketonuria, homocystinuria, classical galactosaemia, maple syrup urine disease, congenital hypothyroidism, cystic fibrosis, glutaric aciduria type 1, medium-chain acyl-CoA Dehydrogenase deficiency, and adenosine deaminase deficiency severe combined immunodeficiency [].
In 2023, following health technology assessments conducted by the Health Information and Quality Authority [,], and recommendations from the National Screening Advisory Committee (NSAC), the Minister of Health approved the addition of Severe Combined Immunodeficiency (SCID) and Spinal Muscular Atrophy (SMA) to the Irish NBS programme. Further expansion of NBS is a priority workstream for the NSAC in Ireland, with over 30 additional conditions under consideration for future inclusion [].
Preparations for implementation of SCID and SMA screening are underway within the National Healthy Childhood Programme, who have clinical governance for NBS in Ireland. As part of these preparations, this review aims to identify evidence on implementation timelines from jurisdictions that have successfully integrated molecular screening for SCID and SMA into NBS. We also sought to identify published evidence from jurisdictions that have added additional metabolic conditions detectable by tandem mass spectrometry, as more conditions may be added to the Irish NBS programme in the coming years.
2. Materials and Methods
2.1. Study Design
This is a scoping review reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews checklist []. A scoping review approach was chosen due to the anticipated heterogeneity in study methods and disciplinary perspectives. This design allows for a comprehensive synthesis of existing evidence to inform practice, programs, and policy, while also identifying gaps to guide future research priorities [,]. We did not pre-register a protocol for this review.
2.2. Eligibility Criteria
Studies were eligible for inclusion if they met the following criteria, structured according to the Population, Intervention, Comparison, Outcome and Study type (PICOS) framework, and were published within the last ten years (2015–2025):
- Population: Newborns in high-income countries.
- Intervention: National, regional or state-level implementation of expanded bloodspot screening for SCID, SMA, or metabolic conditions during the newborn period [].
- Control: Not applicable.
- Outcome: Timeframe for implementation. There is considerable heterogeneity in how this outcome is reported across the literature. Some publications describe timeframes from the point of view of governmental or regulatory approval, or pilot study initiation, while others mention earlier preparatory and political processes. To address this transparently, we report the timing associated with specific steps as described in each publication, where available.
- Study design: Evaluation of the implementation of a real-world screening programme.
Studies were excluded if they focused solely on pilot programmes or if a more recent publication was available with more comprehensive information for a given jurisdiction. The search was limited to English-language publications.
2.3. Information Sources
We searched PubMed, on the 31st of January 2025, for studies published between 2015 and 2025. Additional references were identified through snowball reference searching. Since the International Journal of Neonatal Screening (IJNS) was considered a key source, we hand-searched articles published within the last six months (July 2024–January 2025: IJNS Volume 10, Issues 3 and 4) to account for potential delays in time-to-indexing in PubMed []. A second database, Embase, was searched on the 6th of May 2025.
We also searched the US Health Resources and Services Administration (HRSA) and the United Kingdom’s (UK) official governmental website for grey literature on the implementation of SCID and SMA screening. The US was included due to its earlier adoption of SCID and, subsequently, SMA screening, making it a likely source of key information on implementation. The UK was selected for its known work on a pilot initiative. Both countries were also chosen for the accessibility of English-language materials.
