Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy
Abstract
1. Introduction
2. Review Methods
2.1. Aims of Review
2.2. Search Strategy
2.3. Inclusion Criteria
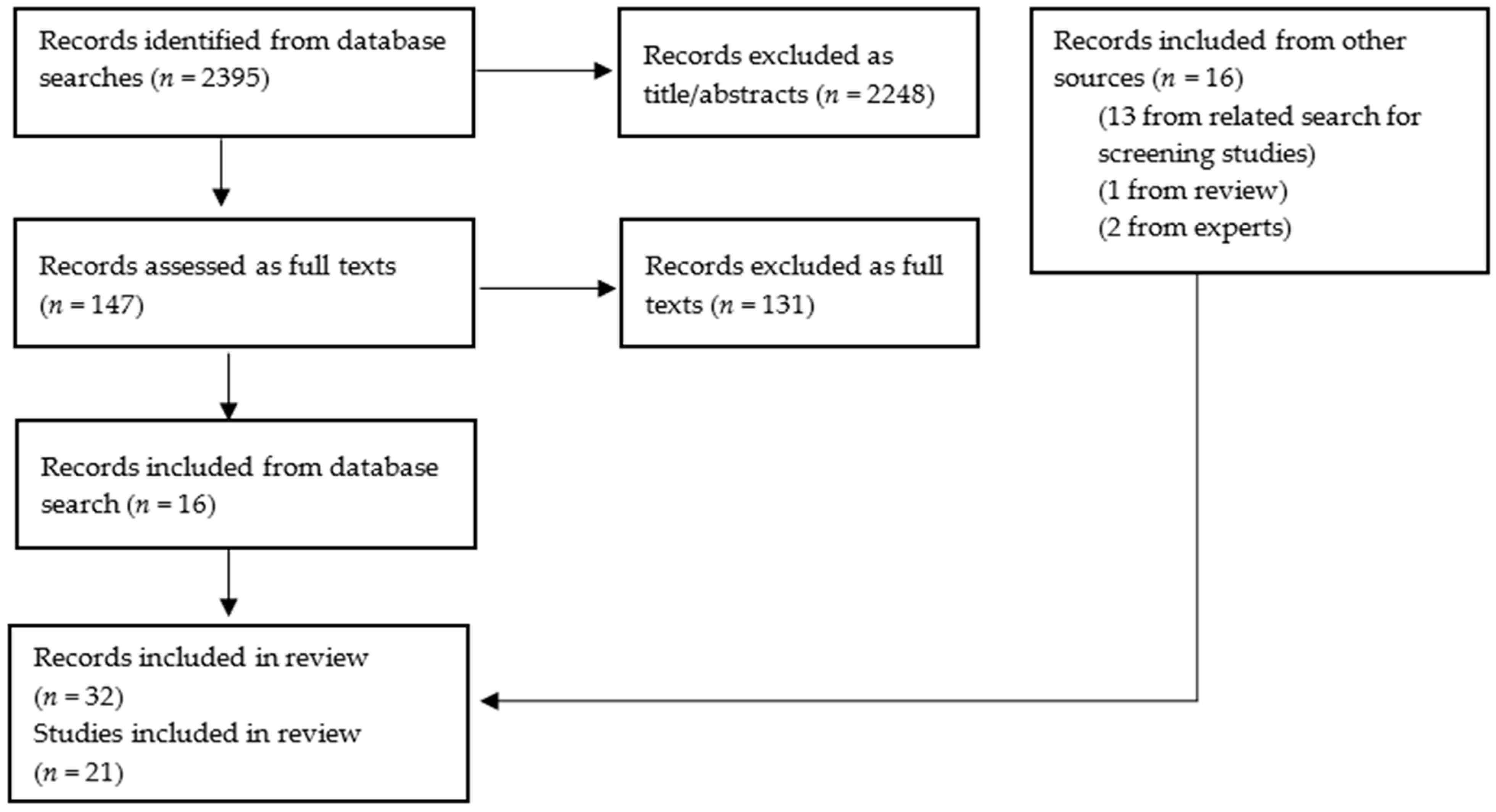
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Approach to Synthesis
3. Results
3.1. Volume and Type of Included Studies
3.2. Interventional Single-Arm Studies
3.2.1. Outcomes in Babies with Two SMN2 Copies
3.2.2. Outcomes in Babies with Three SMN2 Copies
3.3. Comparative Observational Studies
3.3.1. Two SMN2 Copies
3.3.2. Three SMN2 Copies
3.3.3. Four or More SMN2 Copies
3.3.4. Mixed SMN2 Copy Number Cohorts
3.3.5. Respiratory and Feeding Outcomes
3.4. Prospective Follow-Up of Screened Cohorts
3.4.1. One SMN2 Copy
3.4.2. Two SMN2 Copies
3.4.3. Three SMN2 Copies
3.4.4. Four or More SMN2 Copies
3.5. Risk of Bias in the Included Studies
3.6. Quality of Life
3.7. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Medline Search Strategy
- exp “Spinal Muscular Atrophies of Childhood”/
- exp Muscular Atrophy, Spinal/
- (werdnig-hoffman or werdnig hoffman).tw.
- (kugelberg-welander or kugelberg welander).tw.
- Spinal muscular atroph*.tw.
- or/1–5
- randomized controlled trial.pt. or randomized.mp. or placebo.mp.
- (“clinical trial” or “clinical trial, phase i” or “clinical trial, phase ii” or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or “multicenter study” or “randomized controlled trial”).pt. or double-blind method/ or clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ or controlled clinical trials as topic/ or randomized controlled trials as topic/ or early termination of clinical trials as topic/ or multicenter studies as topic/ or ((randomi?ed adj7 trial*) or (controlled adj3 trial*) or (clinical adj2 trial*) or ((single or doubl* or tripl* or treb*) and (blind* or mask*))).ti,ab,kw. or (“4 arm” or “four arm”).ti,ab,kw.
- 7 or 8
- 6 and 9
- Epidemiologic studies/
- exp case control studies/
- exp cohort studies/
- Case control.tw.
- (cohort adj (study or studies)).tw.
- Cohort analy$.tw.
- (Follow up adj (study or studies)).tw.
- (observational adj (study or studies)).tw.
- Longitudinal.tw.
- Retrospective.tw.
- Cross sectional.tw.
- Cross-sectional studies/
- or/11–22
- 6 and 23
- meta analysis.mp,pt. or review.pt. or search:.tw.
- 6 and 25
- 10 or 24 or 26
References
- Wirth, B. Spinal Muscular Atrophy: In the Challenge Lies a Solution. Trends Neurosci. 2021, 44, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Swoboda, K.J.; Scott, H.D.; Hejmanowski, A.Q. Homozygous SMN1 Deletions in Unaffected Family Members and Modification of the Phenotype by SMN2. Am. J. Med. Genet. Part A 2004, 130, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Risdiplam: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Onasemnogene Abeparvovec: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, S.; Wevers, R.A.; Wijburg, F.A.; Leeflang, M.M.G. A Framework for Evaluating Long-Term Impact of Newborn Screening. Eur. J. Hum. Genet. 2024, 32, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Two Copies of SMN2 at Risk for Spinal Muscular Atrophy Type 1: The Phase III SPR1NT Trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef]
- UK National Screening Committee (UK NSC) (Costello Medical). Screening for Spinal Muscular Atrophy: External Review against Programme Appraisal Criteria for the UK National Screening Committee (UK NSC); UK National Screening Committee: London, UK, 2018. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 July 2024).
- Crawford, T.O.; Swoboda, K.J.; De Vivo, D.C.; Bertini, E.; Hwu, W.L.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Nazario, A.N.; Parsons, J.A.; et al. Continued Benefit of Nusinersen Initiated in the Presymptomatic Stage of Spinal Muscular Atrophy: 5-Year Update of the NURTURE Study. Muscle Nerve 2023, 68, 157–170. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen Initiated in Infants during the Presymptomatic Stage of Spinal Muscular Atrophy: Interim Efficacy and Safety Results from the Phase 2 NURTURE Study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Kirschner, J.; Crawford, T.; Ryan, M.; Finkel, R.; Swoboda, K.; De Vivo, D.; Bertini, E.; Hwu, W.; Sansone, V.; Pechmann, A.; et al. Impact of Nusinersen on Caregiver Experience and HRQoL in Presymptomatic SMA: NURTURE Study Results. J. Neuromuscul. Dis. 2022, 9, S113–S114. [Google Scholar] [CrossRef]
- Finkel, R.; Farrar, M.; Vlodavets, D.; Zanoteli, E.; Al-Muhaizea, M.; Nelson, L.; Prufer, A.; Servais, L.; Wang, Y.; Fisher, C.; et al. FP.24 RAINBOWFISH: Preliminary Efficacy and Safety Data in Risdiplam-Treated Infants with Presymptomatic Spinal Muscular Atrophy (SMA). Neuromuscul. Disord. 2022, 32, S85–S86. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of Risdiplam in Infants with Genetically Diagnosed and Presymptomatic Spinal Muscular Atrophy (RAINBOWFISH). Available online: https://classic.clinicaltrials.gov/ct2/show/study/NCT03779334 (accessed on 15 May 2024).
