Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022—Epidemiology and Impact on Clinical Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalonian NBS Program
2.2. Impact of the NBS Program
2.3. Statistical Analysis of Questionnaire Data
3. Results
3.1. Incidence and Epidemiology of SCD in Catalonia
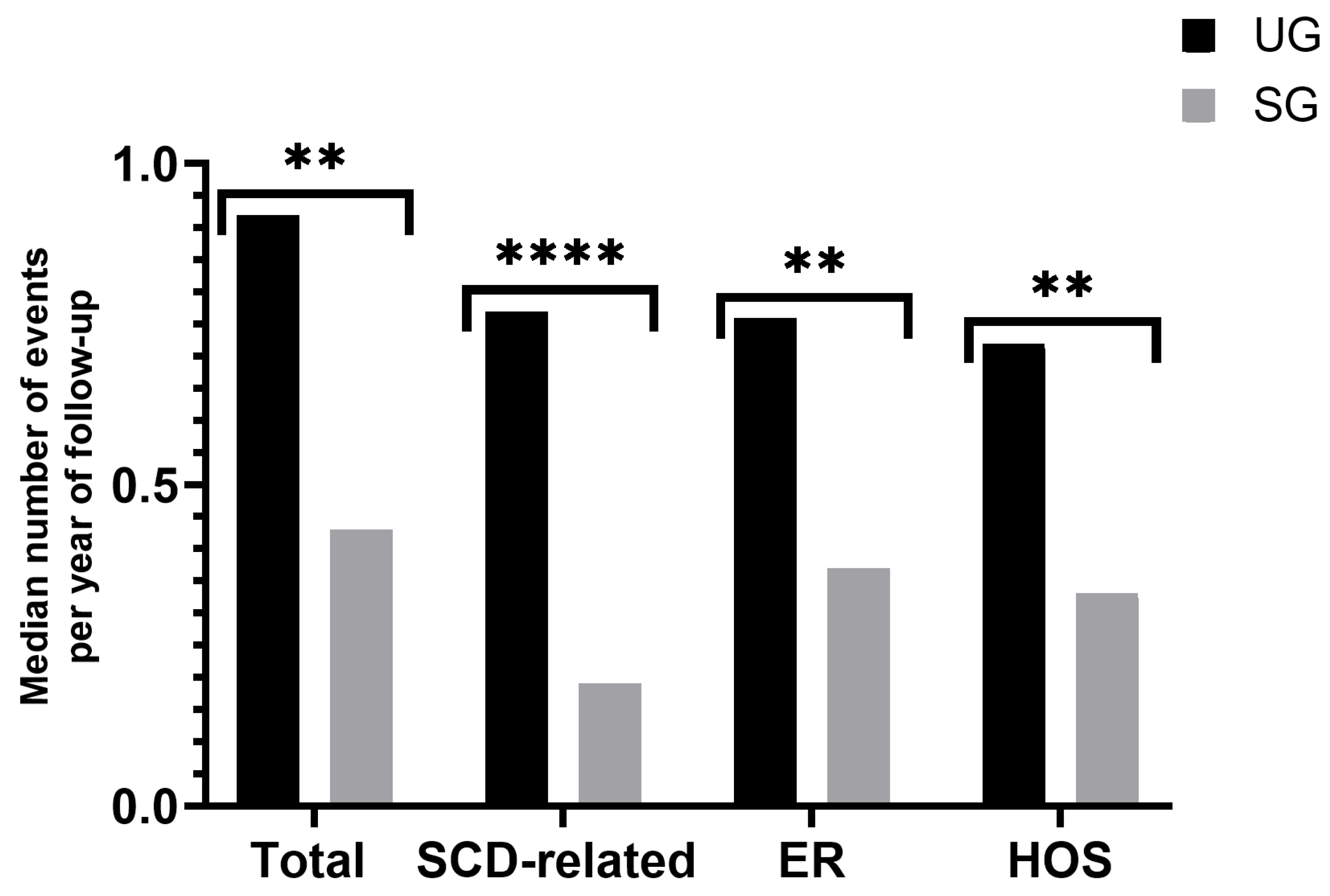
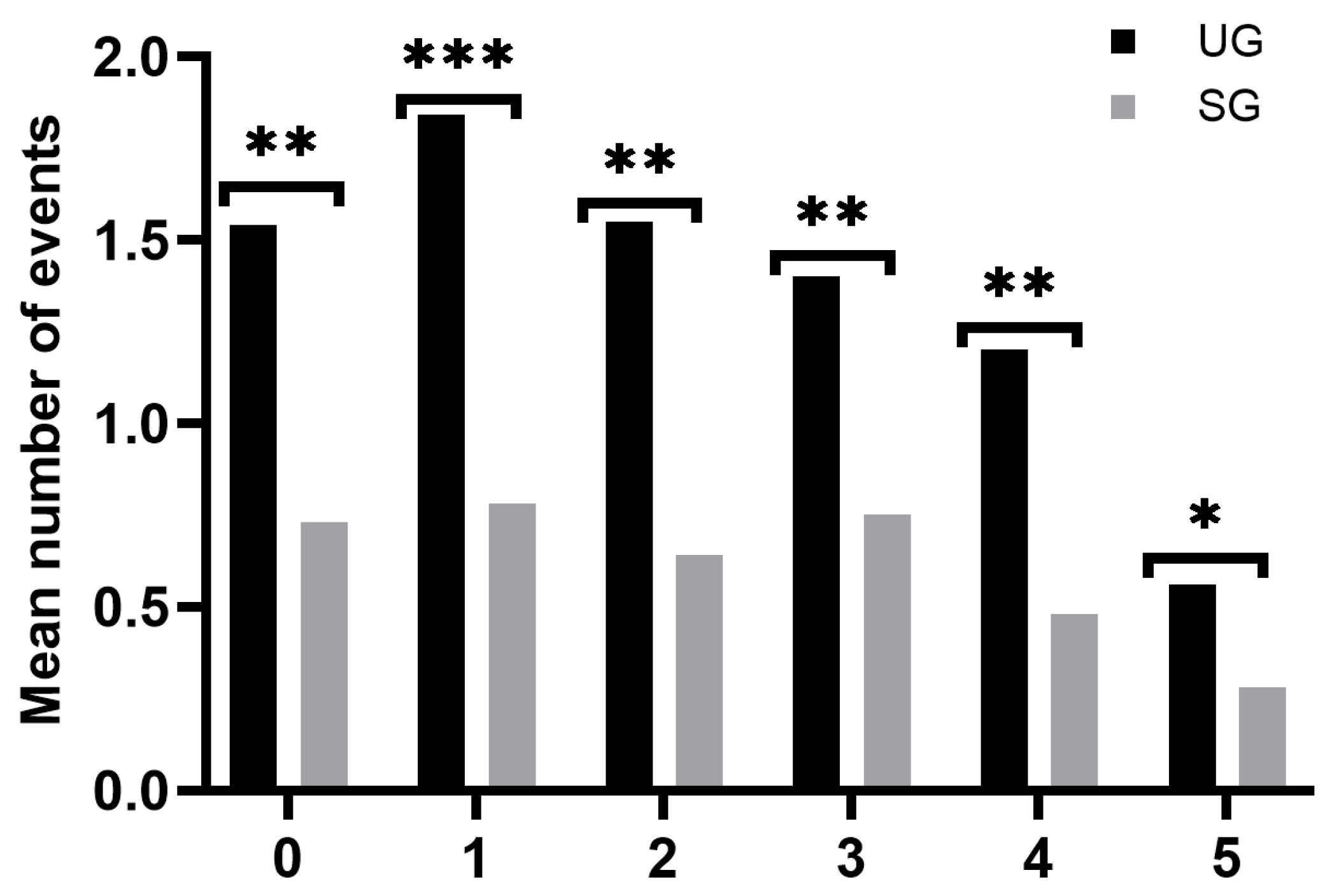
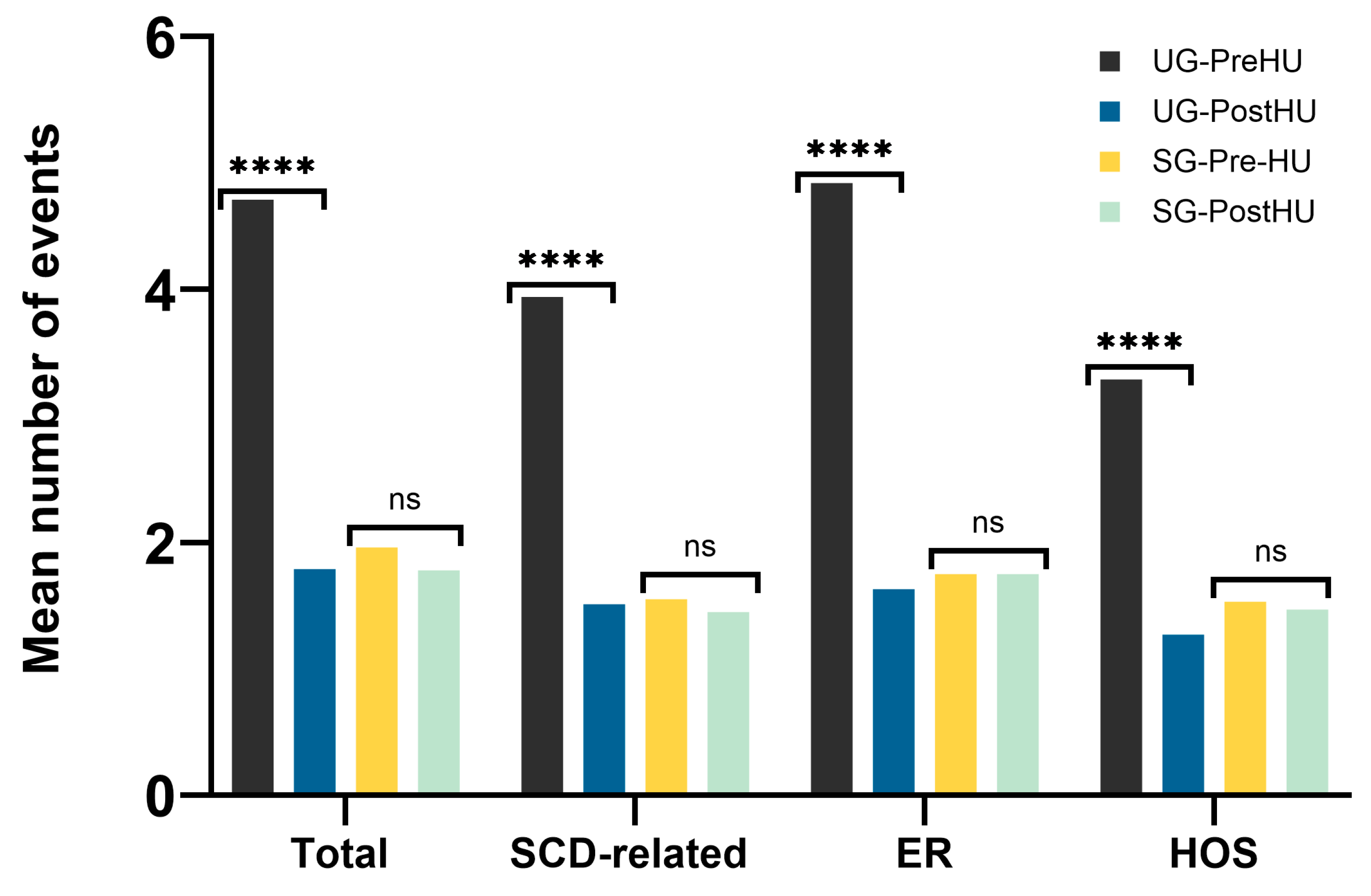
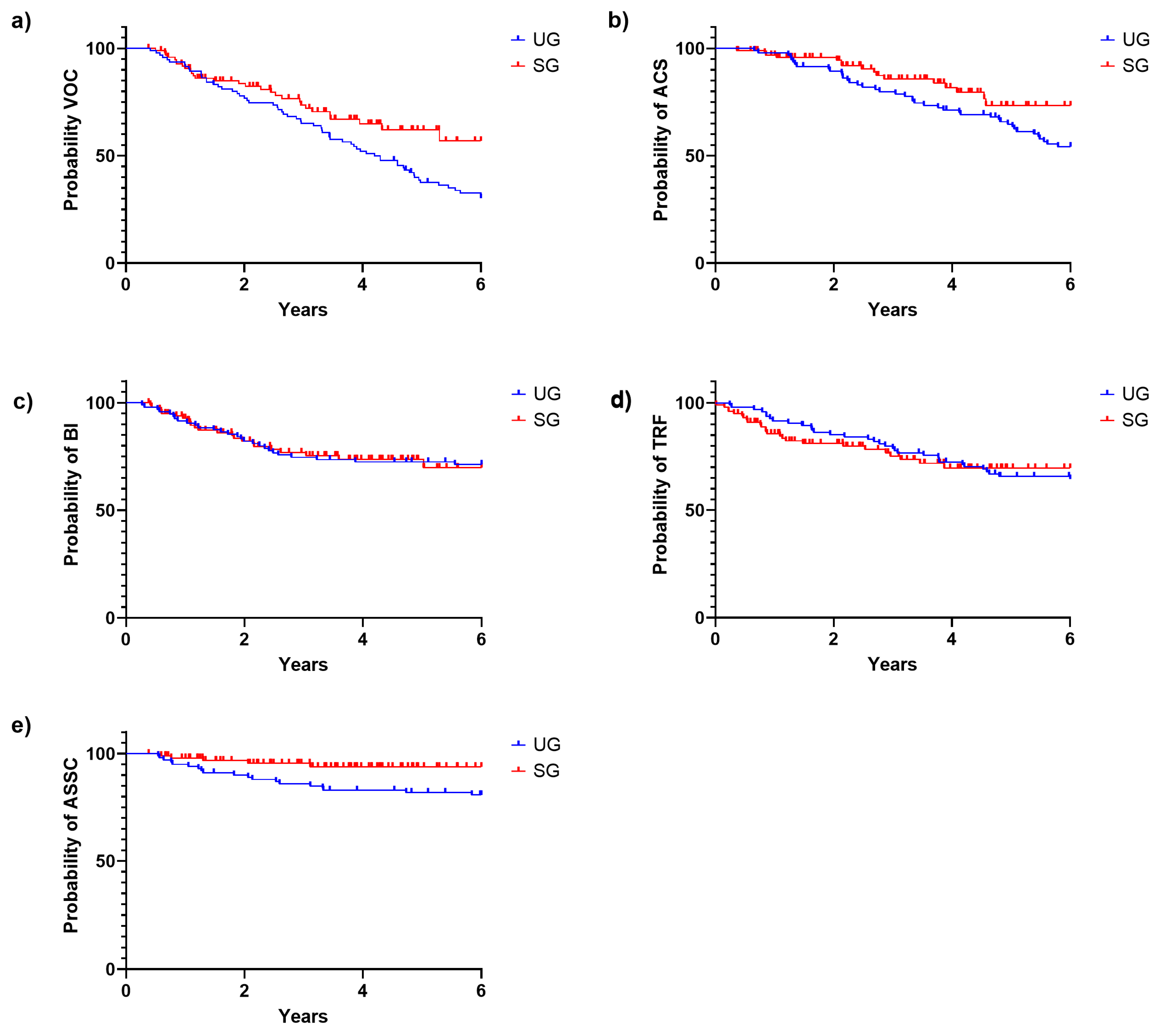
3.2. Clinical Impact of the NBS Program
3.2.1. Demographic Data of the Study Cohort
3.2.2. Clinical Events during the First Six Years of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef]
- Thomson, A.M.; A McHugh, T.; Oron, A.P.; Teply, C.; Lonberg, N.; Tella, V.V.; Wilner, L.B.; Fuller, K.; Hagins, H.; Aboagye, R.G.; et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Grosse, S.D.; Odame, I.; Atrash, H.K.; Amendah, D.D.; Piel, F.B.; Williams, T.N. Sickle cell disease in Africa: A neglected cause of early childhood mortality. Am. J. Prev. Med. 2011, 41 (Suppl. S4), S398–S405. [Google Scholar] [CrossRef]
- Mañú Pereira, M.d.M.; Colombatti, R.; Alvarez, F.; Bartolucci, P.; Bento, C.; Brunetta, A.L.; Cela, E.; Christou, S.; Collado, A.; de Montalembert, M.; et al. Sickle cell disease landscape and challenges in the EU: The ERN-EuroBloodNet perspective. Lancet Haematol. 2023, 10, e687–e694. [Google Scholar] [CrossRef]