2.4. Search
A comprehensive search strategy linked using Boolean operators was developed to capture the literature on implementation timelines for expanded newborn bloodspot screening programmes, provided below:
((((implement* OR implementation OR adopt* OR rollout) AND (newborn OR “new-born” OR neonate OR neonatal)) AND (“bloodspot screen” OR “Guthrie test” OR “heel prick” OR “heel-prick” OR screen* OR screening OR “tandem mass spectrometry”))) AND (“severe combined immunodeficiency” OR SCID OR “spinal muscular atrophy” OR SMA OR phenylketonuria OR “maple syrup urine disease” OR homocystinuria OR tyrosinemia OR “5-oxoprolinuria” OR “glutathione synthetase deficiency” OR citrullinemia OR “argininosuccinic aciduria” OR argininemia OR “short chain acyl-CoA dehydrogenase deficiency” OR “isobutyryl-CoA dehydrogenase deficiency” OR “glutaric aciduria” OR “multiple acyl-CoA dehydrogenase deficiency” OR “Medium/Short chain L-3-hydroxyacyl-CoA dehydrogenase deficiency” OR “medium chain acyl-CoA dehydrogenase deficiency” OR “Long chain 3 hydroxyacyl-CoA dehydrogenase deficiency” OR “trifunctional protein deficiency” OR “Very long chain acyl-CoA dehydrogenase deficiency” OR “carnitine palmitoyl transferase deficiency” OR “carnitine/acylcarnitine translocase deficiency” OR “carnitine uptake defect” OR “propionic acidemia” OR “methylmalonic acidemia” OR “malonic aciduria” OR “multiple carboxylase deficiency” OR “3-hydroxy 3-methylglutaric-CoA lyase deficiency” OR “3-methylcrotonyl-CoA carboxylase deficiency” OR “3-methylglutaconic aciduria” OR “2-methylbutyryl-CoA dehydrogenase deficiency” OR “isovaleric acidemia” OR “glutaric acidemia” OR “beta-ketothiolase deficiency”).
2.5. Data Charting Process
The literature search results were imported into Covidence, a systematic review management software [], which performed automatic deduplication. A single reviewer performed title and abstract screening, then full text review based on the pre-defined eligibility criteria.
2.6. Data Items
The following data were extracted: author, year, country/region, study design, condition screened for, implementation steps and associated timeframes (where available) and time taken to implement new NBS programmes.
After identifying studies reporting on the primary review outcome (implementation timeframes) we decided, post hoc, to extract additional data on challenges and facilitators encountered when implementing SCID and SMA screening, recognising the relevance of these factors for current and future NBS programme expansions in Ireland and other jurisdictions.
2.7. Synthesis of Results
Narrative synthesis was performed.
3. Results
3.1. Selection of Sources of Evidence
We identified 466 publications, with 448 from PubMed and Embase and 18 found through citation searching and grey literature, with 83 duplicates. No additional studies were identified by hand-searching IJNS publications. After title and abstract screening, 304 studies were excluded. We then reviewed 79 full-text articles, excluding 65 that did not meet eligibility criteria in terms of outcomes, interventions, population, or study design, or because a more detailed and recent publication for that jurisdiction was available. Ultimately, 14 publications were included in the review (Figure 1).
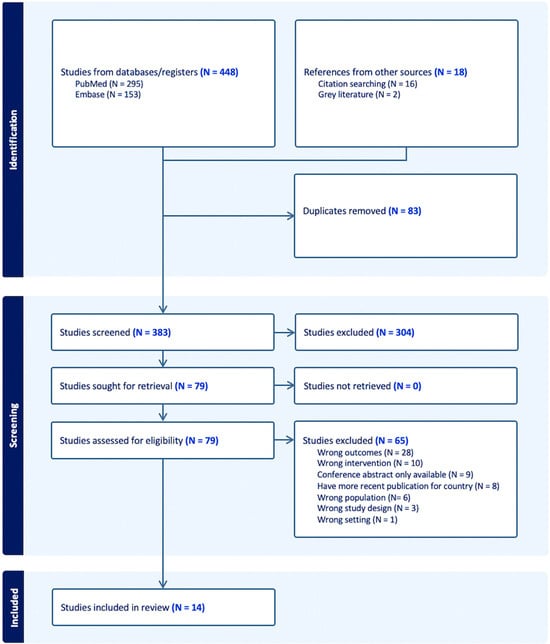
Figure 1.
Flowchart depicting study identification and screening process.
3.2. Characteristics of Sources of Evidence
Seven publications (six peer-reviewed and one grey) described the addition of SCID to NBS programmes [,,,,,]. Four (three peer-reviewed and one grey) focused on SMA [,,,]. Three reported on the addition of multiple conditions [,,].
