The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands
Abstract
1. Introduction
2. Methods
2.1. Case 1—Congenital Hypothyroidism
2.2. Case 2—Congenital Adrenal Hyperplasia
2.3. Case 3—Maple Syrup Urine Disease
2.4. Analysis
3. Results
3.1. Care Pathways
3.1.1. False-Positive NBS Result CH, CAH and MSUD
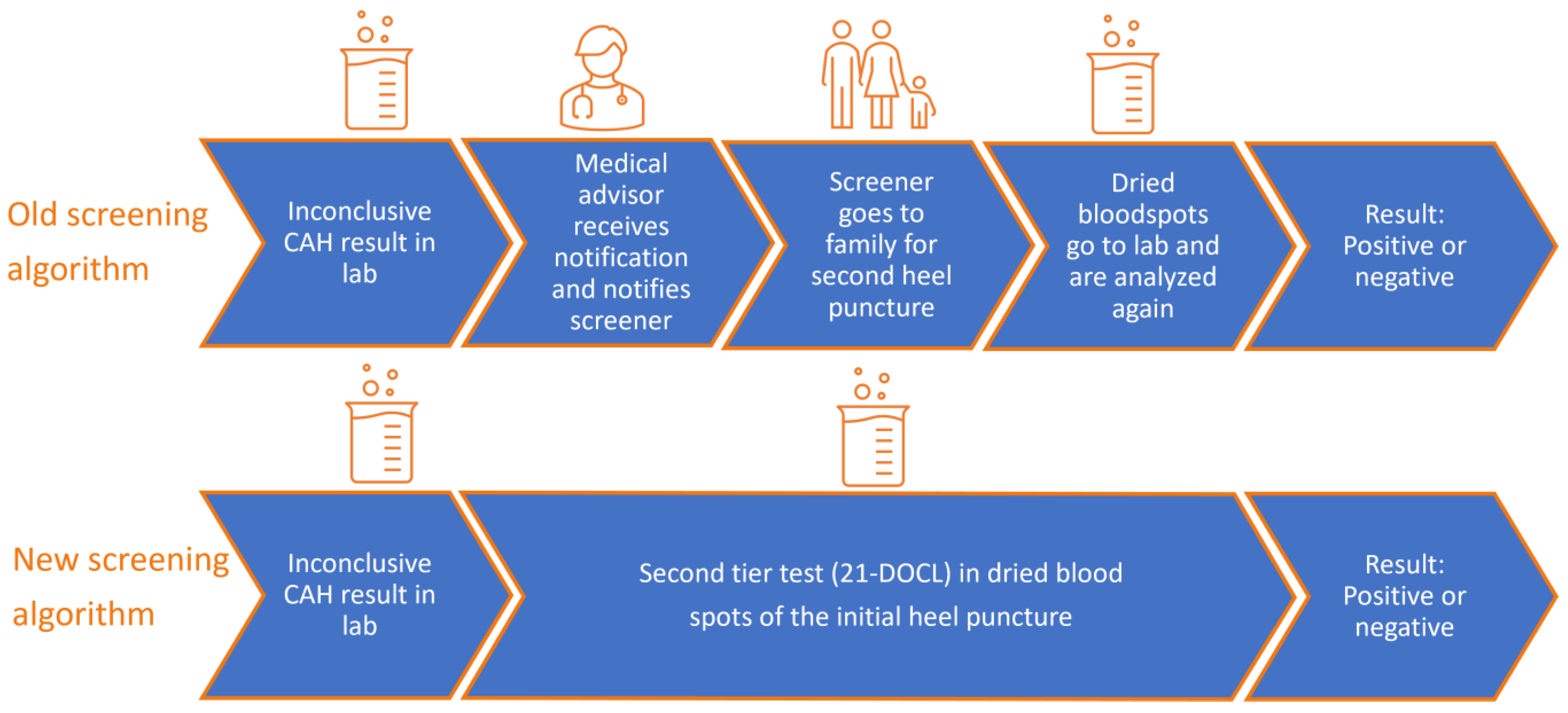
3.1.2. Inconclusive NBS Result CAH
3.2. Case 1—Congenital Hypothyroidism
Volumes and Costs of False-Positive NBS Result CH
3.3. Case 2—Congenital Adrenal Hyperplasia
3.3.1. Volumes and Costs of Inconclusive NBS Result CAH
3.3.2. Volumes and Costs of False-Positive NBS Result CAH
3.3.3. Total Costs of Inconclusive and False-Positive NBS Result CAH
3.4. Case 3—Maple Syrup Urine Disease
Volumes and Costs of False-Positive NBS Result MSUD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uitbreiding van de Hielprik. Available online: https://www.pns.nl/hielprik/professionals/uitbreiding (accessed on 22 November 2023).