- Marco Sánchez, J.M.; Bardón Cancho, E.J.; Benéitez, D.; Payán-Pernía, S.; Gimbert, A.C.; Ruiz-Llobet, A.; Salinas, J.A.; Sebastián, E.; Argilés, B.; Bermúdez, M.; et al. Haemoglobinopathies and other rare anemias in Spain: Ten years of a nationwide registry (REHem-AR). Ann Hematol. 2024, 103, 2743–2755. [Google Scholar] [CrossRef]
- Minkovitz, C.S.; Grason, H.; Ruderman, M.; Casella, J.F. Newborn Screening Programs and Sickle Cell Disease: A Public Health Services and Systems Approach. Am. J. Prev. Med. 2016, 51 (Suppl. S1), S39–S47. [Google Scholar] [CrossRef]
- Daniel, Y.; Elion, J.; Allaf, B.; Badens, C.; Bouva, M.J.; Brincat, I.; Cela, E.; Coppinger, C.; de Montalembert, M.; Gulbis, B.; et al. Newborn Screening for Sickle Cell Disease in Europe. Int. J. Neonatal Screen. 2019, 5, 15. [Google Scholar] [CrossRef]
- Gulbis, B.; Lê, P.-Q.; Ketelslegers, O.; Dresse, M.-F.; Adam, A.-S.; Cotton, F.; Boemer, F.; Bours, V.; Minon, J.-M.; Ferster, A. Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement. Int. J. Neonatal Screen. 2018, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- García-Morín, M.; Bardón-Cancho, E.J.; Beléndez, C.; Zamarro, R.; Béliz-Mendiola, C.; González-Rivera, M.; Vecilla, C.; Llorente-Otones, L.; Pérez-Alonso, V.; Román, S.S.; et al. Fifteen years of newborn sickle cell disease screening in Madrid, Spain: An emerging disease in a European country. Ann. Hematol. 