One publication reported on multiple countries (Belgium, the Czech Republic, Estonia, Germany, Poland, Portugal, Slovakia and the US) [], while three focused on the US only [,,]. Seven described nationwide NBS programmes in six countries: New Zealand [], Slovenia [], Germany [,], Hong Kong [], Norway and Sweden [,]. Three focused on regional programmes in Southern Belgium [], Tuscany and Catalonia [,].
3.3. Narrative Synthesis
Findings identified within the peer-reviewed literature are summarised in Table 1. Reported timeframes for the implementation of official NBS programmes for SCID ranged from eleven months (Catalonia) to eleven years (Germany) [,]. However, most studies indicated that the implementation of national, regional or state-level SCID screening programmes required between two and six years (Norway, New Zealand, Sweden, Tuscany, and US states) [,,,,]. For SMA, implementation timeframes ranged from one to four years (Poland, US, Estonia, Germany, Portugal, Slovakia, Czech Republic and Belgium) [,,,]. Reports from Hong Kong and Slovenia, where NBS for multiple new conditions was implemented, indicated implementation timelines of approximately six years from pilot studies to national programmes [,].

Table 1.
Studies reporting timeframes taken to implement additional conditions (SCID, SMA or multiple) into national, regional or state-level NBS screening programmes published 2015–2025.
3.4. Grey Literature Search
Governmental websites from the US and UK were searched for information on timelines for the implementation of screening programmes for SCID and SMA. Two relevant grey literature reports were identified from the US, but no reports were found for the UK. According to blog posts from the UK National Screening Service, SCID and SMA are not currently recommended as part of the NHS Newborn Blood Spot Screening Programme [,]. However, an in-service evaluation of SCID screening within live NHS services is underway [], and planning has commenced for a similar evaluation of SMA []. The results of these evaluations are expected to inform whether SCID and SMA should become part of the NHS Newborn Screening Programme [,]. Results from the grey literature search are provided in Table 2.

Table 2.
Grey literature on timelines associated with the implementation of newborn screening for SCID and SMA.
3.5. Challenges and Facilitators in Implementing Screening for SCID and SMA
Challenges and facilitators encountered during the implementation of screening for SCID and SMA were reported in eight publications included in this review. Four reported on SCID and five on SMA (one publication reported on both SCID and SMA). A common challenge for both SCID and SMA was the need for dedicated lab space, agreed workflows, new equipment and expertise in molecular technologies. Other challenges and facilitators are detailed below in Table 3.

Table 3.
Challenges and facilitators encountered during implementation of newborn bloodspot screening for SCID and SMA.
4. Discussion
4.1. Summary of Evidence
We identified twelve peer-reviewed and two grey literature publications reporting on timeframes associated with the national, regional or state-level implementation of expanded NBS programmes in the US [,,,], Europe [,,,,,,,], Hong Kong [] and New Zealand [].
The identified publications varied in how they described implementation timeframes, and this outcome was rarely a primary focus. Most reported timelines began with pilot studies [,,,,,,,,], while others referenced the point of inclusion in screening panels or governmental approval [,,]. One mentioned earlier political processes []. Despite these differences, most publications indicate that establishing new national, regional, or state-level newborn screening programs typically takes between two and six years.
The most comparable studies to the Irish context—where the NSAC and Minister for Health have recommended adding SCID and SMA to the NBS programme—appear to be those by Singh et al., (US) [], Heather et al., (New Zealand) [] and Argudo-Ramirez et al., (Catalonia) []. In the US, average implementation times within state-level programmes were 4.3 years for SCID screening, 2.1 years for SMA screening, 2.7 years for CCHD, 4 years for Pompe, 3.2 years for MPS-I and 3.5 years for X-ALD []. However, it is important to note that the timelines for implementing SMA screening in the US were supported by extensive preparatory work conducted by the Centers for Disease Control and Prevention in laboratory settings, which began in 2013, five years before SMA was added to US recommendations in 2018 []. Implementation was also expedited by the fact that SCID screening had already been established, as SCID and SMA can be multiplexed together []. In New Zealand, it took five years from the addition of SCID to the screening panel to achieve nationwide implementation [], whereas, in Catalonia, regional implementation of official NBS screening for SCID was achieved within 11 months from when the Department of Public Health communicated that SCID was approved for inclusion in its NBS programme []. However, the publication from Catalonia did not provide details on any preparatory work that may have taken place prior to this approval [].
