Laboratory Findings in COVID-19—Alterations of Hematological, Immunological, Biochemical, Hormonal and Other Lab Panels: A Narrative Review
Abstract
:Introduction
Discussion
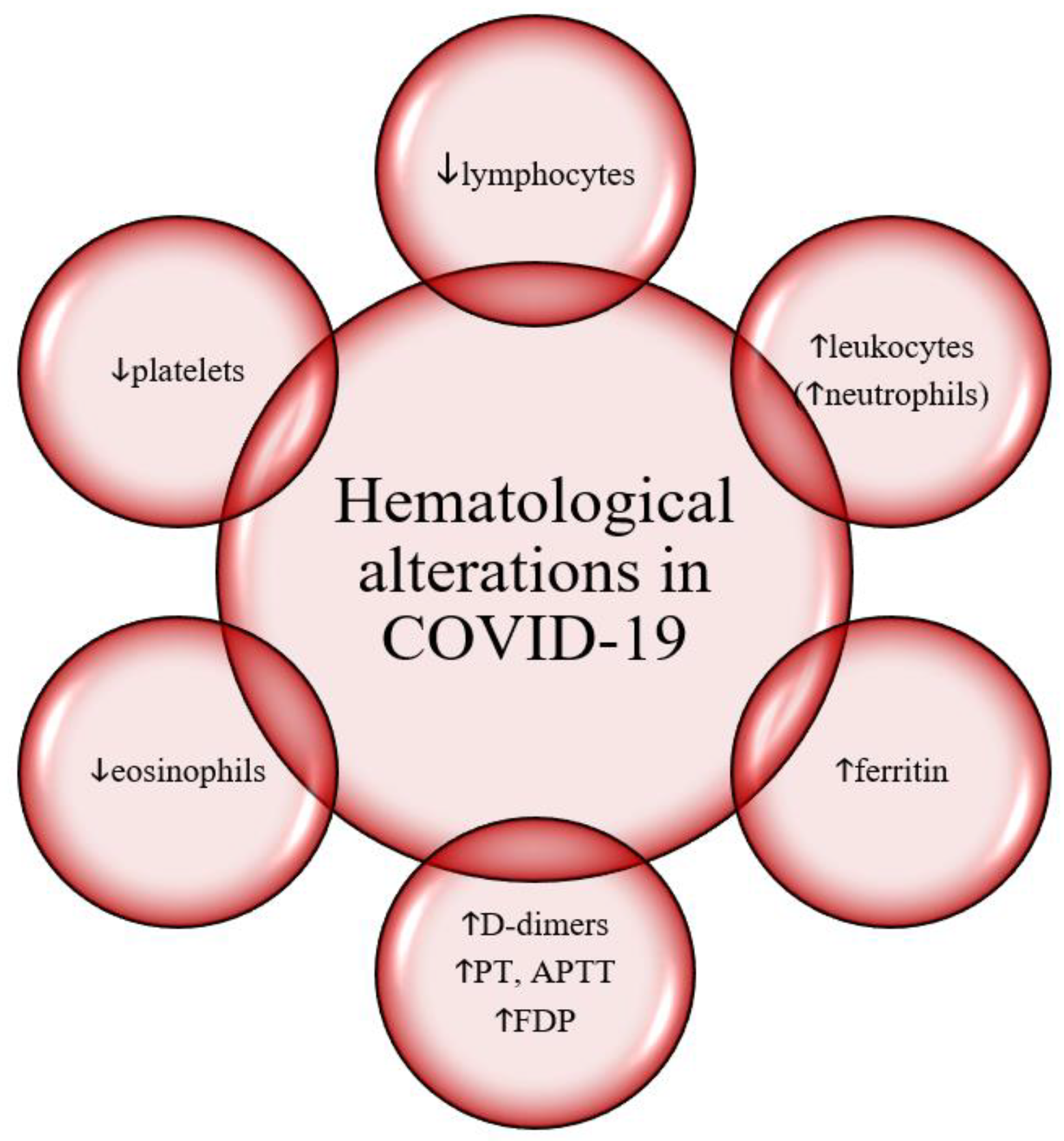
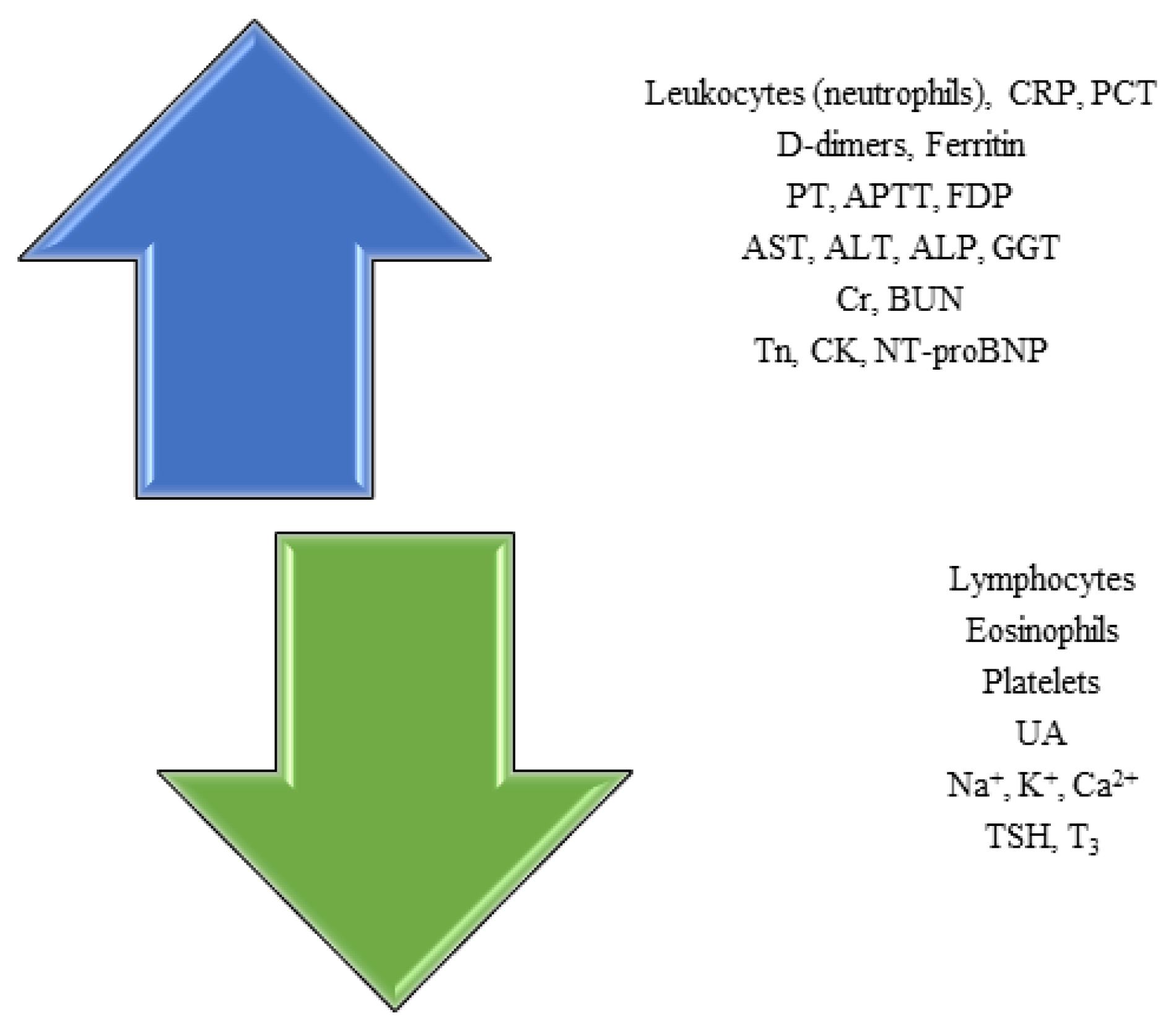
Hematological findings and inflammation markers in COVID-19
Immunological findings in COVID-19
Hormonal changes in COVID-19
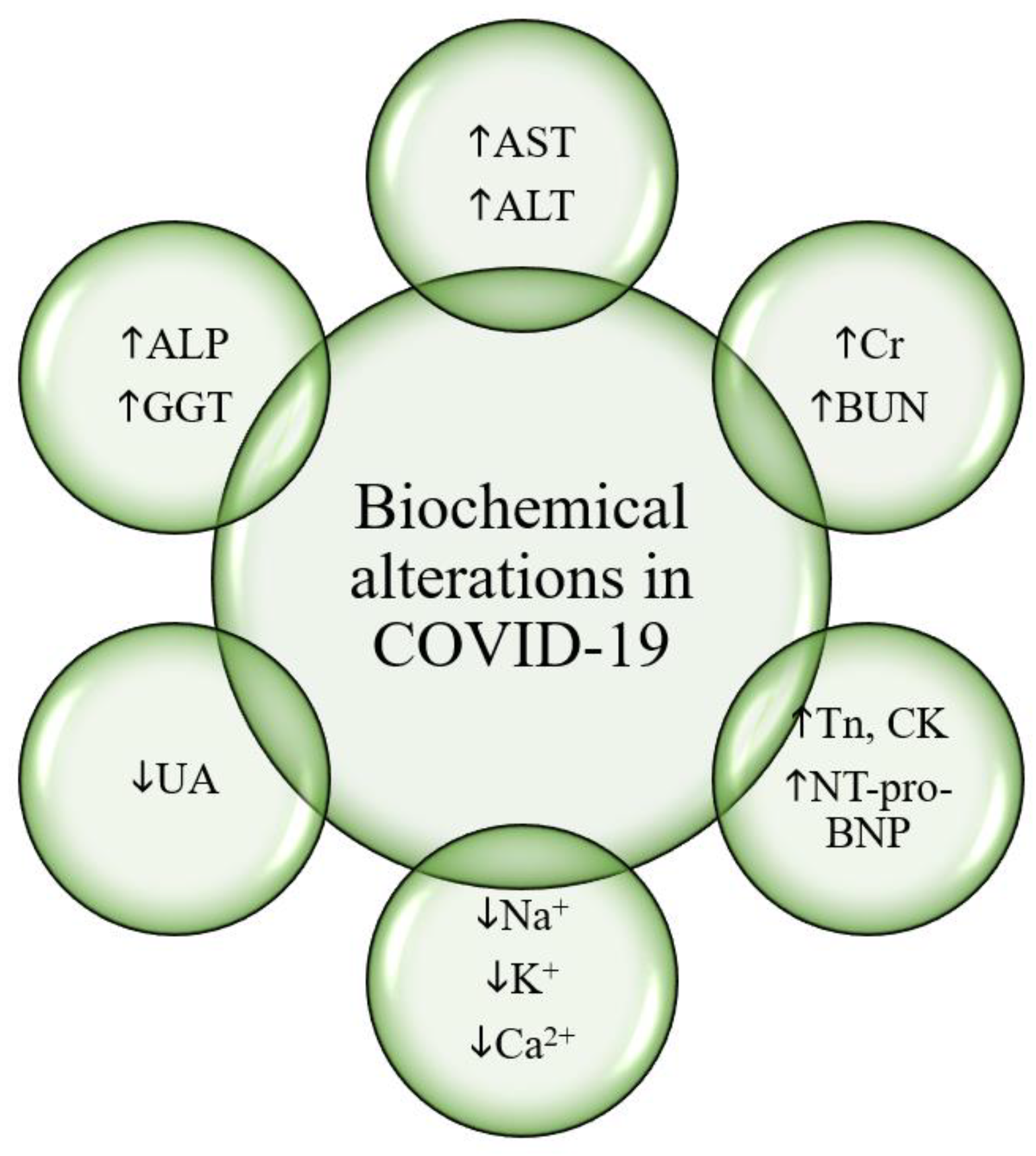
Biochemical findings in COVID-19
Liver involvement in COVID-19
Kidney involvement in COVID-19
Cardiac involvement in COVID-19
Dyselectrolytemia in COVID-19
Muscle involvement in COVID-19
Vitamin D: 25(OH)D
Conclusions
Conflict of interest disclosure
Compliance with ethical standards
References
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 15 August 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; Yu, T.; Xia, J.; Wei, Y.; Wu, W.; Xie, X.; Yin, W.; Li, H.; Liu, M.; Xiao, Y.; Gao, H.; Guo, L.; Xie, J.; Wang, G.; Jiang, R.; Gao, Z.; Jin, Q.; Wang, J.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; Tai, Y.; Bai, C.; Gao, T.; Song, J.; Xia, P.; Dong, J.; Zhao, J.; Wang, F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; Tian, D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; Yan, F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef]
- Akhmerov, A.; Marbán, E. COVID-19 and the Heart. Circ Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef]
- O'Shea, P.M.; Lee, G.R.; Griffin, T.P.; Tormey, V.; Hayat, A.; Costelloe, S.J.; Griffin, D.G.; Srinivasan, S.; O'Kane, M.; Burke, C.M.; Faul, J.; Thompson, C.J.; Curley, G.; Tormey, W.P. COVID-19 in adults: test menu for hospital blood science laboratories. Ir J Med Sci. 2020, 189, 1147–1152. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020, 58, 1063–1069. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Ricardo, D.; Jaloma-Cruz, A.R. Coronavirus Disease 2019: Hematological Anomalies and Antithrombotic Therapy. Tohoku J Exp Med. 2020, 251, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Lippi, G.; Plebani, M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020, 58, 1135–1138. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B.; Mucheli, S.S.; Kuperan, P.; Ong, K.H. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020, 95, E131–E134. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, Y.; Chen, J.; Xin, S.; Zhang, W.; Zhou, X.; Mao, Y.; Hu, L.; Liu, D.; Chang, B.; Chang, W.; Liu, Y.; Ma, X.; Wang, Y.; Liu, X. Prognostic factors for severe acute respiratory syndrome: a clinical analysis of 165 cases. Clin Infect Dis. 2004, 38, 483–489. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; Du, B.; Li, L.J.; Zeng, G.; Yuen, K.Y.; Chen, R.C.; Tang, C.L.; Wang, T.; Chen, P.Y.; Xiang, J.; Li, S.Y.; Wang, J.L.; Liang, Z.J.; Peng, Y.X.; Wei, L.; Liu, Y.; Hu, Y.H.; Peng, P.; Wang, J.M.; Liu, J.Y.; Chen, Z.; Li, G.; Zheng, Z.J.; Qiu, S.Q.; Luo, J.; Ye, C.J.; Zhu, S.Y.; Zhong, N.S. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; Guan, L.; Wei, Y.; Li, H.; Wu, X.; Xu, J.; Tu, S.; Zhang, Y.; Chen, H.; Cao, B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; Wang, C.; Zhao, J.; Sun, X.; Tian, R.; Wu, W.; Wu, D.; Ma, J.; Chen, Y.; Zhang, D.; Xie, J.; Yan, X.; Zhou, X.; Liu, Z.; Wang, J.; Du, B.; Qin, Y.; Gao, P.; Qin, X.; Xu, Y.; Zhang, W.; Li, T.; Zhang, F.; Zhao, Y.; Li, Y.; Zhang, S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Frater, J.L.; Zini, G.; d'Onofrio, G.; Rogers, H.J. COVID-19 and the clinical hematology laboratory. Int J Lab Hematol. 2020, 42 (Suppl. 1), 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020, 505, 190–191. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; Zheng, P.Y.; Tian, L.F.; Qu, H.Q.; Liu, D.C.; Wang, E.Y.; Xiao, X.J.; Li, S.Y.; Ye, F.; Guan, L.; Hu, D.S.; Hakonarson, H.; Liu, Z.G.; Zhong, N.S. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020, 56, 2001526. [Google Scholar] [CrossRef]
- Kowitdamrong, E.; Puthanakit, T.; Jantarabenjakul, W.; Prompetchara, E.; Suchartlikitwong, P.; Putcharoen, O.; Hirankarn, N. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One. 2020, 15, e0240502. [Google Scholar] [CrossRef]