2020, 99, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Martella, M.; Cattaneo, L.; Viola, G.; Cappellari, A.; Bergamo, C.; Azzena, S.; Schiavon, S.; Baraldi, E.; Barba, B.D.; et al. Results of a multicenter universal newborn screening program for sickle cell disease in Italy: A call to action. Pediatr. Blood Cancer 2019, 66, e27657. [Google Scholar] [CrossRef] [PubMed]
- Brousse, V.; Allaf, B.; Benkerrou, M. Newborn screening for sickle cell disease in France. Med. Sci. 2021, 37, 482–490. [Google Scholar] [CrossRef]
- Green, N.S.; Zapfel, A.; Nnodu, O.E.; Franklin, P.; Tubman, V.N.; Chirande, L.F.; Kiyaga, C.; Chunda-Liyoka, C.M.; Awuonda, B.; Ohene-Frempong, K.; et al. The Consortium on Newborn Screening in Africa for sickle cell disease: Study rationale and methodology. Blood Adv. 2022, 6, 6187–6197. [Google Scholar] [CrossRef]
- Zapfel, A.J.; Thompson, A.A.; Bridges, K.; Richardson, J.; Kasongo, L.; Przybylski, C.; Kiyaga, C.; Barango, P.; Odame, I. World Coalition on SCD launches, sparking global focus on SCD diagnosis and care. Blood Adv. 2023, 7, 6812–6814. [Google Scholar] [CrossRef]