Although reporting challenges and facilitators affecting the implementation of SCID and SMA was not the primary focus of this review, several important themes emerged from the studies identified. Implementing NBS for SCID and SMA requires substantial laboratory infrastructure, including new equipment, sufficient and dedicated workspace, IT systems, and personnel with expertise in molecular testing [,,,,]. In the US, the limited availability of experts in immunodeficiency for diagnosis and treatment was flagged as a key challenge []. Given this, the recruitment of clinical and laboratory personnel with the necessary skillset may delay implementation timelines. Developing screening, diagnostic and treatment algorithms that account for the severe and time-sensitive presentation of these conditions is essential [,,]. Complex scenarios—such as cases where parents refuse treatment—should also be considered in advance []. Premature and low-birth-weight infants often yield out-of-range results and require modified algorithms and follow-up protocols [,]. Robust information and communication technology systems are vital for supporting sample tracking within NBS to ensure that newborns who require further testing are not missed []. Ensuring quality assurance and clear governance structures is essential to ensure the success of expanded NBS programmes [,,]. International comparisons to evaluate long-term treatment outcomes and compare screening metrics are important given the rarity of these conditions; however, variability in classifications across regions and over time presents significant challenges [,,].
4.2. Implications for Policy, Practice and Research
In the Irish context, implementing NBS for SCID and SMA is a policy and practice priority. This review highlights that establishing new bloodspot screening programmes in other jurisdictions typically took between two and six years.
From a research perspective, most of the literature screened focused on diagnostic and treatment pathways for the screened conditions or pilot studies, rather than on the implementation of national, regional, or state-level NBS programmes. There is a clear need for more applied implementation research, particularly studies that detail the practical steps required to achieve national, regional or state-level programme coverage, establish robust laboratory processes and elucidate how programmes progress from legislation to implementation. Such work could significantly accelerate and standardise the adoption of expanded NBS across countries. The use of standardised research reporting checklists, such as the Standards for Reporting Implementation Studies (StaRI) in implementation research, could enhance the utility of future implementation research [].
4.3. Limitations
As mentioned previously, none of the studies in this review provided granular, step-by-step timelines for the implementation of expanded NBS programmes, limiting our ability to identify rate-limiting steps or dependencies. Regarding methodology, search, screening, article selection, data extraction and synthesis were conducted by single reviewers, which may introduce bias. Second, our search may not include all conditions for which it is possible to screen for in neonates. Third, we only searched two databases; however, we supplemented this with hand-searching six months of publications within the International Journal of Neonatal Screening and grey literature searches on US and UK websites. Lastly, our searches were restricted to English-language studies due to resource constraints within the research team.
5. Conclusions
Internationally, the implementation of new screening programmes for SCID and SMA typically takes between two and six years. There is a need for more applied implementation research detailing the processes involved in expanding NBS programmes.
Author Contributions
Conceptualisation: A.C. and H.B. Methodology: M.M.B., A.O., A.C. and H.B. Investigation: M.M.B. and A.O., Writing—original draft: M.M.B. Writing—review and editing: A.O., L.O., M.E., J.J.B., P.M., A.C. and H.B. Supervision: A.C. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This review was conducted as part of the NHCP work programme and it did not receive specific funding.
Institutional Review Board Statement
Ethical approval was not required for this scoping review, as it involves secondary analysis of publicly available data.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Health Service Executive (HSE). Heel Prick Screening. 2025. Available online: https://www2.hse.ie/conditions/heel-prick-screening/ (accessed on 25 June 2025).