- Padoan, A.; Sciacovelli, L.; Basso, D.; Negrini, D.; Zuin, S.; Cosma, C.; Faggian, D.; Matricardi, P.; Plebani, M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta. 2020, 507, 164–166. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Bomze, D.; Risch, L.; Brugger, S.D.; Paprotny, M.; Weber, M.; Thiel, S.; Kern, L.; Albrich, W.C.; Kohler, P.; Kahlert, C.R.; Vernazza, P.; Bühler, P.K.; Schüpbach, R.A.; Gómez-Mejia, A.; Popa, A.M.; Bergthaler, A.; Penninger, J.M.; Flatz, L. Severe Coronavirus Disease 2019 (COVID-19) is Associated With Elevated Serum Immunoglobulin (Ig) A and Antiphospholipid IgA Antibodies. Clin Infect Dis. 2021, 73, e2869–e2874. [Google Scholar] [CrossRef]
- Fourati, S.; Hue, S.; Pawlotsky, J.M.; Mekontso-Dessap, A.; de Prost, N. SARS-CoV-2 viral loads and serum IgA/IgG immune responses in critically ill COVID-19 patients. Intensive Care Med. 2020, 46, 1781–1783. [Google Scholar] [CrossRef]
- Figueiredo-Campos, P.; Blankenhaus, B.; Mota, C.; Gomes, A.; Serrano, M.; Ariotti, S.; Costa, C.; Nunes-Cabaço, H.; Mendes, A.M.; Gaspar, P.; Pereira-Santos, M.C.; Rodrigues, F.; Condeço, J.; Escoval, M.A.; Santos, M.; Ramirez, M.; Melo-Cristino, J.; Simas, J.P.; Vasconcelos, E.; Afonso, Â.; Veldhoen, M. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020, 50, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.T.; Campbell, J.A.; Ross, F.; Peña Jiménez, P.; Rudzinski, E.R.; Dickerson, J.A. Acute ANCA Vasculitis and Asymptomatic COVID-19. Pediatrics. 2021, 147, e2020033092. [Google Scholar] [CrossRef]
- Uppal, N.N.; Kello, N.; Shah, H.H.; Khanin, Y.; De Oleo, I.R.; Epstein, E.; Sharma, P.; Larsen, C.P.; Bijol, V.; Jhaveri, K.D. De Novo ANCA-Associated Vasculitis With Glomerulonephritis in COVID-19. Kidney Int Rep. 2020, 5, 2079–2083. [Google Scholar] [CrossRef]
- Moeinzadeh, F.; Dezfouli, M.; Naimi, A.; Shahidi, S.; Moradi, H. Newly Diagnosed Glomerulonephritis During COVID-19 Infection Undergoing Immunosuppression Therapy, a Case Report. Iran J Kidney Dis. 2020, 14, 239–242. [Google Scholar]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; Grondona, A.; Silvestri, T.; Zanuso, S.; Mazzolini, M.; Lalanne, C.; Quarneti, C.; Fusconi, M.; Giostra, F.; Granito, A.; Muratori, L.; Lenzi, M.; Muratori, P. COVID-19 and Immunological Dysregulation: Can Autoantibodies be Useful? Clin Transl Sci. 2021, 14, 502–508. [Google Scholar] [CrossRef]
- Vlachoyiannopoulos, P.G.; Magira, E.; Alexopoulos, H.; Jahaj, E.; Theophilopoulou, K.; Kotanidou, A.; Tzioufas, A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020, 79, 1661–1663. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tamiazzo, S.; Stobbione, P.; Agatea, L.; De Gaspari, P.; Stecca, A.; Lauritano, E.C.; Roveta, A.; Tozzoli, R.; Guaschino, R.; Bonometti, R. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci. 2021, 14, 898–907. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Carlo Stella, N.; Mariani, G.; Nai, C.; Coppola, A.; Naldani, D.; Gallotti, P. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol. 2020, 39, 2095–2097. [Google Scholar] [CrossRef]
- Mantovani Cardoso, E.; Hundal, J.; Feterman, D.; Magaldi, J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020, 39, 2811–2815. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; Zhao, C.; Zhang, J.; Jia, Q.; Zuo, X.; Li, J.; Wang, L.; Cao, Q.; Jia, E. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin Transl Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Varionchik, N.; Zaidenov, V.; Savina, P.; Sarkisova, N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur J Immunol. 2021, 51, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Gallizzi, R.; Sutera, D.; Spagnolo, A.; Bagnato, A.M.; Cannavò, S.P.; Grasso, L.; Guarneri, C.; Nunnari, G.; Mazza, F.; Pajno, G.B. Management of pernio-like cutaneous manifestations in children during the outbreak of COVID-19. Dermatol Ther. 2020, 33, e14312. [Google Scholar] [CrossRef]
- Gagiannis, D.; Steinestel, J.; Hackenbroch, C.; Schreiner, B.; Hannemann, M.; Bloch, W.; Umathum, V.G.; Gebauer, N.; Rother, C.; Stahl, M.; Witte, H.M.; Steinestel, K. Clinical, Serological, and Histopathological Similarities Between Severe COVID-19 and Acute Exacerbation of Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD). Front Immunol. 2020, 11, 587517. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Molinaro, E.; Novara, E.; Bonometti, R.; Sacchi, M.C.; Stobbione, P.; Lauritano, E.C.; Boverio, R. Isolated immune thrombocytopenic purpura in a young adult Covid-19 patient. Eur Rev Med Pharmacol Sci. 2020, 24, 10850–10852. [Google Scholar] [CrossRef]