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Three Copies of SMN2 at Risk for Spinal Muscular Atrophy: The Phase III SPR1NT Trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef]
- Shell, R.D.; McGrattan, K.E.; Hurst-Davis, R.; Young, S.D.; Baranello, G.; Lavrov, A.; O’Brien, E.; Wallach, S.; LaMarca, N.; Reyna, S.P.; et al. Onasemnogene Abeparvovec Preserves Bulbar Function in Infants with Presymptomatic Spinal Muscular Atrophy: A Post-Hoc Analysis of the SPR1NT Trial. Neuromuscul. Disord. 2023, 33, 670–676. [Google Scholar] [CrossRef]
- Chaplin, M.; Bresnahan, R.; Fleeman, N.; Mahon, J.; Houten, R.; Beale, S.; Boland, A.; Dundar, Y.; Marsden, A.; Munot, P. Onasemnogene Abeparvovec for Treating Pre-Symptomatic Spinal Muscular Atrophy: An External Assessment Group Perspective of the Partial Review of NICE Highly Specialised Technology Evaluation 15. PharmacoEcon. Open 2023, 7, 863–875. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.; D’Silva, A.M.; Sampaio, H.; Briggs, N.; Herbert, K.; Wiley, V.; Farrar, M.A. Newborn Screening for Spinal Muscular Atrophy in Australia: A Non-Randomised Cohort Study. Lancet Child Adolesc. Health 2023, 7, 159–170. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.T.; Russell, J.S.; Wiley, V.; Alexander, I.E.; Farrar, M.A. The Implementation of Newborn Screening for Spinal Muscular Atrophy: The Australian Experience. Genet. Med. 2020, 22, 557–565. [Google Scholar] [CrossRef]
- Ngawa, M.; Dal Farra, F.; Marinescu, A.D.; Servais, L. Longitudinal Developmental Profile of Newborns and Toddlers Treated for Spinal Muscular Atrophy. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231154336. [Google Scholar] [CrossRef]
- Stettner, G.M.; Hasselmann, O.; Tscherter, A.; Galiart, E.; Jacquier, D.; Klein, A. Treatment of Spinal Muscular Atrophy with Onasemnogene Abeparvovec in Switzerland: A Prospective Observational Case Series Study. BMC Neurol. 2023, 23, 88. [Google Scholar] [CrossRef]
- Schwartz, O.; Vill, K.; Pfaffenlehner, M.; Behrens, M.; Weiß, C.; Johannsen, J.; Friese, J.; Hahn, A.; Ziegler, A.; Illsinger, S.; et al. Clinical Effectiveness of Newborn Screening for Spinal Muscular Atrophy: A Nonrandomized Controlled Trial. JAMA Pediatr. 2024, 178, 540–547. [Google Scholar] [CrossRef]
- Weiß, C.; Ziegler, A.; Becker, L.-L.; Johannsen, J.; Brennenstuhl, H.; Schreiber, G.; Flotats-Bastardas, M.; Stoltenburg, C.; Hartmann, H.; Illsinger, S.; et al. Gene Replacement Therapy with Onasemnogene Abeparvovec in Children with Spinal Muscular Atrophy Aged 24 Months or Younger and Bodyweight up to 15 Kg: An Observational Cohort Study. Lancet Child Adolesc. Health 2022, 6, 17–27. [Google Scholar] [CrossRef]
- Servais, L.; Day, J.W.; De Vivo, D.C.; Kirschner, J.; Mercuri, E.; Muntoni, F.; Proud, C.M.; Shieh, P.B.; Tizzano, E.F.; Quijano-Roy, S.; et al. Real-World Outcomes in Patients with Spinal Muscular Atrophy Treated with Onasemnogene Abeparvovec Monotherapy: Findings from the RESTORE Registry. J. Neuromuscul. Dis. 2024, 11, 425–442. [Google Scholar] [CrossRef]
- Elkins, K.; Wittenauer, A.; Hagar, A.F.; Logan, R.; Sekul, E.; Xiang, Y.; Verma, S.; Wilcox, W.R. Georgia State Spinal Muscular Atrophy Newborn Screening Experience: Screening Assay Performance and Early Clinical Outcomes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Deng, S.; Chiriboga, C.A.; Kay, D.M.; Irumudomon, O.; Laureta, E.; Delfiner, L.; Treidler, S.O.; Anziska, Y.; Sakonju, A.; et al. Newborn Screening for Spinal Muscular Atrophy in New York State: Clinical Outcomes from the First 3 Years. Neurology 2022, 99, e1527–e1537. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Caberg, J.H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three Years Pilot of Spinal Muscular Atrophy Newborn Screening Turned into Official Program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef] [PubMed]
- Vill, K.; Schwartz, O.; Blaschek, A.; Glaser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Roschinger, W.; Becker, M.; Czibere, L.; et al. Newborn Screening for Spinal Muscular Atrophy in Germany: Clinical Results after 2 Years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, A.; Kolbel, H.; Schwartz, O.; Kohler, C.; Glaser, D.; Eggermann, K.; Hannibal, I.; Schara-Schmidt, U.; Muller-Felber, W.; Vill, K. Newborn Screening for SMA—Can a Wait-and-See Strategy Be Responsibly Justified in Patients With Four SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kolbel, H.; Kopka, M.; Modler, L.; Blaschek, A.; Schara-Schmidt, U.; Vill, K.; Schwartz, O.; Muller-Felber, W. Impaired Neurodevelopment in Children with 5q-SMA—2 Years after Newborn Screening. J. Neuromuscul. Dis. 2024, 11, 143–151. [Google Scholar] [CrossRef]
- Schwartz, O.; Kolbel, H.; Blaschek, A.; Glaser, D.; Burggraf, S.; Roschinger, W.; Schara, U.; Muller-Felber, W.; Vill, K. Spinal Muscular Atrophy—Is Newborn Screening Too Late for Children with Two SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 389–396. [Google Scholar] [CrossRef]
- Wallace, S.; Orstavik, K.; Rowe, A.; Strand, J. National Newborn Screening for SMA in Norway. Neuromuscul. Disord. 2023, 33, S90. [Google Scholar] [CrossRef]
- Hale, J.E.; Darras, B.T.; Swoboda, K.J.; Estrella, E.; Chen, J.Y.H.; Abbott, M.A.; Hay, B.N.; Kumar, B.; Counihan, A.M.; Gerstel-Thompson, J.; et al. Massachusetts’ Findings from Statewide Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2021, 7, 26. [Google Scholar] [CrossRef]
- Matteson, J.; Wu, C.H.; Mathur, D.; Tang, H.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Sharma, S.C.; Neogi, P.; Fitzgibbon, I.; et al. California’s Experience with SMA Newborn Screening: A Successful Path to Early Intervention. J. Neuromuscul. Dis. 2022, 9, 777–785. [Google Scholar] [CrossRef]
- Kucera, K.S.; Taylor, J.L.; Robles, V.R.; Clinard, K.; Migliore, B.; Boyea, B.L.; Okoniewski, K.C.; Duparc, M.; Rehder, C.W.; Shone, S.M.; et al. A Voluntary Statewide Newborn Screening Pilot for Spinal Muscular Atrophy: Results from Early Check. Int. J. Neonatal Screen. 2021, 7, 20. [Google Scholar] [CrossRef]
- Noguchi, Y.; Bo, R.; Nishio, H.; Matsumoto, H.; Matsui, K.; Yano, Y.; Sugawara, M.; Ueda, G.; Wijaya, Y.O.S.; Niba, E.T.E.; et al. PCR-Based Screening of Spinal Muscular Atrophy for Newborn Infants in Hyogo Prefecture, Japan. Genes 2022, 13, 2110. [Google Scholar] [CrossRef]
- Weng, W.C.; Hsu, Y.K.; Chang, F.M.; Lin, C.Y.; Hwu, W.L.; Lee, W.T.; Lee, N.C.; Chien, Y.H. CMAP Changes upon Symptom Onset and during Treatment in Spinal Muscular Atrophy Patients: Lessons Learned from Newborn Screening. Genet. Med. 2021, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Kido, J.; Sugawara, K.; Yoshida, S.; Ozasa, S.; Nomura, K.; Okada, K.; Fujiyama, N.; Nakamura, K. Newborn Screening for Spinal Muscular Atrophy in Japan: One Year of Experience. Mol. Genet. Metab. Rep. 2022, 32, 100908. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.; Benguerba, K.; Gehani, M.; Raju, D.; Faulkner, E.; LaMarca, N.; Servais, L. P68 Outcomes in Patients with Spinal Muscular Atrophy (SMA) and Four or More SMN2 Copies Treated with Onasemnogene Abeparvovec: Findings from RESTORE. Neuromuscul. Disord. 2023, 33, S133. [Google Scholar] [CrossRef]