- Health Information and Quality Authority. Health Technology Assessment of the Addition of Severe Combined Immunodeficiency (SCID) to the National Newborn Bloodspot Screening Programme. 2023. Available online: https://www.hiqa.ie/sites/default/files/2023-01/HTA_addition_of_SCID_to_NNBSP_Jan-2023.pdf (accessed on 31 January 2025).
- Health Information and Quality Authority. Health Technology Assessment of the Addition of Spinal Muscular Atrophy (SMA) to the National Newborn Bloodspot Screening Programme. 2023. Available online: https://www.hiqa.ie/sites/default/files/2023-11/Addition-of-SMA-to-NNBSP.pdf (accessed on 31 January 2025).
- National Screening Advisory Committee (NSAC). Expansion of the National Newborn Bloodspot Screening (NBS) Programme. 2024. Available online: https://assets.gov.ie/static/documents/expansion-of-the-national-newborn-bloodspot-screening-nbs-programme.pdf (accessed on 31 January 2025).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Weill Cornell Medicine Samuel J. Wood Library. Systematic Reviews: Scoping Reviews. 2025. Available online: https://med.cornell.libguides.com/systematicreviews/scopingreviews (accessed on 27 February 2025).
- Illinois Department of Public Health Genetics and Newborn Screening. Tandem Mass Spectrometry Newborn Screening Information for Physicians and Other Health Care Providers. Available online: https://www.idph.state.il.us/HealthWellness/msmsfaq.htm#:~:text=This%20technology%2C%20tandem%20mass%20spectrometry,and%20fatty%20acid%20oxidation%20disorders (accessed on 13 February 2025).
- Irwin, A.N.; Rackham, D. Comparison of the time-to-indexing in PubMed between biomedical journals according to impact factor, discipline, and focus. Res. Soc. Adm. Pharm. 2017, 13, 389–393. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. 2022. Available online: https://www.covidence.org/ (accessed on 31 January 2025).
- Heather, N.; de Hora, M.; Brothers, S.; Grainger, P.; Knoll, D.; Webster, D. Introducing Newborn Screening for Severe Combined Immunodeficiency—The New Zealand Experience. Int. J. Neonatal Screen. 2022, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Strand, J.; Gul, K.A.; Erichsen, H.C.; Lundman, E.; Berge, M.C.; Trømborg, A.K.; Sørgjerd, L.K.; Ytre-Arne, M.; Hogner, S.; Halsne, R.; et al. Second-Tier Next Generation Sequencing Integrated in Nationwide Newborn Screening Provides Rapid Molecular Diagnostics of Severe Combined Immunodeficiency. Front. Immunol. 2020, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, C.; Nennstiel, U.; Hönig, M.; Albert, M.H.; Ghosh, S.; Schuetz, C.; Brockow, I.; Hörster, F.; Niehues, T.; Ehl, S.; et al. Prospective Newborn Screening for SCID in Germany: A First Analysis by the Pediatric Immunology Working Group (API). J. Clin. Immunol. 2023, 43, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Argudo-Ramírez, A.; Martín-Nalda, A.; González de Aledo-Castillo, J.M.; López-Galera, R.; Marín-Soria, J.L.; Pajares-García, S.; Martínez-Gallo, M.; García-Prat, M.; Colobran, R.; Riviere, J.G.; et al. Newborn screening for SCID: Experience in Spain (Catalonia). Int. J. Neonatal Screen. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Göngrich, C.; Ekwall, O.; Sundin, M.; Brodszki, N.; Fasth, A.; Marits, P.; Dysting, S.; Jonsson, S.; Barbaro, M.; Wedell, A.; et al. First year of TREC-based national SCID screening in Sweden. Int. J. Neonatal Screen. 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Guarnieri, V.; Capitanini, F.; Pelosi, C.; Astorino, V.; Boscia, S.; Calistri, E.; Canessa, C.; Cortimiglia, M.; Lippi, F.; et al. Expanded Newborn Screening for Inborn Errors of Immunity: The Experience of Tuscany. J. Allergy Clin. Immunol. Pract. 2024, 12, 1622–1630.e4. [Google Scholar] [CrossRef] [PubMed]
- Müller-Felber, W.; Blaschek, A.; Schwartz, O.; Gläser, D.; Nennstiel, U.; Brockow, I.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; et al. Newbornscreening SMA—From Pilot Project to Nationwide Screening in Germany. J. Neuromuscul. Dis. 2023, 10, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Caberg, J.H.; Dideberg, V.; Dardenne, D.; Bours, V.; Hiligsmann, M.; Dangouloff, T.; Servais, L. Newborn screening for SMA in Southern Belgium. Neuromuscul. Disord. 2019, 29, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Vrščaj, E.; Dangouloff, T.; Osredkar, D.; Servais, L. Newborn screening programs for spinal muscular atrophy worldwide in 2023. J. Neuromuscul. Dis. 2024, 11, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Ream, M.A.; Lam, K.K. Review of Newborn Screening Implementation for Spinal Muscular Atrophy Final Report. Prepared for The Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau. 2020. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/reports-recommendations/sma-nbs-implementation-report.pdf (accessed on 20 February 2025).