- Pilotto, A.; Odolini, S.; Masciocchi, S.; Comelli, A.; Volonghi, I.; Gazzina, S.; Nocivelli, S.; Pezzini, A.; Focà, E.; Caruso, A.; Leonardi, M.; Pasolini, M.P.; Gasparotti, R.; Castelli, F.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Padovani, A. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. Ann Neurol. 2020, 88, 423–427. [Google Scholar] [CrossRef]
- Edén, A.; Kanberg, N.; Gostner, J.; Fuchs, D.; Hagberg, L.; Andersson, L.M.; Lindh, M.; Price, R.W.; Zetterberg, H.; Gisslén, M. CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms: A Case Series. Neurology. 2021, 96, e294–e300. [Google Scholar] [CrossRef]
- Conca, W.; Alabdely, M.; Albaiz, F.; Foster, M.W.; Alamri, M.; Alkaff, M.; Al-Mohanna, F.; Nagelkerke, N.; Almaghrabi, R.S. Serum β2-microglobulin levels in Coronavirus disease 2019 (Covid-19): Another prognosticator of disease severity? PLoS One. 2021, 16, e0247758. [Google Scholar] [CrossRef]
- Cristiano, A.; Fortunati, V.; Cherubini, F.; Bernardini, S.; Nuccetelli, M. Anti-phospholipids antibodies and immune complexes in COVID-19 patients: a putative role in disease course for anti-annexin-V antibodies. Clin Rheumatol. 2021, 40, 2939–2945. [Google Scholar] [CrossRef]
- Karahan, S.; Erol, K.; Yuksel, R.C.; et al. Antiphospholipid antibodies in COVID-19-associated pneumonia patients in intensive care unit. Mod Rheumatol. 2021, 1–10. [Google Scholar] [CrossRef]
- Siguret, V.; Voicu, S.; Neuwirth, M.; Delrue, M.; Gayat, E.; Stépanian, A.; Mégarbane, B. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res. 2020, 195, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; Linskens, E.A.; Benoit, D.; Peperstraete, H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J Thromb Haemost. 2020, 18, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Perricone, C.; Tonello, M.; Bistoni, O.; Cattelan, A.M.; Bursi, R.; Cafaro, G.; De Robertis, E.; Mencacci, A.; Bozza, S.; Vianello, A.; Iaccarino, L.; Gerli, R.; Doria, A.; Bartoloni, E. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol. 2020, 38, 754–759. [Google Scholar]
- Borghi, M.O.; Beltagy, A.; Garrafa, E.; Curreli, D.; Cecchini, G.; Bodio, C.; Grossi, C.; Blengino, S.; Tincani, A.; Franceschini, F.; Andreoli, L.; Lazzaroni, M.G.; Piantoni, S.; Masneri, S.; Crisafulli, F.; Brugnoni, D.; Muiesan, M.L.; Salvetti, M.; Parati, G.; Torresani, E.; Mahler, M.; Heilbron, F.; Pregnolato, F.; Pengo, M.; Tedesco, F.; Pozzi, N.; Meroni, P.L. Anti-Phospholipid Antibodies in COVID-19 Are Different From Those Detectable in the Anti-Phospholipid Syndrome. Front Immunol. 2020, 11, 584241. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; Yadav, V.; Wang, J.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Smith, S.A.; Morrissey, J.H.; Kanthi, Y.; Knight, J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Y.; Zhang, S.; Qin, X.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; Wang, C.; Zhao, J.; Sun, X.; Tian, R.; Wu, W.; Wu, D.; Ma, J.; Chen, Y.; Zhang, D.; Xie, J.; Yan, X.; Zhou, X.; Liu, Z.; Wang, J.; Du, B.; Qin, Y.; Gao, P.; Lu, M.; Hou, X.; Wu, X.; Zhu, H.; Xu, Y.; Zhang, W.; Li, T.; Zhang, F.; Zhao, Y.; Li, Y.; Zhang, S. Antiphospholipid Antibodies in Critically Ill Patients With COVID-19. Arthritis Rheumatol. 2020, 72, 1998–2004. [Google Scholar] [CrossRef]
- Marcos-Jiménez, A.; Sánchez-Alonso, S.; Alcaraz-Serna, A.; Esparcia, L.; López-Sanz, C.; Sampedro-Núñez, M.; Mateu-Albero, T.; Sánchez-Cerrillo, I.; Martínez-Fleta, P.; Gabrie, L.; Del Campo Guerola, L.; Rodríguez-Frade, J.M.; Casasnovas, J.M.; Reyburn, H.T.; Valés-Gómez, M.; López-Trascasa, M.; Martín-Gayo, E.; Calzada, M.J.; Castañeda, S.; de la Fuente, H.; González-Álvaro, I.; Sánchez-Madrid, F.; Muñoz-Calleja, C.; Alfranca, A. Deregulated cellular circuits driving immunoglobulins and complement consumption associate with the severity of COVID-19 patients. Eur J Immunol. 2021, 51, 634–647. [Google Scholar] [CrossRef]
- Tammaro, A.; Adebanjo, G.A.R.; Del Nonno, F.; Pezzuto, A.; Ramirez-Estrada, S.; Parisella, F.R.; Rello, J.; Scarabello, A. Cutaneous Endothelial Dysfunction and Complement Deposition in COVID-19. Am J Dermatopathol. 2021, 43, 237–238. [Google Scholar] [CrossRef]