- De Neonatale Hielprik Screening: Monitor 2022; TNO: The Hague, The Netherlands, 2023.
- van den Heuvel, L.M.; van der Pal, S.M.; Verschoof-Puite, R.K.; Klapwijk, J.E.; Elsinghorst, E.; Dekkers, E.; van der Ploeg, C.P.B.; Henneman, L. Psychosocial Impact of a True-Positive, False-Positive, or Inconclusive Newborn Bloodspot Screening Result: A Questionnaire Study among Parents. Int. J. Neonatal Screen. 2024, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of Expanded Newborn Screening for Biochemical Genetic Disorders on Child Outcomes and Parental Stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Kerr, E.; Tam, K.; Carroll, J.C.; Potter, B.K.; Chakraborty, P.; Davies, C.; et al. Parent Experience with False-Positive Newborn Screening Results for Cystic Fibrosis. Pediatrics 2016, 138, e20161052. [Google Scholar] [CrossRef] [PubMed]
- Tarini, B.A.; Christakis, D.A.; Welch, H.G. State Newborn Screening in the Tandem Mass Spectrometry Era: More Tests, More False-Positive Results. Pediatrics 2006, 118, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. Expanded newborn screening for biochemical disorders: The effect of a false-positive result. Pediatrics 2006, 117, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.R.; Clayton, E.W. False positive newborn screening results are not always benign. Public Health Genom. 2011, 14, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Karaceper, M.D.; Chakraborty, P.; Coyle, D.; Wilson, K.; Kronick, J.B.; Hawken, S.; Davies, C.; Brownell, M.; Dodds, L.; Feigenbaum, A.; et al. The health system impact of false positive newborn screening results for medium-chain acyl-CoA dehydrogenase deficiency: A cohort study. Orphanet. J. Rare Dis. 2016, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Ersig, A.L.; Lee, S. Psychosocial Issues Related to Newborn Screening: A Systematic Review and Synthesis. Int. J. Neonatal Screen. 2022, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, E.A.; Perrin, J.M.; Waisbren, S.E.; Prosser, L.A. Impact of false-positive newborn metabolic screening results on early health care utilization. Genet. Med. 2009, 11, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tarini, B.A.; Clark, S.J.; Pilli, S.; Dombkowski, K.J.; Korzeniewski, S.J.; Gebremariam, A.; Eisenhandler, J.; Grigorescu, V. False-positive newborn screening result and future health care use in a state Medicaid cohort. Pediatrics 2011, 128, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Stroek, K.; Heijboer, A.C.; van Veen-Sijne, M.; Bosch, A.M.; van der Ploeg, C.P.B.; Zwaveling-Soonawala, N.; de Jonge, R.; van Trotsenburg, A.S.P.; Boelen, A. Improving the Dutch Newborn Screening for Central Congenital Hypothyroidism by Using 95% Reference Intervals for Thyroxine-Binding Globulin. Eur. Thyroid. J. 2021, 10, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Stroek, K.; Ruiter, A.; van der Linde, A.; Ackermans, M.; Bouva, M.J.; Engel, H.; Jakobs, B.; Kemper, E.A.; van den Akker, E.L.T.; van Albada, M.E.; et al. Second-tier Testing for 21-Hydroxylase Deficiency in the Netherlands: A Newborn Screening Pilot Study. J. Clin. Endocrinol. Metab. 2021, 106, e4487–e4496. [Google Scholar] [CrossRef] [PubMed]