- Marín Soria, J.L.; González de Aledo Castillo, J.M.; Argudo Ramírez, A.; López Galera, R.M.; Pajares García, S.; Ribes Rubió, A.; García Villoria, J.; Yahyaoui Macías, R.; Álvarez Ríos, A.; Melguizo Madrid, E.; et al. Beginnings, evolution and current situation of the Newborn Screening Programs in Spain. Rev. Esp. Salud Publica 2021, 95, e202102041. [Google Scholar]
- Marín Soria, J.L.; López Galera, R.M.; Argudo Ramírez, A.; González de Aledo, J.M.; Pajares García, S.; Navarro Sastre, A.; Hernandez Pérez, J.M.; Ribes Rubio, A.; Gort Mas, L.; García Villoria, J.; et al. 50 years of the Neonatal Screening Program in Catalonia. Rev. Esp. Salud Publica 2020, 94, e202012177. [Google Scholar]
- Lê, P.-Q.; Ferster, A.; Dedeken, L.; Vermylen, C.; Vanderfaeillie, A.; Rozen, L.; Heijmans, C.; Huybrechts, S.; Devalck, C.; Cotton, F.; et al. Neonatal screening improves sickle cell disease clinical outcome in Belgium. J. Med. Screen. 2017, 25, 57–63. [Google Scholar] [CrossRef]
- García-Morin, M.; Bardón-Cancho, E.J.; Beléndez, C.; Dulín, E.; Blanco-Soto, P.; Puertas-López, C.; Prieto-Medina, M.; Cervera-Bravo, Á.; Llorente-Otones, L.; Pérez-Alonso, V.; et al. Madrid Newborn Sickle Cell Disease Cohort: Clinical outcomes, stroke prevention and survival. Ann. Hematol. 2023, 103, 373–383. [Google Scholar] [CrossRef]
- Poventud-Fuentes, I.; Garnett, E.; Vispo, B.; Elghetany, M.T.; Devaraj, S. Hemoglobin fractionation by Sebia Capillarys 2 Flex Piercing System as primary method for evaluation of hemoglobinopathies. Clin. Chim. Acta 2021, 519, 193–197. [Google Scholar] [CrossRef]