- Pfister, F.; Vonbrunn, E.; Ries, T.; Jäck, H.M.; Überla, K.; Lochnit, G.; Sheriff, A.; Herrmann, M.; Büttner-Herold, M.; Amann, K.; Daniel, C. Complement Activation in Kidneys of Patients With COVID-19. Front Immunol. 2021, 11, 594849. [Google Scholar] [CrossRef]
- Gupta, R.; Gant, V.A.; Williams, B.; Enver, T. Increased Complement Receptor-3 levels in monocytes and granulocytes distinguish COVID-19 patients with pneumonia from those with mild symptoms. Int J Infect Dis. 2020, 99, 381–385. [Google Scholar] [CrossRef] [PubMed]
- de Nooijer, A.H.; Grondman, I.; Janssen, N.A.F.; Netea, M.G.; Willems, L.; van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.J.; Toonen, E.J.M.; Joosten, L.A.B. RCI-COVID-19 study group. Complement Activation in the Disease Course of Coronavirus Disease 2019 and Its Effects on Clinical Outcomes. J Infect Dis. 2021, 223, 214–224. [Google Scholar] [CrossRef] [PubMed]
- D'Alessandro, A.; Thomas, T.; Dzieciatkowska, M.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hod, E.A.; Spitalnik, S.L.; Hansen, K.C. Serum Proteomics in COVID-19 Patients: Altered Coagulation and Complement Status as a Function of IL-6 Level. J Proteome Res. 2020, 19, 4417–4427. [Google Scholar] [CrossRef] [PubMed]
- Holter, J.C.; Pischke, S.E.; de Boer, E.; Lind, A.; Jenum, S.; Holten, A.R.; Tonby, K.; Barratt-Due, A.; Sokolova, M.; Schjalm, C.; Chaban, V.; Kolderup, A.; Tran, T.; Tollefsrud Gjølberg, T.; Skeie, L.G.; Hesstvedt, L.; Ormåsen, V.; Fevang, B.; Austad, C.; Müller, K.E.; Fladeby, C.; Holberg-Petersen, M.; Halvorsen, B.; Müller, F.; Aukrust, P.; Dudman, S.; Ueland, T.; Andersen, J.T.; Lund-Johansen, F.; Heggelund, L.; Dyrhol-Riise, A.M.; Mollnes, T.E. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020, 117, 25018–25025. [Google Scholar] [CrossRef]
- Perea Polak, A.; Romero Madrid, B.; García Ocaña, P.P.; Lomeña Alvarez, G.; Martínez Pilar, L.; Gómez-Moyano, E. Complement-mediated thrombogenic vasculopathy in COVID-19. Int J Dermatol. 2021, 60, 229–232. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Xu, W. Thyroid Function Analysis in 50 Patients with COVID-19: A Retrospective Study. Thyroid. 2021, 31, 8–11. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; Wang, T.; Guo, W.; Chen, J.; Ding, C.; Zhang, X.; Huang, J.; Han, M.; Li, S.; Luo, X.; Zhao, J.; Ning, Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020, 368, m1091. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After Sars-COV-2 Infection. J Clin Endocrinol Metab. 2020, 105, dgaa276. [Google Scholar] [CrossRef]
- Gao, W.; Guo, W.; Guo, Y.; Shi, M.; Dong, G.; Wang, G.; Ge, Q.; Zhu, J.; Zhou, X. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021, 44, 1031–1040. [Google Scholar] [CrossRef]
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020, 183, 381–387. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; Woo, Y.C.; Lam, C.W.; Hung, I.F.N.; Lam, K.S.L. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J Clin Endocrinol Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, F.; Tu, W.; Zhang, J.; Choudhry, A.A.; Ahmed, O.; Cheng, J.; Cui, Y.; Liu, B.; Dai, M.; Chen, L.; Han, D.; Fan, Y.; Zeng, Y.; Li, W.; Li, S.; Chen, X.; Shen, M.; Pan, P. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021, 521, 111097. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C.; Meeran, K.; Comninos, A.N.; Abbara, A.; Dhillo, W.S. Thyroid Function Before, During, and After COVID-19. J Clin Endocrinol Metab. 2021, 106, e803–e811. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.; Gültekin, H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: Results of single-centre pandemic hospital. Int J Clin Pract. 2021, 75, e14129. [Google Scholar] [CrossRef]
- Malik, J.; Malik, A.; Javaid, M.; Zahid, T.; Ishaq, U.; Shoaib, M. Thyroid function analysis in COVID-19: A retrospective study from a single center. PLoS One. 2021, 16, e0249421. [Google Scholar] [CrossRef]
- Wang, W.; Su, X.; Ding, Y.; Fan, W.; Zhou, W.; Su, J.; Chen, Z.; Zhao, H.; Xu, K.; Ni, Q.; Xu, X.; Qiu, Y.; Teng, L. Thyroid Function Abnormalities in COVID-19 Patients. Front Endocrinol (Lausanne). 2021, 11, 623792. [Google Scholar] [CrossRef]