- Dangouloff, T.; Hiligsmann, M.; Deconinck, N.; D’Amico, A.; Seferian, A.M.; Boemer, F.; Servais, L. Financial Cost and Quality of Life of Patients with Spinal Muscular Atrophy Identified by Symptoms or Newborn Screening. Dev. Med. Child Neurol. 2023, 65, 67–77. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Spinraza (Nusinersen): Summary of Product Characteristics (SmPC); EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- US Food and Drug Administration (FDA). Spinraza (Nusinersen) Product Label; FDA: Silver Spring, MD, USA, 2016. [Google Scholar]
- European Medicines Agency (EMA). Evrysdi (Risdiplam) Summary of Product Characteristics (SmPC); EMA: Amsterdam, The Netherlands, 2021. [Google Scholar]
- US Food and Drug Administration (FDA). Evrysdi (Risdiplam) Product Label; FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- European Medicines Agency (EMA). Zolgensma (Onasemnogene Abeparvovec) Summary of Product Characteristics (SmPC); EMA: Amsterdam, The Netherlands, 2020. [Google Scholar]
- US Food and Drug Administration (FDA). Zolgensma (Onasemnogene Abeparvovec) Product Label; FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Aragon-Gawinska, K.; Mouraux, C.; Dangouloff, T.; Servais, L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes 2023, 14, 1377. [Google Scholar] [CrossRef]
- Albrechtsen, S.S.; Born, A.P.; Boesen, M.S. Nusinersen Treatment of Spinal Muscular Atrophy—A Systematic Review. Dan. Med. J. 2020, 67, A02200100. [Google Scholar]
- Chiriboga, C.A. Pharmacotherapy for Spinal Muscular Atrophy in Babies and Children: A Review of Approved and Experimental Therapies. Paediatr. Drugs 2022, 24, 585–602. [Google Scholar] [CrossRef]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment of Patients with Spinal Muscular Atrophy: Current Perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef]
- Institute for Quality and Efficiency in Health Care (IQWiG). Newborn Screening for 5q-Linked Spinal Muscular Atrophy IQWiG Reports; Commission No. S18-02; Institute for Quality and Efficiency in Health Care (IQWiG): Berlin, Germany, 2020. [Google Scholar]
- Jedrzejowska, M. Advances in Newborn Screening and Presymptomatic Diagnosis of Spinal Muscular Atrophy. Degener. Neurol. Neuromuscul. Dis. 2020, 10, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Markati, T.; Fisher, G.; Ramdas, S.; Servais, L. Risdiplam: An Investigational Survival Motor Neuron 2 (SMN2) Splicing Modifier for Spinal Muscular Atrophy (SMA). Expert Opin. Investig. Drugs 2022, 31, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Awano, H.; Tanaka, S.; Toro, W.; Zhang, S.; Dabbous, O.; Igarashi, A. Systematic Literature Review of Clinical and Economic Evidence for Spinal Muscular Atrophy. Adv. Ther. 2022, 39, 1915–1958. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ruan, Y.; Chen, Y. Safety and Efficacy of Gene Therapy with Onasemnogene Abeparvovec in the Treatment of Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis. J. Paediatr. Child Health 2023, 59, 431–438. [Google Scholar] [CrossRef]
- Harada, Y.; Rao, V.K.; Arya, K.; Kuntz, N.L.; DiDonato, C.J.; Napchan-Pomerantz, G.; Agarwal, A.; Stefans, V.; Katsuno, M.; Veerapandiyan, A. Combination Molecular Therapies for Type 1 Spinal Muscular Atrophy. Muscle Nerve 2020, 62, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.M.; Mercuri, E.; Finkel, R.S.; Kirschner, J.; De Vivo, D.C.; Muntoni, F.; Saito, K.; Tizzano, E.F.; Desguerre, I.; Quijano-Roy, S.; et al. Combination Disease-modifying Treatment in Spinal Muscular Atrophy: A Proposed Classification. Ann. Clin. Transl. Neurol. 2023, 10, 2155–2160. [Google Scholar] [CrossRef]
| Nusinersen | Risdiplam | Onasemnogene Abeparvovec | |
|---|---|---|---|
| Brand name | Spinraza | Evrysdi | Zolgensma |
| Manufacturer | Biogen Idec | Roche | Novartis Gene Therapies |
| Mechanism of action | Antisense oligonucleotide designed to modify the product of the SMN2 gene to produce more functional SMN protein | Small molecule SMN2 splicing modifier which targets the SMN2 gene to produce more SMN protein | Gene therapy product within a recombinant viral vector, which expresses human SMN protein |
| Mode of delivery | Multiple intrathecal injections (four loading doses followed by maintenance dose every 4 months) | Oral administration on a daily basis | Administered once as a single-dose intravenous infusion |
| Marketing authorisation (EMA) | Treatment of 5q SMA (2017; updated 2022) | Treatment of 5q SMA in patients with a clinical diagnosis of SMA type 1, type 2, or type 3, or with one to four SMN2 copies (2021) | Treatment of 5q SMA with a bi-allelic mutation in the SMN1 gene and either a clinical diagnosis of SMA type 1, or up to 3 copies of the SMN2 gene (2020; updated 2022) |
| Marketing authorisation (FDA) | Treatment of SMA in paediatric and adult patients (2016) | Treatment of SMA in patients 2 months of age and older (2020) | Treatment of paediatric patients less than 2 years of age with SMA with bi-allelic mutations in the SMN1 gene (2019; updated 2023) |
| NICE recommendation (symptomatic SMA) | Recommended in 2019 for treatment of 5q SMA types 1, 2, or 3 subject to a managed access agreement and further data collection (TA588). Guidance under review (expected Dec 2024) | Recommended in 2021 for treatment of 5q SMA types 1, 2, or 3, subject to a managed access agreement and further data collection (TA755). Guidance under review (expected Dec 2024) | Recommended in 2021 for treatment of 5q SMA type 1 with a bi-allelic SMN1 mutation in babies aged 6 months or younger (or 7 to 12 months if agreed by national multidisciplinary team), if not requiring tracheostomy or permanent ventilation for >16 h/day, and subject to a commercial arrangement (HST15) |
| SMC recommendation (symptomatic SMA) | Recommended in 2018 for treatment of symptomatic type 1 5q SMA, and in 2019 for treatment of types 2 and 3 SMA (for up to 3 years while further evidence generated) | Recommended in 2022 for treatment of 5q SMA in patients aged 2 months and older with a clinical diagnosis of SMA types 1, 2, or 3 | Recommended in 2021 for treatment of 5q SMA with a bi-allelic mutation in the SMN1 gene and a clinical diagnosis of SMA type 1 |
| NICE recommendation (presymptomatic SMA) | Recommended in 2019 for treatment of presymptomatic 5q SMA, subject to a managed access agreement and further data collection (TA588). Guidance under review (expected Dec 2024) | Recommended in 2021 for treatment of presymptomatic 5q SMA and 1 to 4 SMN2 copies, subject to a managed access agreement and further data collection (TA755). Guidance under review (expected Dec 2024) | Recommended in 2023 for treatment of presymptomatic 5q SMA with a bi-allelic SMN1 mutation and up to 3 copies of the SMN2 gene in babies aged 12 months and under, subject to a commercial arrangement (HST24) |
| SMC recommendation (presymptomatic SMA) | No specific recommendations for presymptomatic SMA or patients with specific SMN2 copy numbers | Recommended in 2022 for treatment of 5q SMA in patients aged 2 months and older with 1 to 4 SMN2 copies | Recommended in 2021 for treatment of presymptomatic 5q SMA with a bi-allelic mutation in the SMN1 gene and up to 3 copies of the SMN2 gene (expected to develop SMA type 1) |
| Study | Study Type Setting | Population | Interventions Follow-Up | Survival | Sitting Independently a | Crawling a | Standing with Assistance a | Standing Independently a | Walking with Assistance a | Walking Independently a |
|---|---|---|---|---|---|---|---|---|---|---|
| Two SMN2 copies | ||||||||||