- Puehringer, H.; Najmabadi, H.; Law, H.-Y.; Krugluger, W.; Viprakasit, V.; Pissard, S.; Baysal, E.; Taher, A.; Farra, C.; Al-Ali, A.; et al. Validation of a reverse-hybridization StripAssay for the simultaneous analysis of common alpha-thalassemia point mutations and deletions. Clin. Chem. Lab. Med. 2007, 45, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Yoo, E.-H.; Kim, J.-Y.; Kim, J.-W.; Ki, C.-S. Multiplex ligation-dependent probe amplification screening of isolated increased HbF levels revealed three cases of novel rearrangements/deletions in the beta-globin gene cluster. Br. J. Haematol. 2010, 148, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Roellinger, S.E.; Lundquist, P.A.; Highsmith, W.E.; Dawson, D.B. Development and clinical implementation of a combination deletion PCR and multiplex ligation-dependent probe amplification assay for detecting deletions involving the human α-globin gene cluster. J. Mol. Diagn. 2011, 13, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Cela, E.; Ruiz-Llobet, A.; Cervera, Á.; Guía de Práctica Clínica de la Enfermedad de Células Falciformes. Sociedad Española de Hematología y Oncología Pediátrica (SEHOP). 2019. Available online: https://www.sehop.org/wp-content/uploads/2019/03/Guía-SEHOP-Falciforme-2019.pdf (accessed on 17 October 2023).