- Sen, K.; Sinha, A.; Sen, S.; Chakraborty, S.; Alam, M.S. Thyroid Function Test in COVID-19 Patients: A Cross-Sectional Study in a Tertiary Care Hospital. Indian J Endocrinol Metab. 2020, 24, 532–536. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front Endocrinol (Lausanne). 2021, 11, 593179. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Mukhtar, N.; Aljomaiah, A.; Aljamei, H.; Bakhsh, A.; Alsudani, N.; Elsayed, T.; Alrashidi, N.; Fadel, R.; Alqahtani, E.; Raef, H.; Butt, M.I.; Sulaiman, O. The Impact of COVID-19 Viral Infection on the Hypothalamic-Pituitary-Adrenal Axis. Endocr Pract. 2021, 27, 83–89. [Google Scholar] [CrossRef]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; Comninos, A.N.; Abbara, A.; Dhillo, W.S. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660. [Google Scholar] [CrossRef]
- Ramezani, M.; Simani, L.; Karimialavijeh, E.; Rezaei, O.; Hajiesmaeili, M.; Pakdaman, H. The Role of Anxiety and Cortisol in Outcomes of Patients With Covid-19. Basic Clin Neurosci. 2020, 11, 179–184. [Google Scholar] [CrossRef]
- Rieder, M.; Wirth, L.; Pollmeier, L.; Jeserich, M.; Goller, I.; Baldus, N.; Schmid, B.; Busch, H.J.; Hofmann, M.; Kern, W.; Bode, C.; Duerschmied, D.; Lother, A. Serum ACE2, Angiotensin II, and Aldosterone Levels Are Unchanged in Patients With COVID-19. Am J Hypertens. 2021, 34, 278–281. [Google Scholar] [CrossRef]
- Villard, O.; Morquin, D.; Molinari, N.; Raingeard, I.; Nagot, N.; Cristol, J.P.; Jung, B.; Roubille, C.; Foulongne, V.; Fesler, P.; Lamure, S.; Taourel, P.; Konate, A.; Maria, A.T.J.; Makinson, A.; Bertchansky, I.; Larcher, R.; Klouche, K.; Le Moing, V.; Renard, E.; Guilpain, P. The Plasmatic Aldosterone and C-Reactive Protein Levels, and the Severity of Covid-19: The Dyhor-19 Study. J Clin Med. 2020, 9, 2315. [Google Scholar] [CrossRef]
- Gu, W.T.; Zhou, F.; Xie, W.Q.; Wang, S.; Yao, H.; Liu, Y.T.; Gao, L.; Wu, Z.B. A potential impact of SARS-CoV-2 on pituitary glands and pituitary neuroendocrine tumors. Endocrine. 2021, 72, 340–348. [Google Scholar] [CrossRef]
- Ding, T.; Wang, T.; Zhang, J.; Cui, P.; Chen, Z.; Zhou, S.; Yuan, S.; Ma, W.; Zhang, M.; Rong, Y.; Chang, J.; Miao, X.; Ma, X.; Wang, S. Analysis of Ovarian Injury Associated With COVID-19 Disease in Reproductive-Aged Women in Wuhan, China: An Observational Study. Front Med (Lausanne). 2021, 8, 635255. [Google Scholar] [CrossRef]
- Kadihasanoglu, M.; Aktas, S.; Yardimci, E.; Aral, H.; Kadioglu, A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. J Sex Med. 2021, 18, 256–264. [Google Scholar] [CrossRef]
- Okçelik, S. COVID-19 pneumonia causes lower testosterone levels. Andrologia. 2021, 53, e13909. [Google Scholar] [CrossRef]
- Rastrelli, G.; Di Stasi, V.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Greco, G.F.; Cervi, G.; Pecoriello, A.; Magini, A.; Todisco, T.; Cipriani, S.; Maseroli, E.; Corona, G.; Salonia, A.; Lenzi, A.; Maggi, M.; De Donno, G.; Vignozzi, L. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021, 9, 88–98. [Google Scholar] [CrossRef]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Ye, G.; Mao, Y.; Xiong, Y.; Sun, H.; Zheng, F.; Chen, Z.; Qin, J.; Lyu, J.; Zhang, Y.; Zhang, M. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021, 93, 456–462. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: diagnostic value in organ injuries. Eur J Clin Microbiol Infect Dis. 2020, 39, 2447–2455. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Karslioglu, B. Did Covid-19 pandemic narrow the spectrum of surgical indications? J Clin Invest Surg. 2021, 6, 58–63. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Li, J.; Cheng, X.; Yang, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020, 18, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Schattenberg, J.M. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020, 8, 509–519. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection [Internet]. bioRxiv. 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.02.03.9 31766v1 (accessed on 4 October 2021).
- Feng, G.; Zheng, K.I.; Yan, Q.Q.; Rios, R.S.; Targher, G.; Byrne, C.D.; Poucke, S.V.; Liu, W.Y.; Zheng, M.H. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020, 8, 18–24. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases (AASLD). Clinical insights for hepatology and liver transplant providers during the covid-19 pandemic [Internet]. 2020. Available online: www.aasld.org (accessed on 4 October 2021).