| NURTURE Crawford 2023 [10] De Vivo 2019 [11] | - Ph2, OL, MC, single-arm trial - 15 sites in 7 countries | Two SMN2 copies (n = 15) - Babies (≤6 weeks) with presympt SMA - NR how diagnosed | - Nusinersen (intrathecally every 4 mo) - Median age at first dose: 19 days (range 8–41) - Median FU 4.9 yr (data Feb 2021) | - 15/15 (100%) alive at FU | - 15/15 (100%) sat independently - 11/15 (73%) within normal window | - 14/15 (93%) crawled - 6/15 (40%) within normal window | - 15/15 (100%) stood with assistance - 9/15 (60%) within normal window | - 13/15 (87%) stood independently - 4/15 (27%) within normal window | - 14/15 (93%) walked with assistance - 6/15 (40%) within normal window | - 13/15 (87%) walked independently - 6/15 (40%) within normal window |
| SPR1NT Strauss 2022 [7] Chaplin 2023 [17] | - Ph3, OL, MC, single-arm trial - 16 sites in 6 countries | Two SMN2 copies (n = 14) - Babies (≤6 weeks) with presympt SMA - 5 via prenatal screening, 9 via newborn screening | - Onasemnogene abeparvovec via one-off infusion - Median age at infusion: 21 days (range 8–34) - FU to age 18 mo Compared with: - Untreated matched PNCR cohort (n = 23) - Symptomatic SMA type 1 studies b | - 14/14 (100%) alive at 14 mo | - 14/14 (100%) sat independently by 18 mo - 11/14 (79%) within normal window Untreated: - 0/23 (0%) (p < 0.0001) Symptomatic b - START 9/12 (75%) - STR1VE-US 14/22 (64%) - STR1VE-EU 14/32 (44%) c | - 9/14 (64%) crawled - 4/14 (29%) within normal window Untreated: NR | - 14/14 (100%) stood with assistance - 6/14 (43%) within normal window Untreated: NR | - 11/14 (79%) stood independently by 18 mo - 7/14 (50%) within normal window Untreated: - 0/23 (0%) Symptomatic b - START 2/12 (17%) - STR1VE-US 1/22 (5%) - STR1VE-EU 1/33 (3%) c | - 11/14 (79%) walked with assistance - 6/14 (43%) within normal window Untreated: NR | - 9/14 (64%) walked independently by 18 mo - 5/14 (36%) within normal window Untreated: - 0/23 (0%) Symptomatic SMA b - START 2/12 (17%) - STR1VE-US 1/22 (5%) - STR1VE-EU 1/33 (3%) c |
| RAINBOW-FISH Finkel 2022 [13,14] (abst) | - OL, MC, single-arm trial - 7 sites in 7 countries | Two SMN2 copies (n = 4) - Babies (≤6 weeks) with presympt SMA - NR how diagnosed | - Risdiplam orally once daily - Median age at first dose: NR - Analysed pts with ≥12 mo FU (data July 2021) | - 4/4 (100%) alive at FU | - 4/4 sat independently; 2/4 within normal window | - 2/4 crawled - 2/4 within normal window - Remaining 2/4 still within window (may achieve milestone) | NR | - 2/4 stood independently - 1/4 within normal window - Remaining 2/4 still within window (may achieve milestone) | NR | - 1/4 walked independently - 1/4 within normal window - Remaining 2/4 still within window (may achieve milestone) |
| Three SMN2 copies | ||||||||||
| NURTURE Crawford 2023 [10] De Vivo 2019 [11] | - Ph2, OL, MC, single-arm trial - 15 sites in 7 countries | Three SMN2 copies (n = 10) - Babies (≤6 weeks) with presympt SMA - NR how diagnosed | - Nusinersen (intrathecally every 4 mo) - Median age at first dose: 23 days (range 3–42) - Median FU 4.9 yr (data Feb 2021) | - 10/10 (100%) alive at FU | - 10/10 (100%) sat independently - 10/10 (100%) within normal window | - 10/10 (100%) crawled - 10/10 (100%) within normal window | - 10/10 (100%) stood with assistance - 10/10 (100%) within normal window | - 10/10 (100%) stood independently - 10/10 (100%) within normal window | - 10/10 (100%) walked with assistance - 9/10 (90%) within normal window | - 10/10 (100%) walked independently - 10/10 (100%) within normal window |
| SPR1NT Strauss 2022 [15] | - Ph3, OL, MC, single-arm trial - 16 sites in 6 countries | Three SMN2 copies (n = 15) - Babies (≤6 weeks) with presympt SMA - 13 via newborn screening; 1 via prenatal screening; 1 NR | - Onasemnogene abeparvovec via one-off infusion - Median age at infusion: 32 days (range 9–43) - FU to age 24 mo Compared with: - Untreated matched PNCR cohort (n = 81) | - 15/15 (100%) alive at 14 mo | - 14/15 (93%) sat independently by 24 mo - 11/15 (73%) within normal window Untreated cohort: NR | - 14/15 (93%) crawled - 13/15 (87%) within normal window Untreated: NR | - 14/15 (93%) stood with assistance - 11/15 (73%) within normal window Untreated: NR | - 15/15 (100%) stood independently by 24 mo - 14/15 (93%) within normal window Untreated: - 19/81 (24%) (p < 0.0001) | - 14/15 (93%) walked with assistance - 13/15 (87%) within normal window Untreated: NR | - 14/15 (93%) walked independently by 24 mo (1 additional not captured on video) - 11/15 (73%) within normal window Untreated: - 17/81 (21%) (p < 0.0001) |
| RAINBOW-FISH Finkel 2022 [13,14] (abst) | - OL, MC, single-arm trial - 7 sites in 7 countries | Three+ SMN2 copies (n = 3) - Babies (≤6 weeks) with presympt SMA - NR how diagnosed | - Risdiplam orally once daily - Median age at first dose: NR - Analysed pts with ≥12 mo FU (data July 2021) | - 3/3 (100%) alive at FU | - 3/3 sat independently - 1/3 within normal window | - 3/3 crawled - 3/3 within normal window | NR | - 3/3 stood independently - 3/3 within normal window | NR | - 3/3 walked independently - 3/3 within normal window |
| Study | Population | Interventions, FU | Respiratory Outcomes | Feeding Outcomes | Other Motor and Neurological Outcomes |
|---|---|---|---|---|---|
| Two SMN2 copies | |||||
| NURTURE Crawford 2023 [10]; De Vivo 2019 [11]; Kirschner 2022 [12] (abst) | Two SMN2 copies (n = 15) - Babies with presympt SMA | - Nusinersen - Median FU 4.9 yr | - None required tracheostomy or permanent ventilation - 4/15 (27%) had respiratory support (≥6 h/day for ≥7 consecutive days), initiated during acute, reversible illnesses | - 5/15 (33%) required gastrostomy tube Reasons for tube placement: dysphagia (n = 3; 1 used as needed); low weight (n = 2) - 15/15 (100%) continued to grow and gain weight | - 12/15 (80%) achieved maximum CHOP INTEND score (score 64) - 10/15 at age 13 mo and 7/15 at age 24 mo had protocol-defined SMA symptoms. The 7 with symptoms all continued to grow, gain weight and achieve motor milestones |
| SPR1NT Strauss 2022 [7] Shell 2023 [16] | Two SMN2 copies (n = 14) - Babies with presympt SMA | - Onasemnogene abeparvovec - FU to age 18 mo Compared with: - Untreated matched PNCR cohort (n = 23) - Symptomatic SMA type 1 studies a | - 14/14 (100%) survived without permanent ventilation at 14 mo as per protocol - No mechanical respiratory support Untreated: - 6/23 (26%) survived without permanent ventilation (p < 0.0001) Symptomatic SMA a - STR1VE-US 20/22 (91%); - STR1VE-EU 31/32 (97%) survived without permanent ventilation b | - None required nutritional support - 13/14 (93%) maintained body weight (≥3rd WHO percentile) through 18 mo - 14/14 (100%) swallowed normally, full oral nutrition, maintained pulmonary stability [Shell] Untreated: NR Symptomatic SMA a - STR1VE-US 14/22 (64%) maintained weight - STR1VE-EU 15/33 (65%) maintained weight b | - CHOP INTEND scores increased rapidly during initial 3 mo after infusion, reached a median of 60 (range: 51–64) by 6 mo, and all (14/14, 100%) reached score of at least 58 by 18 mo |
| RAINBOWFISH Finkel 2022 [13] (abst) | Two SMN2 copies (n = 4) - Babies with presympt SMA | - Risdiplam - Analysed pts with ≥12 mo FU | - 4/4 (100%) were alive without permanent ventilation at FU | - 4/4 (100%) maintained swallowing and feeding abilities and had not required hospitalisation | - Most achieved near-maximum CHOP INTEND score |
| Three SMN2 copies | |||||