- Thornburg, C.D.; Files, B.A.; Luo, Z.; Miller, S.T.; Kalpatthi, R.; Iyer, R.; Seaman, P.; Lebensburger, J.; Alvarez, O.; Thompson, B.; et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012, 120, 4304–4448. [Google Scholar] [CrossRef]
- Bardón Cancho, E.J.; García-Morín, M.; Beléndez, C.; Velasco, P.; Benéitez, D.; Ruiz-Llobet, A.; Berrueco, R.; Argilés, B.; Cervera, Á.; Salinas, J.A.; et al. Update of the Spanish registry of haemoglobinopathies in children and adults. Med. Clin. 2020, 155, 95–103. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística. Available online: https://www.ine.es/jaxi/Tabla.htm?path=/t20/e245/p08/l0/&file=02005.px&L=0 (accessed on 3 December 2023).
- Institut d’Estadística de Catalunya. Available online: https://www.idescat.cat/pub/?id=naix&n=5094&lang=es (accessed on 3 December 2023).
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 2010, 1, 104. [Google Scholar] [CrossRef]
- Sánchez-Villalobos, M.; Campos Baños, E.; Juan Fita, M.J.; Mellado, J.M.E.; Gallego, I.G.; Videla, A.B.; Piqueras, M.B.; Cortés, M.B.; Jiménez, J.M.M.; Navarro, E.G.; et al. A Newborn Screening Program for Sickle Cell Disease in Murcia (Spain). Int. J. Neonatal Screen. 2023, 9, 55. [Google Scholar] [CrossRef]
- Streetly, A.; Sisodia, R.; Dick, M.; Latinovic, R.; Hounsell, K.; Dormandy, E. Evaluation of newborn sickle cell screening programme in England: 2010-2016. Arch. Dis. Child. 2018, 103, 648–653. [Google Scholar] [CrossRef]
- Therrell, B.L.J.; Lloyd-Puryear, M.A.; Eckman, J.R.; Mann, M.Y. Newborn screening for sickle cell diseases in the United States: A review of data spanning 2 decades. Semin. Perinatol. 2015, 39, 238–251. [Google Scholar] [CrossRef]
- Lobitz, S.; Telfer, P.; Cela, E.; Allaf, B.; Angastiniotis, M.; Johansson, C.B.; Badens, C.; Bento, C.; Bouva, M.J.; Canatan, D.; et al. Newborn screening for sickle cell disease in Europe: Recommendations from a Pan-European Consensus Conference. Br. J. Haematol. 2018, 183, 648–660. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard NBS01; Dried Blood Spot Specimen Collection for Newborn Screening, 7th ed. Clinical and Laboratory Standards Institute CLSI: Wayne, PA, USA, 2021.
- Frömmel, C. Newborn Screening for Sickle Cell Disease and Other Hemoglobinopathies: A Short Review on Classical Laboratory Methods-Isoelectric Focusing, HPLC, and Capillary Electrophoresis. Int. J. Neonatal Screen. 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Kazadi, C.; Ducruet, T.; Forté, S.; Robitaille, N.; Pastore, Y. Positive impacts of universal newborn screening on the outcome of children with sickle cell disease in the province of Quebec: A retrospective cohort study. eJHaem 2024, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Fraser, R.; Forbes, M.; Grindley, M.; Ali, S.; Reid, M. Newborn sickle cell disease screening: The Jamaican experience (1995–2006). J. Med. Screen. 2007, 14, 117–122. [Google Scholar] [CrossRef]
- Quinn, C.T.; Rogers, Z.R.; McCavit, T.L.; Buchanan, G.R. Improved survival of children and adolescents with sickle cell disease. Blood 2010, 115, 3447–3452. [Google Scholar] [CrossRef]
- Upadhye, D.S.; Jain, D.L.; Trivedi, Y.L.; Nadkarni, A.H.; Ghosh, K.; Colah, R.B. Neonatal Screening and the Clinical Outcome in Children with Sickle Cell Disease in Central India. PLoS ONE 2016, 11, e0147081. [Google Scholar] [CrossRef]
- Thaker, P.; Colah, R.B.; Patel, J.; Raicha, B.; Mistry, A.; Mehta, V.; Italia, Y.; Desai, S.; Dave, K.; Shanmugam, R.; et al. Newborn Screening for Sickle Cell Disease Among Tribal Populations in the States of Gujarat and Madhya Pradesh in India: Evaluation and Outcome Over 6 Years. Front. Med. 2021, 8, 731884. [Google Scholar] [CrossRef]