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the liver. J Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; Liu, Y.; Liu, Y.; Wang, G.; Yuan, Z.; Hou, X.; Ren, L.; Wu, Y.; Chen, Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021, 12, 2506. [Google Scholar] [CrossRef]
- Joannidis, M.; Forni, L.G.; Klein, S.J.; Honore, P.M.; Kashani, K.; Ostermann, M.; Prowle, J.; Bagshaw, S.M.; Cantaluppi, V.; Darmon, M.; Ding, X.; Fuhrmann, V.; Hoste, E.; Husain-Syed, F.; Lubnow, M.; Maggiorini, M.; Meersch, M.; Murray, P.T.; Ricci, Z.; Singbartl, K.; Staudinger, T.; Welte, T.; Ronco, C.; Kellum, J.A. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020, 46, 654–672. [Google Scholar] [CrossRef]
- Asgharpour, M.; Zare, E.; Mubarak, M.; Alirezaei, A. COVID-19 and Kidney Disease: Update on Epidemiology, Clinical Manifestations, Pathophysiology and Management. J Coll Physicians Surg Pak. 2020, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Aleebrahim-Dehkordi, E.; Reyhanian, A.; Saberianpour, S.; Hasanpour-Dehkordi, A. Acute kidney injury in COVID-19; a review on current knowledge. J Nephropathol. 2020, 9, e31. [Google Scholar] [CrossRef]
- Gagliardi, I.; Patella, G.; Michael, A.; Serra, R.; Provenzano, M.; Andreucci, M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J Clin Med. 2020, 9, 2506. [Google Scholar] [CrossRef]
- Coll, E.; Botey, A.; Alvarez, L.; Poch, E.; Quintó, L.; Saurina, A.; Vera, M.; Piera, C.; Darnell, A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000, 36, 29–34. [Google Scholar] [CrossRef]
- Nisha, R.; Srinivasa Kannan, S.R.; Thanga Mariappan, K.; Jagatha, P. Biochemical evaluation of creatinine and urea in patients with renal failure undergoing hemodialysis. J Clin Path Lab Med. 2017, 1, 1–5. [Google Scholar]
- Liu, Y.M.; Xie, J.; Chen, M.M.; Zhang, X.; Cheng, X.; Li, H.; Zhou, F.; Qin, J.J.; Lei, F.; Chen, Z.; Lin, L.; Yang, C.; Mao, W.; Chen, G.; Lu, H.; Xia, X.; Wang, D.; Liao, X.; Yang, J.; Huang, X.; Zhang, B.H.; Yuan, Y.; Cai, J.; Zhang, X.J.; Wang, Y.; Zhang, X.; She, Z.G.; Li, H. Kidney Function Indicators Predict Adverse Outcomes of COVID-19. Med (N Y). 2021, 2, 38–48.e2. [Google Scholar] [CrossRef]
- Soleimani, M. Acute Kidney Injury in SARS-CoV-2 Infection: Direct Effect of Virus on Kidney Proximal Tubule Cells. Int J Mol Sci. 2020, 21, 3275. [Google Scholar] [CrossRef]
- Popescu, B.; Doinița, O.I.; Balalau, C.; Scaunasu, V.; et al. Fibroscopic examination on ENT patients in COVID-19 era. J Clin Invest Surg. 2020, 5, 63–65. [Google Scholar] [CrossRef]
- Macedo, E. Blood urea nitrogen beyond estimation of renal function. Crit Care Med. 2011, 39, 405–406. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Thum, T. SARS-CoV-2 receptor ACE2 expression in the human heart: cause of a post-pandemic wave of heart failure? Eur Heart J. 2020, 41, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Tsang, W.K.; Tang, C.S.; Lam, M.F.; Lai, F.M.; To, K.F.; Fung, K.S.; Tang, H.L.; Yan, W.W.; Chan, H.W.; Lai, T.S.; Tong, K.L.; Lai, K.N. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005, 67, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Marques, M.; López-Sánchez, P.; de Valdenebro, M.; Muñez, E.; Serrano, M.L.; Malo, R.; García, E.; Cuervas, V. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020, 35, 1353–1361. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Werion, A.; Belkhir, L.; Perrot, M.; Schmit, G.; Aydin, S.; Chen, Z.; Penaloza, A.; De Greef, J.; Yildiz, H.; Pothen, L.; Yombi, J.C.; Dewulf, J.; Scohy, A.; Gérard, L.; Wittebole, X.; Laterre, P.F.; Miller, S.E.; Devuyst, O.; Jadoul, M.; Morelle, J. Cliniques universitaires Saint-Luc (CUSL) COVID-19 Research Group. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020, 98, 1296–1307. [Google Scholar] [CrossRef]
- Wu, V.C.; Huang, J.W.; Hsueh, P.R.; Yang, Y.F.; Tsai, H.B.; Kan, W.C.; Chang, H.W.; Wu, K.D. SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital. Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am J Kidney Dis. 2005, 45, 88–95. [Google Scholar] [CrossRef]
- Inker, L.A.; Tighiouart, H.; Coresh, J.; Foster, M.C.; Anderson, A.H.; Beck, G.J.; Contreras, G.; Greene, T.; Karger, A.B.; Kusek, J.W.; Lash, J.; Lewis, J.; Schelling, J.R.; Navaneethan, S.D.; Sondheimer, J.; Shafi, T.; Levey, A.S. GFR Estimation Using β-Trace Protein and β2-Microglobulin in CKD. Am J Kidney Dis. 2016, 67, 40–48. [Google Scholar] [CrossRef]