| NURTURE Crawford 2023 [10]; De Vivo 2019 [11] | Three SMN2 copies (n = 10) - Babies with presympt SMA | - Nusinersen - Median FU 4.9 yr | - None required tracheostomy or permanent ventilation - None used respiratory support (≥6 h/day for ≥7 consecutive days) | - 0/10 (0%) required gastrostomy tube - 10/10 (100%) continued to grow and gain weight | - 10/10 (100%) achieved maximum CHOP INTEND score (score 64) - Mean CHOP INTEND scores increased steadily from baseline; stabilized around maximum score - Mean CHOP INTEND scores were higher in NURTURE than in ENDEAR (symptomatic SMA) - 2/10 at age 13 mo and 0/10 at age 24 mo had protocol-defined SMA symptoms |
| SPR1NT Strauss 2022 [15] Shell 2023 [16] | Three SMN2 copies (n = 15) - Babies with presympt SMA | - Onasemnogene abeparvovec - FU to age 24 mo | - 15/15 (100%) survived without permanent ventilation at 14 mo - No mechanical respiratory support Untreated: NR | - None required feeding tube - 10/15 (67%) maintained body weight (≥3rd WHO percentile) without feeding support at all study visits through 24 mo - 15/15 (100%) reached ≥3rd WHO percentile for body weight by study end - 15/15 (100%) swallowed normally, full oral nutrition, maintained pulmonary stability [Shel] Untreated: NR | - [CHOP INTEND not reported] |
| RAINBOWFISH Finkel 2022 [13] (abst) | Three+ SMN2 copies (n = 3) - Babies with presympt SMA | - Risdiplam - Analysed pts with ≥12 mo FU | - 3/3 (100%) were alive without permanent ventilation at FU | - 3/3 (100%) maintained swallowing and feeding abilities and had not required hospitalisation | - 3/3 (100%) achieved maximum CHOP INTEND score |
| Study | SMN2 Copies | Presymptomatic and/or Screened Cohorts | Symptomatic Cohorts | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Interventions | Survival | Sitting | Standing | Walking | Group | Interventions | Survival | Sitting | Standing | Walking | ||
| Kariyawasam 2023 [18]; 2020 [19] Australia (1 centre) FU 24 mo | Two copies | Screened N = 9 (4 presympt, 5 sympt) | - 8 Nus or OA (median 1 mo) - 1 untreated (SMA+ comorbidities) | - 8/8 alive - 0/1 alive | - 8/8 sat - 0/1 sat | - 8/8 stood with asst; 7/8 stood alone - 0/1 stood | - 5/8 walked with asst; 3/8 walked alone - 0/1 walked | Sympt N = 9 | - 7 Nus (median 12 mo) - 2 untreated (SMA + comorbidities) | - 7/7 alive - 0/2 alive | - 6/7 sat - 0/2 sat | - 1/7 stood with asst - 0/2 stood | - 0/7 walked - 0/2 walked |
| Three copies | Screened N = 5 (4 presympt, 1 sympt) | - 5 Nus or OA (median 1 mo) | - 5/5 alive | - 5/5 sat | - 5/5 stood with asst; 5/5 stood alone | - 5/5 walked with asst; 5/5 walked alone | Sympt N = 8 | - 8 Nus (median 12 mo) | - 8/8 alive | - 8/8 sat | - 3/8 stood with asst; 3/8 stood alone | - 1/8 walked with asst; 0/8 walked alone | |
| Four + copies | Screened N = 1 (presympt) | - 1 untreated | - 1/1 alive | - 1/1 sat | - 1/1 stood with asst; 1/1 stood alone | - 1/1 walked with asst; 1/1 walked alone | Sympt N = 1 | - 1 Nu (median 12 mo) | - 1/1 alive | - 1/1 sat | - 1/1 stood with asst; 1/1 stood alone | - 0/1 walked with asst; 0/1 walked alone | |
| Ngawa 2023 [20] Belgium (1 centre) FU 10–61 mo | Two copies | Sympt N = 8 Via scr/sympt Type 1 | - 5 Nus + Ris (1–5 mo) - 3 OA (2–5 mo) | - 5/5 alive - 3/3 alive | - 4/5 sat - 3/3 sat | - 1/5 stood - 1/3 stood | - 0/5 walked - 0/3 walked | ||||||
| Three copies | Presympt N = 5 Via scr/FH | - 1 Nus+Ris (1 mo) - 2 Nus (1–6 mo) - 1 OA (1 mo) - 1 Ris (1 mo) | - 1/1 alive - 2/2 alive - 1/1 alive - 1/1 alive | - 1/1 sat - 2/2 sat - 1/1 sat - 1/1 sat | - 1/1 stood - 2/2 stood - 1/1 stood - 0/1 stood | - 1/1 walked - 1/2 walked - 1/1 walked - 0/1 walked | Sympt N = 3 Via scr/sympt Type 1 | - 2 Nus + Ris (10–12 mo) - 1 Nus (16 mo) | - 2/2 alive - 1/1 alive | - 1/2 sat - 1/1 sat | - 0/2 stood - 0/1 stood | - 0/2 walked - 0/1 walked | |
| Four copies | Presympt N = 2 Via scr/FH | - 2 Ris (1 mo) | - 2/2 alive | - 2/2 sat | - 1/2 stood | - 1/2 walked | |||||||
| Stettner 2023 [21] Switzerland (registry) FU 6–20 mo | Two copies | Sympt N = 6 Type 1 | - 4 OA (2–6 mo) - 2 Nus + OA (1–3 mo + 3–10 mo) | - 6/6 alive | - 3/6 sat | - 0/6 stood | - 0/6 walked | ||||||
| Three copies | Presympt N = 2 Via FH | - 2 OA (1 mo) | - 2/2 alive | - 2/2 sat | - 2/2 stood indep | - 2/2 walked indep | Sympt N = 1 Type 2 | - 1 OA (17 mo) | - 1/1 alive | - 1/1 sat | - 1/1 stood with asst | - 0/1 walked | |
| Schwartz 2024 [22] Germany, Austria, Switzerland (SMArtCARE registry; 70 centres) FU ≥ 18 mo | Two/three copies | Screened N = 44 (33 presympt, 11 sympt) SMN2: - Two: 31 - Three: 13 | - 12 Nus - 7 OA - 21 Nus + OA - 2 Nus + Ris - 2 untreated (mean 1 mo) | NR | - 40/44 (91%) sat | NR | - 28/44 (64%) walked indep, 18 (41%) in normal window; latter all presympt at start | Sympt N = 190 SMN2: One: 1 Two: 110 Three: 79 | - 21 Nus - 66 OA - 4 Ris - 72 Nus + OA - 13 Nus + Ris - 5 untreated (mean 11 mo) | NR | - 141/190 (74%) sat | NR | - 28/190 (15%) walked indep, 11 (6%) in normal window |
| Weiss 2022 [23] Germany, Austria (SMArtCARE; 18 centres) FU 6 mo | Two/three copies | Presympt N = 6 NR how identified | - 6 OA (NR) | - 6/6 alive | NR | NR | NR | Sympt N = 50 Type 1 (N = 45) Type 2 (N = 5) | - 50 OA ± Nus (1–59 mo) | - 50/50 alive | NR | NR | NR |
| Servais 2024 [24] (RESTORE; 7 countries, mainly USA) FU 0–37 mo | 1/2/3/4 copies | Screened N = 32 (presympt or sympt) | - 32 OA (0–72 mo) | NR | NR | - 16/32 stood indep (within window) | - 16/32 walked indep (10/32 within window); 20/32 walked with asst | Sympt N = NR | - OA (0–28 mo) (N = NR) | NR | NR | NR | - None walked indep |
| Study | SMN2 Copies | Presymptomatic and/or Screened Cohorts | Symptomatic Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Interventions | Other Motor Outcomes | Respiratory | Feeding | Group | Interventions | Other Motor Outcomes | Respiratory | Feeding | ||
| Kariyawasam 2023 [18]; 2020 [19] Australia FU 24 mo | Two/ three/ four copies | Screened N = 15 (9 presympt, 6 sympt) | - 13 Nus or OA (median 1 mo) - 2 untreated | NR | - NIV: 1/14 at baseline and 1/14 at 2 yr FU | - Supplemental feeding: 1/14 at baseline and 1/14 at 2 yr FU | Sympt N = 18 | - 16 Nus (median 12 mo) - 2 untreated | NR | - NIV: 3/16 at baseline and 6/16 at 2 yr FU | - Supplemental feeding: 2/16 at baseline and 6/16 at 2 yr FU |
| Stettner 2023 [21] Switzerland (registry) FU 6–20 mo | Two copies | Sympt N = 6 Type 1 | - 4 OA (2–6 mo) - 2 Nus + OA (1–3 mo + 3–10 mo) | - CHOP-INTEND mean increase of 28 | - 1/6 night ventilation | - 3/6 required nasogastric tube or gastrostomy at end of FU | |||||
| Three copies | Presympt N = 2 Via FH | - 2 OA (1 mo) | - 2/2 normal motor development; max CHOP INTEND | - 2/2 no respiratory support | - 2/2 no nutritional support | Sympt N = 1 Type 2 | - 1 OA (17 mo) | - 1/1 reached max CHOP INTEND | - 1/1 no respiratory support | - 1/1 no nutritional support | |
| Schwartz 2024 [22] Germany, Austria, Switzerland (SMArtCARE registry; 70 centres) FU ≥ 18 mo | Two/three copies | Screened N = 44 (33 presympt, 11 sympt) SMN2: - Two: 31 - Three: 13 | - 12 Nus - 7 OA - 21 Nus + OA - 2 Nus + Ris - 2 untreated (mean 1 mo) | Two SMN2: 7/31 (23%) asymptomatic at FU Three SMN2: 10/13 (77%) asymptomatic at FU | - Baseline: 3/44 (7%) occasional ventilation - After treatment start, 2 (5%) stopped ventilation | - Baseline: 1/44 (2%) supplemental feeding - After treatment start, 1/44 (2%) exclusive tube feeding | Sympt N = 190 SMN2: One: 1 Two: 110 Three: 79 | - 21 Nus - 66 OA - 4 Ris - 72 Nus + OA - 13 Nus + Ris - 5 untreated (mean 11 mo) | NR | - Baseline: 11/190 (6%) perm vent, 19/190 (10%) occasional ventilation - After treatment start, 5/190 (2.6%) started perm vent + 6 (3%) stopped, and 32/190 (17%) occasional ventilation | - Baseline: 14/190 (7%) exclusive tube feeding, 9 (5%) supplemental - After treatment start, 29 (15%) started tube feeding (12 exclusive, 17 supplemental) |