- Brousse, V.; Arnaud, C.; Lesprit, E.; Quinet, B.; Odièvre, M.-H.; Etienne-Julan, M.; Guillaumat, C.; Elana, G.; Belloy, M.; Garnier, N.; et al. Evaluation of Outcomes and Quality of Care in Children with Sickle Cell Disease Diagnosed by Newborn Screening: A Real-World Nation-Wide Study in France. J. Clin. Med. 2019, 8, 1594. [Google Scholar] [CrossRef]
- Odey, F.; Okomo, U.; Oyo-Ita, A. Vaccines for preventing invasive salmonella infections in people with sickle cell disease. Cochrane Database Syst. Rev. 2018, 12, CD006975. [Google Scholar] [CrossRef]
- Telfer, P.; Coen, P.; Chakravorty, S.; Wilkey, O.; Evans, J.; Newell, H.; Smalling, B.; Amos, R.; Stephens, A.; Rogers, D.; et al. Clinical outcomes in children with sickle cell disease living in England: A neonatal cohort in East London. Haematologica 2007, 92, 905–912. [Google Scholar] [CrossRef]
- Figueiredo, M.S. The compound state: Hb S/beta-thalassemia. Rev. Bras. Hematol. Hemoter. 2015, 37, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Gualandro, S.F.M.; Fonseca, G.H.H.; Yokomizo, I.K.; Gualandro, D.M.; Suganuma, L.M. Cohort study of adult patients with haemoglobin SC disease: Clinical characteristics and predictors of mortality. Br. J. Haematol. 2015, 171, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gaston, M.H.; Verter, J.I.; Woods, G.; Pegelow, C.; Kelleher, J.; Presbury, G.; Zarkowsky, H.; Vichinsky, E.; Iyer, R.; Lobel, J.S.; et al. Prophylaxis with Oral Penicillin in Children with Sickle Cell Anemia. N. Engl. J. Med. 1986, 314, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Ware, R.E.; Miller, S.T.; Iyer, R.V.; Casella, J.F.; Minniti, C.P.; Rana, S.; Thornburg, C.D.; Rogers, Z.R.; Kalpatthi, R.V.; et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (BABY HUG). Lancet 2011, 377, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report. 2014. Available online: https://www.nhlbi.nih.gov/sites/default/files/media/docs/sickle-cell-disease-report%20020816_0.pdf (accessed on 21 July 2024).
- Opoka, R.O.; Ndugwa, C.M.; Latham, T.S.; Lane, A.; Hume, H.A.; Kasirye, P.; Hodges, J.S.; Ware, R.E.; John, C.C. Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM): A trial for children with sickle cell anemia. Blood 2017, 130, 2585–2593. [Google Scholar] [CrossRef]
- John, C.C.; Opoka, R.O.; Latham, T.S.; Hume, H.A.; Nabaggala, C.; Kasirye, P.; Ndugwa, C.M.; Lane, A.; Ware, R.E. Hydroxyurea Dose Escalation for Sickle Cell Anemia in Sub-Saharan Africa. N. Engl. J. Med. 2020, 382, 2524–2533. [Google Scholar] [CrossRef]
- de Montalembert, M.; Galactéros, F.; Oevermann, L.; Cannas, G.; Joseph, L.; Loko, G.; Elenga, N.; Benkerrou, M.; Etienne-Julan, M.; Castex, M.-P.; et al. Hydroxyurea Is Associated with Later Onset of Occurrence of Acute Splenic Sequestration Episodes in Sickle Cell Disease: Lessons from the European Sickle Cell Disease Cohort—Hydroxyurea (ESCORT-HU) Study. Blood 2022, 140 (Suppl. S1), 449–450. [Google Scholar] [CrossRef]
- Allali, S.; Galactéros, F.; Oevermann, L.; Cannas, G.; Joseph, L.; Loko, G.; Elenga, N.; Benkerrou, M.; Etienne-Julan, M.; Castex, M.; et al. Hydroxyurea is associated with later onset of acute splenic sequestration crisis in sickle cell disease: Lessons from the European Sickle Cell Disease Cohort-Hydroxyurea (ESCORT-HU) study. Am. J. Hematol. 2024, 99, 555–561. [Google Scholar] [CrossRef]


| Confirmatory Diagnosis by Genetic Analysis (Sanger and MLPA if Necessary) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype by CE | HbSS | HbSβ0 | HbSβ+ | HbSC | HbSA Ϯ | HbSX Ϯ | NA | n |