- Sun, D.Q.; Wang, T.Y.; Zheng, K.I.; Targher, G.; Byrne, C.D.; Chen, Y.P.; Zheng, M.H. Subclinical Acute Kidney Injury in COVID-19 Patients: A Retrospective Cohort Study. Nephron. 2020, 144, 347–350. [Google Scholar] [CrossRef]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; Richardson, D.C.; Rosella, L.C.; Simor, A.; Smieja, M.; Zahariadis, G.; Gubbay, J.B. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; Huang, H.; Yang, B.; Huang, C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; Maroldi, R.; Adamo, M.; Ammirati, E.; Sinagra, G.; Lombardi, C.M.; Metra, M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; Zhao, Y.; Li, Y.; Wang, X.; Peng, Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef]
- Zazzo, J.F.; Troché, G.; Ruel, P.; Maintenant, J. High incidence of hypophosphatemia in surgical intensive care patients: efficacy of phosphorus therapy on myocardial function. Intensive Care Med. 1995, 21, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Lindner, G.; Wolzt, M.; Ahmad, S.S.; Sauter, T.; Leichtle, A.B.; Fiedler, G.M.; Fuhrmann, V.; Exadaktylos, A.K. Hyperphosphatemia Is an Independent Risk Factor for Mortality in Critically Ill Patients: Results from a Cross-Sectional Study. PLoS One. 2015, 10, e0133426. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, C.; Su, F.; Dai, J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). MedRxiv. 2020. [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; Zhang, Z.; Wang, L.; Peng, L.; Chen, L.; Qin, Y.; Zhao, D.; Tan, S.; Yin, L.; Xu, J.; Zhou, C.; Jiang, C.; Liu, L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; Jin, Y.; Niu, X.; Ping, R.; Du, Y.; Li, T.; Xu, G.; Hu, Q.; Tu, L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Butt, I.; Sawlani, V.; Geberhiwot, T. Prolonged confusional state as first manifestation of COVID-19. Ann Clin Transl Neurol. 2020, 7, 1450–1452. [Google Scholar] [CrossRef]
- Berni, A.; Malandrino, D.; Parenti, G.; Maggi, M.; Poggesi, L.; Peri, A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. 2020, 43, 1137–1139. [Google Scholar] [CrossRef]
- Post, A.; Dullaart, R.P.F.; Bakker, S.J.L. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur J Intern Med. 2020, 75, 109. [Google Scholar] [CrossRef]
- Post, A.; Dullaart, R.F.; Bakker, S.L. Sodium status and kidney involvement during COVID-19 infection. Virus Res 2020, 286, 198034. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, H.; Hu, J.; Lian, J.; Gu, J.; Zhang, S.; Ye, C.; Lu, Y.; Jin, C.; Yu, G.; Jia, H.; Zhang, Y.; Sheng, J.; Li, L.; Yang, Y. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. 2020, 94, 81–87. [Google Scholar] [CrossRef]
- Griffin, T.P.; Wall, D.; Blake, L.; Griffin, D.G.; Robinson, S.M.; Bell, M.; Mulkerrin, E.C.; O'Shea, P.M. Vitamin D Status of Adults in the Community, in Outpatient Clinics, in Hospital, and in Nursing Homes in the West of Ireland. J Gerontol A Biol Sci Med Sci. 2020, 75, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Komolmit, P.; Charoensuk, K.; Thanapirom, K.; Suksawatamnuay, S.; Thaimai, P.; Chirathaworn, C.; Poovorawan, Y. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: A randomised double-blinded, placebo-control trial. PLoS One. 2017, 12, e0174608. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of Vitamin D Status for Enhanced Immuno-protection Against Covid-19. Ir Med J. 2020, 113, 58. [Google Scholar]
- Dara, N.; Hosseini, A.; Sayyari, A.; et al. Gastrointestinal Manifestations and Dynamics of Liver Enzymes in Children and Adolescents with COVID-19 Infection: A Systematic Review and Meta-Analysis. Iran J Pediatr. 2020, 30, e106935. [Google Scholar] [CrossRef]
- Trifonova, K.Z.; Slaveykov, K.S.; Dochev, I.S.; Parusheva, P.O.; Pekova, L.M. COVID-19 presenting with central retinal vein occlusion: a case report. Arch Balk Med Union. 2021, 56, 268–272. [Google Scholar] [CrossRef]
- Diaconu, C. Thromboembolism in patients with COVID-19. Arch Balk Med Union. 2021, 56, 281–283. [Google Scholar] [CrossRef]
- Vural, A.; Kahraman Ahmet, N. D-dimer levels and acute pulmonary embolism development in COVID-19 patients. J Mind Med Sci. 2021, 8, 133–138. [Google Scholar] [CrossRef]
- Diaconu, C. Cardiovascular complications of COVID-19. Arch Balk Med Union. 2021, 56, 139–141. [Google Scholar] [CrossRef]