| Weiss 2022 [23] Germany, Austria (SMArtCARE; 18 centres) FU 6 mo | Two/three copies | Presympt N = 6 NR how identified | - 6 OA (NR) | - CHOP INTEND increased from Tx to 6 mo FU (p < 0.0001) | NR | NR | Sympt N = 50 Type 1 (N = 45) Type 2 (N = 5) | - 50 OA ± Nus (1–59 mo) | - CHOP INTEND at 6 mo: sig increase in type 1 (p = 0.016) but not sig in type 2 (p = 0.515) | NR | NR |
| Servais 2024 [24] (RESTORE; 7 countries, mainly USA) FU 0–37 mo | 1/2/3/4 copies | Screened N = 20 (presympt or sympt) | - 20 OA (0–72 mo) | - 17/20 CHOP INTEND ≥ 4-point increase - 17/20 had CHOP INTEND ≥ 40 points | NR | NR | Sympt N = 21 | - 21 OA (0–28 mo) | - 20/21 CHOP INTEND ≥4-point increase - 19/21 had CHOP INTEND ≥ 40 points | NR | NR |
| Study Follow-Up | Populations Interventions | Survival | Sitting Independently | Standing | Walking | Other Motor and Neurological Outcomes | Respiratory | Feeding |
|---|---|---|---|---|---|---|---|---|
| One SMN2 copy | ||||||||
| USA (Georgia) Elkins 2022 [25] FU median 5 mo | One SMN2 copy - 2 untreated (sympt) | - Untreated: 2/2 died (at 10d and 22 mo) | NR | NR | NR | NR | ||
| USA (New York State) Lee 2022 [26] FU median 12 mo | One SMN2 copy - 1 Ris at 2 mo (severely sympt) | - Ris: 1/1 alive at FU | NR | NR | NR | - 1 Ris: severe motor symptoms at FU | - Ris: 1/1 ventilator-dependent | - Ris: 1/1 unable to feed orally |
| Two SMN2 copies | ||||||||
| Belgium Boemer 2021 [27] FU 12–33 mo | Two SMN2 copies - 4 Nus (3 at 1 mo, 1 at 5 mo) (sympt) - 1 OA at 2 mo (sympt) | - Nus: 4/4 alive - OA: 1/1 alive | - Nus: 3/4 sat indep - OA: 1/1 sat indep | - Nus: NR - OA: 1/1 stood | - Nus: 1/4 walked with asst; 3/4 not walking - OA: 1/1 not walking | - Nus or OA: 5/5 early sympt at treatment; 5/5 developmental delays despite treatment | NR | NR |
| Germany Vill 2021 [28]; Kolbel 2023 [30]; Schwartz 2022 [31] - FU med 13 mo [Vill]; 10 mo-3.5 yr [Schwartz] | Two SMN2 copies Vill: - 15 Nus at 0.5–1 mo (8 presympt, 7 sympt) - 2 untreated Schwartz: - 11 Nus at ≤6 wk - 1 OA at ≤6 wk - 9 Nus + OA at ≤6 wk | Vill: - Nus: 15/15 alive - Untreated: 2/2 died Schwartz: - Nus/OA: 21/21 alive | Schwartz: - Nus/OA: 15/21 sat indep in window; 4/21 delayed; 2/21 not met | NR | Schwartz: Nus/OA: - 12/20 walked w asst in window; 5/20 delayed; 3/20 not met - 10/19 walked indep in window; 3/19 delayed; 6/19 not met | Vill: - Nus (presympt): 8/8 met milestones - Nus (sympt, n = 7): milestones delayed Schwartz: Nus/OA: - 12/21 met milestones; 3/21 initial delay; 6/21 proximal weakness | Vill: - Nus: 15/15 no respiratory issues - Untreated: 2/2 died (respiratory failure) Schwarz: Nus/OA: - 20/21 no respiratory issues; 1/21 NIV, cough assist | Vill: - Nus: 15/15 no tube feeding - Untreated: NR Schwarz: Nus/OA: - 16/21 no feeding issues; 3/21 mild chewing problems, 2/21 tube feeding |
| Norway Wallace 2023 [32] (abst) FU NR | Two SMN2 copies - 5 OA at 0.5 mo (3 sympt, 2 presympt) | - OA (presympt): 2/2 alive - OA (sympt): 2/3 alive | NR | NR | NR | - OA (presympt): 2/2 motor improvements - OA (sympt): 1/3 died, 2/3 NR | NR | NR |
| USA (California) Matteson 2022 [34] FU to ≥1 yr of age | Two SMN2 copies - 8 Nus and/or OA (at median 1 mo) | NR | NR | NR | NR | - Nus and/or OA: 6/8 had SMA symptoms at FU (incl 3 presympt); 3 had delays or barriers to Tx | NR | NR |
| USA (Georgia) Elkins 2022 [25] FU median 5 mo | Two SMN2 copies - 3 OA at 1–6 mo (2 presympt, 1 sympt) - 2 untreated (sympt) | - OA (presym): 1/1 alive - OA (sympt): 1/1 alive - Untr: 2/2 died | NR | NR | NR | - OA (presympt): 1/1 had symptoms - OA (sympt): 1/1 low CHOP-INTEND - Untreated: 2/2 died | NR | NR |
| USA (Massachusetts) Hale 2021 [33] FU median 13 mo | Two SMN2 copies - 1 Nus at 1 mo (presympt) - 4 OA at 0.4–1 mo (1 presympt, 3 sympt) - 1 Nus at 0.4 mo + OA at 3 mo (sympt) - 1 Nus (0.5 mo) + OA (1 mo) + Ris (10 mo) (sympt) | - Treated: 7/7 alive at follow-up | NR | NR | NR | - 1 Nus (presympt): normal - 1 OA (presympt): mild motor delays - 3 OA (sympt): 2 mild-to-mod delays, 1 improved - 1 Nus + OA: normal at FU - 1 Nus + OA + Ris: normal | NR | NR |
| USA (New York State) Lee 2022 [26] FU median 12 mo | Two SMN2 copies (10 presym, 8 sympt) - 11 OA at 0.4–2 mo - 1 Nus at 3 mo - 4 Nus (1–2 mo) + OA (2–6 mo) - 2 OA (1 mo) + Ris 6 mo) | - Treated: 18/18 alive at FU | NR | NR | NR | - Treated (presympt): 4/10 symptoms at FU but achieved motor milestones; 6/10 no symptoms - Treated (sympt): 8/8 symptoms at FU | - 10 presympt: no respiratory issues - 8 sympt: 5/8 required NIV | - 10 presympt: no feeding issues - 8 sympt: 3/8 required feeding assistance; no gastrostomy tubes |
| USA (North Carolina) Kucera 2021 [35] FU 12 wk | Two SMN2 copies - 1 Nus at 1 mo (sympt) | - Nus: 1/1 alive at 12 wk | NR | NR | NR | - Nus: 1/1 motor symptoms initially worsened but improved by 12 wk | NR | NR |
| Japan (Hyogo) Noguchi 2022 [36] FU 12 + 24 wk | Two SMN2 copies - 1 Nus at 1 mo (presympt) - 1 Nus at 1 mo + OA at 4 mo (sympt) | - 2/2 alive at FU | NR | NR | NR | - Nus (presympt): 1/1 little change at 12 wk - Nus + OA (sympt): 1/1 improvements slowed | NR | - 1 Nus (presympt): NR - 1 Nus + OA (sympt): tube feeding |
| Taiwan Weng 2021 [37] FU median 3 yr | Two SMN2 copies - 3 Nus at 0.4–3 mo (sympt) - 3 untreated (sympt) | - Nus: 3/3 alive at FU - Untreated: 3/3 died | NR | NR | NR | - Nus: 1/3 walked at 4 yr, 1/3 sitter (supported stand) at 3.3 yr, 1/3 sitter (supported walk) at 1.5 yr - Untreated: 3/3 died | - Nus: 2/3 required ventilatory support - Untreated: NR | - Nus: 2/3 required gastrostomy - Untreated: NR |
| Three SMN2 copies | ||||||||
| Belgium Boemer 2021 [27] FU 12–33 mo | Three SMN2 copies - 2 Nus at 1 mo (presympt) - 1 OA at 1 mo (presympt) | - Nus: 2/2 alive - OA: 1/1 alive | - Nus: 2/2 sat indep - OA: 1/1 sat indep | - Nus: 2/2 stood - OA: 1/1 stood | - Nus: 2/2 walked indep - OA: 1/1 walked indep | - Nus or OA: 3/3 hit motor milestones at usual ages | NR | NR |
| Germany Vill 2021 [28] - FU med 13 mo | Three SMN2 copies - 6 Nus at 1 mo (presympt) - 4 untreated | - Nus; 6/6 alive - Untreated: 4/4 alive | NR | NR | NR | - Nus (presympt): 5/6 normal motor milestones; 1/6 minimal delay - Untreated: 3/4 proximal weakness; 1/4 motor deterioration | - Nus: 6/6 no respiratory issues - 4 untreated: NR | NR |
| Norway Wallace 2023 [32] (abst) FU NR | Three SMN2 copies - 3 OA at 0.5 mo (presympt) | - OA: 3/3 alive | NR | NR | NR | - OA: 3/3 had improvements in motor function, all remained asymptomatic | NR | NR |
| USA (California) Matteson 2022 [34] FU to ≥1 yr of age | Three SMN2 copies - 7 Nus and/or OA (at median 1 mo) | NR | NR | NR | NR | - Nus and/or OA: 7/7 no SMA symptoms at FU | NR | |
| USA (Georgia) Elkins 2022 [25] FU median 5 mo | Three SMN2 copies - 6 OA at 1–6 mo (presympt) - 1 Nus at 20 mo (sympt) | - OA: 3 alive (3 no data) - Nus: 1/1 alive | NR | NR | NR | - OA: 1/6 some symptoms at 9 mo; 2/6 normal; 3/6 no data - Nus (at 20 mo): 1/1 symptoms progressed; low CHOP-INTEND at 22 mo | NR | |