- Stroek, K.; Boelen, A.; Bouva, M.J.; De Sain-van der Velden, M.; Schielen, P.; Maase, R.; Engel, H.; Jakobs, B.; Kluijtmans, L.A.J.; Mulder, M.F.; et al. Evaluation of 11 years of newborn screening for maple syrup urine disease in the Netherlands and a systematic review of the literature: Strategies for optimization. JIMD Rep. 2020, 54, 68–78. [Google Scholar] [CrossRef]
- Stroek, K.; Heijboer, A.C.; Bouva, M.J.; van der Ploeg, C.P.B.; Heijnen, M.A.; Weijman, G.; Bosch, A.M.; de Jonge, R.; Schielen, P.; van Trotsenburg, A.S.P.; et al. Critical evaluation of the newborn screening for congenital hypothyroidism in the Netherlands. Eur. J. Endocrinol. 2020, 183, 265–273. [Google Scholar] [CrossRef]
- Hakkaart-van Roijen, L.P.S.; Kanters, T. Kostenhandleiding voor Economische Evaluaties in de Gezondheidszorg: Methodologie en Referentieprijzen Herziene Versie 2024; Opdracht van Zorginstituut Nederland: Diemen, The Netherlands, 2024. [Google Scholar]
- Zorgautoriteit, N. DBC-Zorgproducten. Available online: https://www.opendisdata.nl/msz/zorgproduct (accessed on 8 January 2024).
- NVK. Werkboek Congenitale Hypothyreoïdie; Nederlandse Vereniging voor Kindergeneeskunde: Utrecht, The Netherlands, 2010. [Google Scholar]
- NVK. Werkboek: Adrenogenitaal. Diagnostiek bij Verdenking op AGS. Available online: https://nvk.medonline.nl/adrenogenitaal-syndroom/diagnostiek-bij-verdenking-op-ags/ (accessed on 20 July 2024).
- VKS. Zorgpaden voor behandelaren. Diagnostiek Maple Syrup Urine Disease. Available online: https://zorgpaden.stofwisselingsziekten.nl/zorgpaden/behandelaren/maple-syrup-urine-disease/diagnostiek/ (accessed on 8 April 2024).
- Boelen, A.; Zwaveling-Soonawala, N.; Heijboer, A.C.; van Trotsenburg, A.S.P. Neonatal screening for primary and central congenital hypothyroidism: Is it time to go Dutch? Eur. Thyroid. J. 2023, 12, e230041. [Google Scholar] [CrossRef]
- Jansen, H.I.; van Haeringen, M.; Bouva, M.J.; den Elzen, W.P.J.; Bruinstroop, E.; van der Ploeg, C.P.B.; van Trotsenburg, A.S.P.; Zwaveling-Soonawala, N.; Heijboer, A.C.; Bosch, A.M.; et al. Optimizing the Dutch newborn screening for congenital hypothyroidism by incorporating amino acids and acylcarnitines in a machine learning-based model. Eur. Thyroid. J. 2023, 12, e230141. [Google Scholar] [CrossRef] [PubMed]
- Stroek, K.; Visser, A.; van der Ploeg, C.P.B.; Zwaveling-Soonawala, N.; Heijboer, A.C.; Bosch, A.M.; van Trotsenburg, A.S.P.; Boelen, A.; Hoogendoorn, M.; de Jonge, R. Machine learning to improve false-positive results in the Dutch newborn screening for congenital hypothyroidism. Clin. Biochem. 2023, 116, 7–10. [Google Scholar] [CrossRef]
- Vonk, R.; Hilderink, H.; Plasmans, M.; Kommer, G.; Polder, J. Health Care Expenditures Foresight 2015–2060: Quantitative Preliminary Study at the Request of the Scientific Council for Government Policy (WRR). Part 1: Future Projections. 2020. Available online: https://rivm.openrepository.com/handle/10029/623734 (accessed on 8 April 2024).