- Li, A.; Kuderer, N.M.; Hsu, C.Y.; Shyr, Y.; Warner, J.L.; Shah, D.P.; Kumar, V.; Shah, S.; Kulkarni, A.A.; Fu, J.; Gulati, S.; Zon, R.L.; Li, M.; Desai, A.; Egan, P.C.; Bakouny, Z.; Kc, D.; Hwang, C.; Akpan, I.J.; McKay, R.R.; Girard, J.; Schmidt, A.L.; Halmos, B.; Thompson, M.A.; Patel, J.M.; Pennell, N.A.; Peters, S.; Elshoury, A.; de Lima Lopes, G.; Stover, D.G.; Grivas, P.; Rini, B.I.; Painter, C.A.; Mishra, S.; Connors, J.M.; Lyman, G.H.; Rosovsky, R.P. CCC19 consortium. The CoVID-TE risk assessment model for venous thromboembolism in hospitalized patients with cancer and COVID-19. J Thromb Haemost. 2021, 19, 2522–2532. [Google Scholar] [CrossRef]
- Moisa, C.; Gaman, M.A.; Diaconu, C.C.; et al. Oxidative Stress Levels, JAK2V617F Mutational Status and Thrombotic Complications in Patients with Essential Thrombocythemia. Rev. Chim. 2019, 70, 2822–2825. [Google Scholar] [CrossRef]
- Gaman, A.M.; Moisa, C.; Diaconu, C.C.; et al. Crosstalk between Oxidative Stress, Chronic Inflammation and Disease Progression in Essential Thrombocythemia. Rev. Chim. 2019, 70, 3486–3489. [Google Scholar] [CrossRef]
- Prasad, S.K.; Pradeep, S.; Shimavallu, C.; Kollur, S.P.; Syed, A.; Marraiki, N.; Egbuna, C.; Gaman, M.A.; Kosakowska, O.; Cho, W.C.; Patrick-Iwuanyanwu, K.C.; Ortega Castro, J.; Frau, J.; Flores-Holguín, N.; Glossman-Mitnik, D. Evaluation of Annona muricata Acetogenins as Potential Anti-SARS-CoV-2 Agents Through Computational Approaches. Front Chem. 2021, 8, 624716. [Google Scholar] [CrossRef]
- Eibensteiner, F.; Ritschl, V.; Nawaz, F.A.; Fazel, S.S.; Tsagkaris, C.; Kulnik, S.T.; Crutzen, R.; Klager, E.; Völkl-Kernstock, S.; Schaden, E.; Kletecka-Pulker, M.; Willschke, H.; Atanasov, A.G. People's Willingness to Vaccinate Against COVID-19 Despite Their Safety Concerns: Twitter Poll Analysis. J Med Internet Res. 2021, 23, e28973. [Google Scholar] [CrossRef]
- Găman, M.A.; Ryan, P.M.; Bonilla-Escobar, F.J. To Stay at Port or to Go to Sea: Are Clinical Clerkships a Double-Edged Sword During the COVID-19 Pandemic? Where Do We Go From Here? Int J Med Students. 2020, 8, 92–95. [Google Scholar] [CrossRef]
- Sakka, S.; Nikopoulou, V.; Bonti, E.; et al. Assessing test anxiety and resilience among Greek adolescents during COVID-19 pandemic. J Mind Med Sci. 2020, 7, 173–178. [Google Scholar] [CrossRef]

© 2022 by the author. 2022 Yousef Rasmi, Lucas Paulo Jacinto Saavedra, Matei-Alexandru Cozma, Heba A.S. El-Nashar, Shaza H. Aly, Nouran M. Fahmy, Omayma A. Eldahshan, Mohamed El-Shazly, Elena-Codruța Dobrică, Hamed Kord-Varkaneh, Camelia Cristina Diaconu, Mihnea-Alexandru Găman
Share and Cite
Rasmi, Y.; Saavedra, L.P.J.; Cozma, M.-A.; El-Nashar, H.A.S.; Aly, S.H.; Fahmy, N.M.; Eldahshan, O.A.; El-Shazly, M.; Dobrică, E.-C.; Kord-Varkaneh, H.; et al. Laboratory Findings in COVID-19—Alterations of Hematological, Immunological, Biochemical, Hormonal and Other Lab Panels: A Narrative Review. J. Mind Med. Sci. 2022, 9, 38-55. https://doi.org/10.22543/7674.91.P3855
Rasmi Y, Saavedra LPJ, Cozma M-A, El-Nashar HAS, Aly SH, Fahmy NM, Eldahshan OA, El-Shazly M, Dobrică E-C, Kord-Varkaneh H, et al. Laboratory Findings in COVID-19—Alterations of Hematological, Immunological, Biochemical, Hormonal and Other Lab Panels: A Narrative Review. Journal of Mind and Medical Sciences. 2022; 9(1):38-55. https://doi.org/10.22543/7674.91.P3855
Chicago/Turabian StyleRasmi, Yousef, Lucas Paulo Jacinto Saavedra, Matei-Alexandru Cozma, Heba A.S. El-Nashar, Shaza H. Aly, Nouran M. Fahmy, Omayma A. Eldahshan, Mohamed El-Shazly, Elena-Codruța Dobrică, Hamed Kord-Varkaneh, and et al. 2022. "Laboratory Findings in COVID-19—Alterations of Hematological, Immunological, Biochemical, Hormonal and Other Lab Panels: A Narrative Review" Journal of Mind and Medical Sciences 9, no. 1: 38-55. https://doi.org/10.22543/7674.91.P3855
APA StyleRasmi, Y., Saavedra, L. P. J., Cozma, M.-A., El-Nashar, H. A. S., Aly, S. H., Fahmy, N. M., Eldahshan, O. A., El-Shazly, M., Dobrică, E.-C., Kord-Varkaneh, H., Diaconu, C. C., & Găman, M.-A. (2022). Laboratory Findings in COVID-19—Alterations of Hematological, Immunological, Biochemical, Hormonal and Other Lab Panels: A Narrative Review. Journal of Mind and Medical Sciences, 9(1), 38-55. https://doi.org/10.22543/7674.91.P3855