| USA (New York State) Lee 2022 [26] FU median 12 mo | Three SMN2 copies - 10 OA at 0.4–3 mo (presympt) - 1 Nus (1 mo) + OA (NR) (presympt) | - OA/Nus: 11/11 alive | NR | NR | NR | - OA/Nus: 11/11 asymptomatic at FU, meeting milestones | - OA/Nus: 11/11 no respiratory issues | - OA/Nus: 11/11 no feeding issues |
| Japan (Kumamoto) Sawada 2022 [38] FU 11 mo | Three SMN2 copies - 1 OA at 1.4 mo (presympt) | - OA: 1/1 alive | NR | NR | NR | - OA: 1/1 normal motor development at 11 mo | NR | NR |
| Taiwan Weng 2021 [37] FU median 3 yr | Three SMN2 copies - 3 Nus at 3–6 mo (sympt) - 1 untreated (sympt) | - Nus: 3/3 alive - Untreated: 1/1 alive | NR | NR | NR | - Nus: 1/3 sitter (supported walk) at 2.4 yr, 1/3 walker at 1.3 yr, 1/3 sittter (supported stand) at 0.9 yr - Untreated: 1/1 sitter at 5.3 yr | - Nus: 3/3 no ventilatory support - Untreated: 1/1 required ventilatory support | - Nus: 3/3 no feeding support - Untreated: 1/1 no feeding support |
| Four+ SMN2 copies | ||||||||
| Belgium Boemer 2021 [27] FU 12–33 mo | Four+ SMN2 copies - 1 Nus at 2 mo (presympt) - 1 Ris at 1 mo (presympt) | - Nus: 1/1 alive - Ris: 1/1 alive | - Nus: 1/1 sat indep - Ris: 1/1 sat indep | - Nus: 1/1 stood - Ris: 1/1 stood | - Nus: 1/1 walked indep - Ris: 1/1 walked indep | - Nus or Ris: 2/2 asymptomatic at treatment; 2/2 hit motor milestones at usual ages | NR | NR |
| Germany Vill 2021 [28]; Blaschek 22 [29] - FU med 13 mo [Vill] - FU 10 mo-3.5 y [Schwartz] | Four+ SMN2 copies - 8 treated at 3–36 mo (presympt) - 7 untreated (presympt) | - Treated: 8/8 alive - Untreated: 7/7 alive | NR | NR | NR | - Treated: 8/8 asymptomatic - Untreated: 5/7 symptomatic (at 1.5 to 4 yr); 2 of 5 no complete recovery despite symptomatic treatment | NR | NR |
| Norway Wallace 2023 [32] (abst) FU NR | Four+ SMN2 copies - 2 OA (timing NR) (presympt) | - OA: 2/2 alive | NR | NR | NR | - OA: 2/2 improvements in motor function | NR | NR |
| USA (California) Matteson 2022 [34] FU to ≥1 yr of age | Four+ SMN2 copies - 1 Nus and/or OA (at median 2 mo) | NR | NR | NR | NR | - Nus/OA: 1/1 had SMA symptoms at FU | NR | NR |
| USA (Georgia) Elkins 2022 [25] FU median 5 mo | Four+ SMN2 copies - 2 untreated (presympt) | - Untreated: 1 alive (1 no data) | NR | NR | NR | - Untreated: 1 normal exam at 1.5 mo (1 no data) | NR | NR |
| USA (Massachusetts) Hale 2021 [33] FU median 13 mo | Four+ SMN2 copies - 1 Nus at 0.3 mo (presympt) - 1 OA at 6 mo (sympt) | - Nus/OA: 2/2 alive at follow-up | NR | NR | NR | - 1 Nus (presympt): no symptoms at FU - 1 OA (sympt): no symptoms at FU | NR | NR |
| USA (New York State) Lee 2022 [26] FU median 12 mo | Four+ SMN2 copies - 2 OA at 6 mo (presympt) - 2 untreated (presympt) | - OA: 2/2 alive - Untreated: 2/2 alive | NR | NR | NR | - OA: 2/2 asymptomatic at FU - Untreated: 2/2 asymptomatic at FU | - OA: 2/2 no respiratory issues - Untreated: 22 no respiratory issues | - OA: 2/2 no feeding issues - Untreated: 22 no feeding issues |
| 7 countries, mainly USA (RESTORE) Finkel 2023 [39] (abst) FU mean 14 mo | Four+ SMN2 copies - 19 OA at 1–11 mo | - OA: 19/19 alive at FU | NR | NR | NR | - OA: Of 12 with data, 12/12 achieved new motor milestones - OA: Of 13 with data, 7/13 CHOP INTEND max (64) | - OA (n = 19): No respiratory support | - OA (n = 19): No nutritional support |
| Taiwan Weng 2021 [37] FU median 3 yr | Four+ SMN2 copies - 4 untreated (3 presympt, 1 sympt) | - Untreated: 4/4 alive at FU | NR | NR | NR | - Untreated (presympt): 3/3 no symptoms at FU - Untreated (sympt): 1/1 walker at 3.4 yr | - Untreated: 4/4 no ventilatory support | - Untreated: 4/4 no feeding support |
| Study Ref(s) | Population: Representative Cohort? a | Intervention: Fidelity to Intended Intervention? | Outcomes: Blinded Outcome Assessment? | Outcomes: Sufficient Follow-Up? (≥1 yr) | Outcomes: At Least 90% Analysed? |
|---|---|---|---|---|---|
| Single-arm interventional studies | |||||
| NURTURE; Crawford 2023 [10,11,12] | U | Y | N | Y | Y (25/25 = 100%) |
| SPR1NT; Strauss 2022 [7,15,16,17] | U | Y | N | Y | Y (29/29 = 100%) |
| RAINBOWFISH; Finkel 2022 [13] | U | Y | N | Y | N (7/18 = 39%) |
| Prospective comparative studies | |||||
| Australia; Kariyawasam 2023 [18,19] | Y | U | N | Y | Y (33/33 = 100%) |
| Belgium; Ngawa 2023 [20] | Y | U | N | Y | Y (18/18 = 100%) |
| Switzerland; Stettner 2023 [21] | Y | U | N | N | Y (9/9 = 100%) |
| Schwartz 2024 [22] | Y | U | N | Y | Y (234/234 = 100%) |
| Germany + Austria; Weiss 2022 [23] | Y | U | N | N | N (56/76 = 74%) |
| 7 countries (RESTORE); Servais 2024 [24] | Y | U | N | Y | N (41/168 = 24%) |
| Screening studies with follow-up | |||||
| Belgium; Boemer 2021 [20,27,40] | Y | U | N | Y | Y (10/10 = 100%) |
| Germany; Vill 2021 [28,29,30,31] | Y | U | N | Y | Y (43/43 = 100%) |
| Norway; Wallace 2023 [32] (abst) | Y | U | N | U | Y (10/10 = 100%) |
| USA (California); Matteson 2022 [34] | Y | U | N | Y | N (16/34 = 47%) |
| USA (Georgia); Elkins 2022 [25] | Y | U | N | N | N (11/16 = 69%) |
| USA (Massachusetts); Hale 2021 [33] | Y | U | N | Y | Y (9/9 = 100%) |
| USA (New York State); Lee 2022 [26] | Y | U | N | Y | Y (34/34 = 100%) |
| USA (North Carolina); Kucera 2021 [35] | Y | U | N | N | Y (1/1 = 100%) |
| 7 countries (RESTORE); Finkel 2023 [39] | Y | U | N | Y | N (12/19 = 63%) |
| Japan (Kumamoto); Sawada 2022 [38] | Y | U | N | N | Y (1/1 = 100%) |
| Japan (Hyogo); Noguchi 2022 [36] | Y | U | N | N | Y (2/2 = 100%) |
| Taiwan; Weng 2021 [37] | Y | U | N | Y | N (14/21 = 67%) |
| Nusinersen: NURTURE Crawford 2023 [10] | Risdiplam: RAINBOWFISH Finkel 2022 [13,14] | Onasemnogene abeparvovec: SPR1NT Strauss 2022 [7]; Strauss 2022 [15] | Onasemnogene abeparvovec: screening RESTORE registry [24]; Swiss study [21] |
| Special warnings and precautions [41,42] - Risk of adverse effects of lumbar puncture - Thrombocytopenia and coagulation abnormalities have been observed with other antisense oligonucleotides - Renal toxicity has been observed with other antisense oligonucleotides - Hydrocephalus after nusinersen has been reported in the post-marketing setting | Special warnings and precautions [43,44] - Embryo–foetal toxicity has been observed in animal studies - Effects on male fertility have been observed in animal studies - Retinal toxicity has been observed in non-clinical safety studies, but not in clinical studies in SMA patients | Special warnings and precautions [45,46] - Hepatotoxicity: cases of acute liver failure with fatal outcomes; acute serious liver injury and elevated aminotransferases; may be immune-mediated; corticosteroid recommended - Systemic immune response: possible risk for patients with underlying infection - Thrombocytopenia: transient decreases in platelet counts frequently observed - Thrombotic microangiopathy: cases observed - Cardiac effects: increases in cardiac troponin-I levels observed in clinical trials, and cardiac toxicity in animal studies - Risk of tumorigenicity (theoretical) due to integration of AAV vector into genome | See left |