| FS | 103 (89.6%) | 2 (1.7%) | 5 (4.3%) | 1 (0.9%) | 1 (0.9%) * | 3 (2.6%) | 115 | |
| FSa | 3 (42.9%) | 1 (14.3%) | 1 (14.3%) | 1 (14.3%) # | 1 (14.3%) | 7 | ||
| FSC | 40 (100%) | 40 | ||||||
| FAS Ω | 1 (100%) | 1 | ||||||
| FSC Ω | 1 (100%) | 1 | ||||||
| Total | 164 | |||||||
| UG | SG | p | |
|---|---|---|---|
| N | 95 | 100 | |
| Median time of follow-up (y) | 8.67 (0.66–18.2) | 3.58 (0.25–8.1) | p < 0.0001 * |
| Median time of follow-up, up to six years of life (y) | 4.46 (0.66–6) | 3.58 (0.25–6) | p = 0.02 * |
| Total years of follow-up (up to six years of life) | 399.35 | 340.76 | |
| Deaths | 0 | 1 | |
| HSC transplantation | 2 | 3 | |
| Median age at diagnostics (y) (range) | 1.68 (0.1–5.69) | 0.1 (0–1.99) | p < 0.0001 * |
| Median age at last follow-up (y) (range) | 10.43 (1.48–20.97) | 3.79 (0.38–8.2) | p < 0.0001 * |
| ATB prophylaxis (%) | 94.7 | 100 | p = 0.03 * |
| Median age at ATB onset, (y) (range) | 1.86 (0–11.42) | 0.12(0.01–4.44) | p < 0.0001 * |
| HU treatment (%) | 93.8 | 80.3 | p = 0.013 * |
| Median age at HU onset, (y) (range) | 4.5 (1.23–11.91) | 1.42 (0.76–3.96) | p < 0.0001 * |
| HU-related neutropenia (%) | 6.6 | 9.4 | p = 0.21 |
| HU-related adverse effects (%) | 5.3 | 5.7 | p = 0.1 |
| Age at first VOC (y) | 2.96 (0.4–5.7) | 1.91 (0.5–5.3) | 0.007 * |
| Age at first ACS (y) | 3.33 (0.7–5.8) | 2.58 (0.4–4.6) | 0.074 |
| Age at first BI (y) | 1.80 (0.3–5.6) | 1.22 (0.4–5.0) | 0.52 |
| Age at first TRF (y) | 2.72 (0.2–6) | 0.85 (0.1–3.4) | 0.001 * |
| Age at first ASSC (y) | 1.98 (0.6–5.8) | 1.30 (0.5–3.1) | 0.355 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González de Aledo-Castillo, J.M.; Argudo-Ramírez, A.; Beneitez-Pastor, D.; Collado-Gimbert, A.; Almazán Castro, F.; Roig-Bosch, S.; Andrés-Masó, A.; Ruiz-Llobet, A.; Pedrals-Portabella, G.; Medina-Santamaria, D.; et al. Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022—Epidemiology and Impact on Clinical Events. Int. J. Neonatal Screen. 2024, 10, 69. https://doi.org/10.3390/ijns10040069
González de Aledo-Castillo JM, Argudo-Ramírez A, Beneitez-Pastor D, Collado-Gimbert A, Almazán Castro F, Roig-Bosch S, Andrés-Masó A, Ruiz-Llobet A, Pedrals-Portabella G, Medina-Santamaria D, et al. Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022—Epidemiology and Impact on Clinical Events. International Journal of Neonatal Screening. 2024; 10(4):69. https://doi.org/10.3390/ijns10040069
Chicago/Turabian StyleGonzález de Aledo-Castillo, José Manuel, Ana Argudo-Ramírez, David Beneitez-Pastor, Anna Collado-Gimbert, Francisco Almazán Castro, Sílvia Roig-Bosch, Anna Andrés-Masó, Anna Ruiz-Llobet, Georgina Pedrals-Portabella, David Medina-Santamaria, and et al. 2024. "Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022—Epidemiology and Impact on Clinical Events" International Journal of Neonatal Screening 10, no. 4: 69. https://doi.org/10.3390/ijns10040069
APA StyleGonzález de Aledo-Castillo, J. M., Argudo-Ramírez, A., Beneitez-Pastor, D., Collado-Gimbert, A., Almazán Castro, F., Roig-Bosch, S., Andrés-Masó, A., Ruiz-Llobet, A., Pedrals-Portabella, G., Medina-Santamaria, D., Nadal-Rey, G., Espigares-Salvia, M., Coll-Sibina, M. T., Algar-Serrano, M., Torrent-Español, M., Leoz-Allegretti, P., Rodríguez-Pebé, A., García-Bernal, M., Solà-Segura, E., ... on behalf of the Sickle Cell Disease Newborn Screening Group of Catalonia. (2024). Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022—Epidemiology and Impact on Clinical Events. International Journal of Neonatal Screening, 10(4), 69. https://doi.org/10.3390/ijns10040069