- Singh, S.; Ojodu, J.; Kemper, A.R.; Lam, W.K.K.; Grosse, S.D. Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018. Int. J. Neonatal Screen. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Belaramani, K.M.; Chan, T.C.H.; Hau, E.W.L.; Yeung, M.C.W.; Kwok, A.M.K.; Lo, I.F.M.; Law, T.H.F.; Wu, H.; Na Wong, S.S.; Lam, S.W.; et al. Expanded Newborn Screening for Inborn Errors of Metabolism in Hong Kong: Results and Outcome of a 7 Year Journey. Int. J. Neonatal Screen. 2024, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Repič Lampret, B.; Remec, Ž.I.; Drole Torkar, A.; Žerjav Tanšek, M.; Šmon, A.; Koračin, V.; Čuk, V.; Perko, D.; Ulaga, B.; Jelovšek, A.M.; et al. Expanded newborn screening program in Slovenia using tandem mass spectrometry and confirmatory next generation sequencing genetic testing. Zdr. Varst. 2020, 59, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Newborn Screening for Severe Combined Immunodeficiency Disorder. 2011. Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/reports-recommendations/newborn-screening-scid-report.pdf (accessed on 20 February 2025).
- Boemer, F.; Caberg, J.H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef] [PubMed]
- UK National Screening Committee. Antenatal and Newborn Screening Programme: Spinal Muscular Atropy. 2023. Available online: https://view-health-screening-recommendations.service.gov.uk/sma/ (accessed on 20 February 2025).
- UK National Screening Committee. Newborn Screening Programme: SCID. 2017. Available online: https://view-health-screening-recommendations.service.gov.uk/scid/ (accessed on 20 February 2025).
- UK National Screening Committee. SCID Screening In-Service Evaluation: Analysis of Results Under Way. 2024. Available online: https://nationalscreening.blog.gov.uk/2024/05/21/scid-screening-in-service-evaluation-analysis-of-results-under-way/ (accessed on 20 February 2025).
- UK National Screening Committee. SMA Screening In-Service Evaluation Update. 2024. Available online: https://nationalscreening.blog.gov.uk/2024/07/10/sma-screening-in-service-evaluation-update/ (accessed on 20 February 2025).
- Lee, F.K.; Greene, C.; Mercer, K.; Taylor, J.; Yazdanpanah, G.; Vogt, R.; Lee, R.; Cuthbert, C.; Cordovado, S. CDC’s Laboratory Activities to Support Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, H.; Barwick, M.; Carpenter, C.R.; Eldridge, S.; Grandes, G.; Griffiths, C.J.; Rycroft-Malone, J.; Meissner, P.; Murray, E.; Patel, A.; et al. Standards for Reporting Implementation Studies (StaRI): Explanation and elaboration document. BMJ 2017, 356, i6795. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).