| AE summary (NURTURE) [10] - Any AE: 25/25 (100%) - Moderate or severe AE: 19/25 (76%) - Severe AE: 6/25 (24%) - Serious AE: 12/25 (48%) - AE leading to discontinuation of drug/study: 0 - AE, considered study-drug related by investigators: 0 (0%) - AE, considered possibly study-drug related by investigators: 10/25 (40%) - Serious AE, considered study-drug-related by investigators: 0 (0%) - AE related or possibly related to lumbar puncture: 13/25 (52%) - All resolved despite continued treatment, except for proteinuria (n = 1) and clonus (n = 1) | AE summary (RAINBOWFISH) [13] - At least one AE: 14/18 (78%) - AEs considered treatment-related: 2/18 (11%) (1 diarrhoea, 1 skin discoloration) - Grade 3–5 AEs: 2/18 (11%), both Gd3, neither considered treatment-related (1 gastroenteritis norovirus; 1 cystoid macular oedema) - Serious AEs: 0/18 (0%) [Finkel 2022] - Serious AEs: 8/26 (31%) [ClinicalTrials.gov] [14] - Deaths: 0 (0%) - AEs leading to treatment withdrawal: 0 (0%) - AEs leading to dose modification or interruption: 2/18 (11%), neither considered treatment-related (AE type not specified) | AE summary (SPR1NT, two + three copy cohorts) [7,15] - AEs: 14/14 (100%); 15/15 (100%) - Treatment-related AEs: 10/14 (71%); 8/15 (53%) - Serious AEs: 5/14 (36%); 3/15 (20%) - Treatment-related serious AEs: 0 (0%); 0 (0%) - To attenuate the inflammatory response, all patients commenced oral prednisolone 1 day before infusion and completed a median of 60 days [two SMN2 copy cohort] or 63 days [three copy cohort] | AE summary (RESTORE registry; screened + symptomatic cohorts) [24] (screened cohort; symptomatic cohort) - Any AE: 35/97 (36%); 46/70 (66%) - Grade 3+ AE: 11/97 (11%); 29/70 (41%) - Serious AE: 9/97 (9%); 22/70 (31%) - Treatment-related AE: 26/97 (27%); 28/70 (40%) - Serious treatment-related AE: 4/97 (4%); 4/70 (6%) |
| - | Serious AEs (RAINBOWFISH) [14] - Urinary tract infection: 2/26 (8%) - Gastroenteritis: 2/26 (8%) - Constipation: 1/26 (4%) - Femur fracture: 1/26 (4%) - Soft tissue injury: 1/26 (4%) - Jaundice, neonatal: 1/26 (4%) | AEs of special interest (SPR1NT, two + three copy cohorts) [7,15] - Hepatotoxicity: 3/14 (21%); 4/15 (27%) - Thrombocytopenia: 3/14 (21%); 2/15 (13%) - Cardiac AEs: 2/14 (14%); 3/15 (20%) - Thrombotic microangiopathy: 2/14 (14%); 0/15 (0%) - Sensory abnormalities suggestive of dorsal root ganglionopathy: 3/14 (21%); 1/15 (7%) | AEs of special interest (RESTORE registry; screened + symptomatic cohorts) [24] - Hepatotoxicity: 19/97 (20%); 30/70 (43%) - Transient thrombocytopenia: 5/97 (5%); 18/70 (26%) - Cardiac AEs: 8/97 (8%); 14/70 (20%) - Thrombotic microangiopathy: 0/97 (0%); 1/70 (1.4%) |
| AEs, potentially treatment-related (NURTURE) [10] - ALT increased, AST increased, eosinophil count increased, lymphocyte count increased, WBC count increased, pyrexia: 1/25 (4%) - Blood alkaline phosphatase increased, blood calcium increased, protein urine present: 1/25 (4%) - Clonus, extensor plantar response, muscular weakness, weight-bearing difficulty: 1/25 (4%) - Dermatitis, allergic: 1/25 (4%) - Headache: 1/25 (4%) - Hyperreflexia, tachycardia: 1/25 (4%) - Platelet count increased: 1/25 (4%) - Protein urine present: 1/25 (4%) - Proteinuria: 1/25 (4%) - Rash: 1/25 (4%) | - | AEs, potentially treatment-related (SPR1NT, two + three copy cohorts) [7,15] - Gastrointestinal disorders: 5/14 (36%); 3/15 (20%) - Aspartate aminotransferase increased: 3/14 (21%); 4/15 (27%) - Rash or skin disorders: 2/14 (14%); 3/15 (20%) - Alanine aminotransferase increased: 1/14 (7%); 3/15 (20%) - Blood creatinine phosphokinase MB increased: 1/14 (7%); 2/15 (13%) - Troponin increased: 1/14 (7%); 2/15 (13%) - Gamma-glutamyl transferase increased: 1/14 (7%); 1/15 (7%) - Platelet count increased: 1/14 (7%); 1/15 (7%) - Blood creatinine phosphokinase increased: 1/14 (7%); NR - Blood alkaline phosphatase increased: NR; 1/15 (7%) - Platelet count decreased: 1/14 (7%); NR - Thrombocytopenia: 1/14 (7%); NR - Eye discharge: 1/14 (7%); NR - Malaise: 1/14 (7%); NR - Motor development delay: 1/14 (7%); NR - Feeding or weight gain poor: NR; 2/15 (13%) - Agitation: NR; 1/15 (7%) - Cough: NR; 1/15 (7%) - Iron deficiency anaemia: NR; 1/15 (7%) - Cushingoid: NR; 1/15 (7%) - Pyrexia: NR; 1/15 (7%) - Nasopharyngitis: NR; 1/15 (7%) | - |
| Most common AEs (in ≥5 infants) (NURTURE) [10] - Pyrexia: 21/25 (84%) - Upper respiratory tract infection: 18/25 (72%) - Cough: 16/25 (64%) - Nasopharyngitis: 16/25 (64%) - Vomiting: 12/25 (48%) - Rhinorrhea: 11/25 (44%) - Fall: 10/25 (40%) - Muscular weakness: 10/25 (40%) - Diarrhoea: 9/25 (36%) - Influenza: 9/25 (36%) - Nasal congestion: 8/25 (32%) - Otitis media: 8/25 (32%) - Tremor: 8/25 (32%) - Constipation: 7/25 (28%) - Pneumonia: 7/25 (28%) - Seasonal allergy: 7/25 (28%) - Anaemia: 6/25 (24%) - Dehydration: 6/25 (24%) - Gait disturbance: 6/25 (24%) - Gastroenteritis, viral: 6/25 (24%) - Dermatitis, diaper: 5/25 (20%) - Ear infection: 5/25 (20%) - Respiratory tract infection: 5/25 (20%) - Rhinitis: 5/25 (20%) - Speech disorder, developmental: 5/25 (20%) - Tachycardia: 5/25 (20%) | Most common AEs (in ≥3 infants) (RAINBOWFISH) [13] - Teething: 6/18 (33%) - Nasal congestion: 5/18 (28%) - Pyrexia: 5/18 (28%) - Diarrhoea: 4/18 (22%) - Viral infection: 4/18 (22%) - Vomiting: 4/18 (22%) - Constipation: 3/18 (17%) - Cough: 3/18 (17%) - Eczema: 3/18 (17%) | Most common AEs (in ≥2 infants) (SPR1NT, two + three copy cohorts) [7,15] - Pyrexia: 7/14 (50%); 11/15 (73%) - Upper respiratory tract infection: 5/14 (36%); 9/15 (60)% - Aspartate aminotransferase increased: 3/14 (21%); 4/15 (27%) - Diarrhoea: 3/14 (21%); 4/15 (27%) - Teething: 2/14 (14%); 5/15 (33%) - Gastroesophageal reflux disease: 3/14 (21%); 3/15 (20%) - Rash: 3/14 (21%); 2/15 (13%) - Nasal congestion: 3/14 (21%); 2/15 (13%) - Hypotonia: 3/14 (21%); 2/15 (13%) - Vomiting: 3/14 (21%); 2/15 (13%) - Nasopharyngitis: 2/14 (14%); 3/15 (20%) - Constipation: 4/14 (29%); NR - Cough: NR; 4/15 (27%) - Viral upper respiratory tract infection: 3/14 (21%); NR - Tremor: 3/14 (21%); NR - Muscle contractions, involuntary: 3/14 (21%); NR - Dermatitis, diaper: NR; 3/15 (20%) - Alanine aminotransferase increased: NR; 3/15 (20%) - Otitis media: NR; 3/15 (20%) - Ear infection: 2/14 (14%); NR - Areflexia: 2/14 (14%); NR - Eczema: 2/14 (14%); NR - Influenza: 2/14 (14%); NR - Rhinovirus infection: 2/14 (14%); NR - Blood calcium increased: NR; 2/15 (13%) - Blood creatinine phosphate MB increased: NR; 2/15 (13%) - Microcytic anaemia: NR; 2/15 (13%) - Gastroenteritis: NR; 2/15 (13%) - Hand-foot-and-mouth disease: NR; 2/15 (13%) - Troponin increased: NR; 2/15 (13%) - Urinary tract infection: NR; 2/15 (13%) | Swiss cohort [21] (presymptomatic patients with OA and three SMN2 copies; n = 9) - 100% transient decrease of platelet count - 3/9 (33%) thrombocytopenia - 100% transaminase increase - Troponin-T elevated prior to OA in 100% and showed fluctuations in 57% thereafter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 56. https://doi.org/10.3390/ijns10030056
Cooper K, Nalbant G, Sutton A, Harnan S, Thokala P, Chilcott J, McNeill A, Bessey A. Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy. International Journal of Neonatal Screening. 2024; 10(3):56. https://doi.org/10.3390/ijns10030056
Chicago/Turabian StyleCooper, Katy, Gamze Nalbant, Anthea Sutton, Sue Harnan, Praveen Thokala, Jim Chilcott, Alisdair McNeill, and Alice Bessey. 2024. "Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy" International Journal of Neonatal Screening 10, no. 3: 56. https://doi.org/10.3390/ijns10030056
APA StyleCooper, K., Nalbant, G., Sutton, A., Harnan, S., Thokala, P., Chilcott, J., McNeill, A., & Bessey, A. (2024). Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy. International Journal of Neonatal Screening, 10(3), 56. https://doi.org/10.3390/ijns10030056