- National Institute for Public Health and the Environment (RIVM). Integraal Zorgakkoord: Samen Werken aan Gezonde Zorg September 2022. Available online: https://www.rijksoverheid.nl/documenten/rapporten/2022/09/16/integraal-zorgakkoord-samen-werken-aan-gezonde-zorg (accessed on 8 April 2024).
- Wat is Zorgevaluatie en Wat Is Gepast Gebruik? Available online: https://zorgevaluatiegepastgebruik.nl/over-ze-gg/over-ze-gg/ (accessed on 8 April 2024).

| Type of Costs | Costs | Source |
|---|---|---|
| Medical advisor | EUR 150 | Tariff From RIVM |
| Visit to GP | EUR 29 | Reference price [17] |
| Visit to pediatrician and diagnostic tests to confirm CH | EUR 1024 | Dutch DBC tariff [18] |
| Visit to pediatric endocrinologist and diagnostic tests to confirm CAH | EUR 1049 | Dutch DBC tariff [18] |
| Visit to pediatrician for metabolic diseases and diagnostic tests to confirm MSUD | EUR 2001 | Dutch DBC tariff [18] |
| Screener | EUR 26 | Tariff from RIVM |
| Set for DBS | EUR 3 | Tariff from RIVM |
| Analysis by laboratory | EUR 94 | Tariff from RIVM |
| Second-tier analysis | EUR 290 | Tariff from RIVM |
| Type of Costs | Costs |
|---|---|
| Medical advisor | EUR 150 |
| Visit GP | EUR 29 |
| Visit pediatrician and diagnostic test to confirm CH | EUR 1024 |
| Total | EUR 1203 |
| Type of Costs | Costs Second Heel Puncture | Costs Second Tier |
|---|---|---|
| Screener | EUR 26 | |
| Set for DBS | EUR 3 | |
| Analysis by laboratory | EUR 94 | |
| Medical advisor | EUR 150 | |
| Second-tier analysis | EUR 290 | |
| Total | EUR 272 | EUR 290 |
| Type of Costs | Costs |
|---|---|
| Medical advisor | EUR 150 |
| Visit GP | EUR 29 |
| Visit pediatric endocrinologist and diagnostic tests to confirm CAH | EUR 1049 |
| Total | EUR 1228 |
| Type of Costs | Costs |
|---|---|
| Medical advisor | EUR 150 |
| Visit GP | EUR 29 |
| Visit pediatrician for metabolic diseases and diagnostic tests to confirm MSUD | EUR 2001 |
| Total | EUR 2180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, R.C.; Boelen, A.; van der Kemp, M.H.; Bosch, A.M.; Berghout, E.M.; Weijman, G.; Zwaveling-Soonawala, N.; Verschoof-Puite, R.K.; de Jonge, R.; Hannema, S.E.; et al. The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands. Int. J. Neonatal Screen. 2024, 10, 70. https://doi.org/10.3390/ijns10040070
Martens RC, Boelen A, van der Kemp MH, Bosch AM, Berghout EM, Weijman G, Zwaveling-Soonawala N, Verschoof-Puite RK, de Jonge R, Hannema SE, et al. The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands. International Journal of Neonatal Screening. 2024; 10(4):70. https://doi.org/10.3390/ijns10040070
Chicago/Turabian StyleMartens, Rosalie C., Anita Boelen, Michèle H. van der Kemp, Annet M. Bosch, Eveline M. Berghout, Gert Weijman, Nitash Zwaveling-Soonawala, Rendelien K. Verschoof-Puite, Robert de Jonge, Sabine E. Hannema, and et al. 2024. "The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands" International Journal of Neonatal Screening 10, no. 4: 70. https://doi.org/10.3390/ijns10040070
APA StyleMartens, R. C., Boelen, A., van der Kemp, M. H., Bosch, A. M., Berghout, E. M., Weijman, G., Zwaveling-Soonawala, N., Verschoof-Puite, R. K., de Jonge, R., Hannema, S. E., Bosmans, J. E., & Heijboer, A. C. (2024). The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands. International Journal of Neonatal Screening, 10(4), 70. https://doi.org/10.3390/ijns10040070







