Abstract
Objective. Low-Level Laser Therapy encourages the healing process, reduces inflammation and pain. The aim of this study is to identify the impact of Low-Level Laser Therapy on tissue regeneration with special attention to hard tissues and to compare the effect of several wave lengths in the proliferation and differentiation of cells. Methods. The keywords used were “bone regeneration”, “laser therapy”, “photobiomodulation” OR “bio-stimulation”, “Low-Level Laser therapy” OR “LLLT”, “osteoblast proliferation” AND “differentiation”. Results. The bio-stimulation with Low-Level Laser Therapy also seems to interfere with the osseous integration of implants, by increasing its adherence on the bone-implant surfaces. Evidence has shown that Low-Level Laser Therapy influences the cellular proliferation and differentiation. Conclusions. Low-Level Laser Therapy is a promising therapy in the field of regeneration, but further studies are needed in order to define the standard protocol.
Introduction
LASER (Light Amplification by Stimulated Emission of Radiation) is becoming more and more widespread in everyday practice in medicine, both as a single source of light and combined with other therapies [1,2]. Its implications in human health include the diagnosis, the treatment and the maintenance of the condition of the biological system. For the first time in the history of medicine, Leon Goldman used the laser in 1960, thus being referred to as "the father of the laser". He used a ruby laser to remove the tattoos by irradiating the pigmented skin until the pigmented granulates are melted.
The use of the laser in medicine has brought many advantages, since the laser treatment offers atraumatic conditions and a cleaner operation field from the blood. The postoperative pain and edema are notably reduced [3,4]. Differing from other techniques, the use of laser does not leave any scarring and it offers better aesthetic effects by restoring a "restitutio-ad integrum" of the site [5,6,7,8], either as a single therapy or associated with other topical substances [9,10]. Moreover, in the past years, its applications have spread to other fields of dentistry, such as the treatment of rare vascular abnormalities, efficient antimicrobial activity, endodontic disinfection, the early treatment of inflammatory and premalignant oral lesions [11,12,13,14,15]. Its expertise field has been extended even to dental aesthetic and prosthetic rehabilitation [16,17]. Laser implications in the control of chronic periodontal disease [18,19] has been evidenced in literature studies, as a non-surgical treatment option to conventional root planning. Photodynamics is a non-thermal photochemical and biological interaction. Photobiomodulation (PBM), also called LLLT (Low-Level Laser Therapy), is a non-invasive and safe method. Laser photobiomodulation uses red light (600-700) nm and infrared light (770-1200) nm in most cases to reduce inflammation, pain and to eventually stimulate the healing process [20,21].
Given the multiple implications of laser in the management of general and oral diseases, the purpose of this study is to identify the effects of Low-Level Laser Therapy (LLLT) on regeneration, as well as its effects on the first steps of the cell differentiation and proliferation of osteogenic cells, and the ways through which this treatment may interact with the biomaterials used as a bone replacement.
Materials and Methods
A number of 733 papers from PubMed/Medline, Web of Science and Google Scholar were included for the preparation of this study. The key words used were: “Bone regeneration”, “Laser therapy”, “Photobiomodulation” or “bio-stimulation”, “Low-level Laser therapy” OR “LLLT”, “Osteoblast proliferation” AND “differentiation”. For this research, we have also used the Boolean operator (AND, OR, NOT). The selected papers are both in vitro and in vivo studies. After having read the abstracts, 307 papers were chosen for peer review, out of which 255 papers were excluded from this study for several reasons, including the lack of data shown, duplicated items, language barriers. Therefore, 52 papers were included in the final research (Figure 1).
Figure 1.
PRISMA chart evaluation of the included papers.
Results
Photobiomodulation and its use in oral pathologies
The action of LLLT is based on the interaction between light laser and the chromophore cells as target (especially cytochrome C oxidase, the enzyme of the mitochondrial respiratory chain). From the interaction of the absorbed light of these chromophores, more energy is synthetized (Adenosine-Triphosphate - ATP), which plays an important role in the production of nitric oxide by increasing it and modulating the calcium levels [21].
The patients who were diagnosed with cancer and who underwent high doses of chemotherapy, systematically experience severe complications, such as oral mucositis (OM). The methods used to impede this disease have proven useless. The Multinational Association of Supportive Care in Cancer/The International Society of Oral Oncology for the treatment of oral mucositis in adult oncological patients recommends the use of photobiomodulation (PBM). Even if there is no protocol for the LLLT, the suggested wave length of 650 nm for each square centimeter, the power must be 40 mW with an energy 2J/cm2 [22]. This process of treatment of OM with LLLT has been used on adult patients, but it was revealed that the LLLT also reduces severe oral mucositis in the young oncological patients, with a prevalence of 52%-80% of the disease [23].
The LLLT is used in many other oral diseases, where it has proven more efficient than the previous treatments so far [24] (also shown in Figure 2).
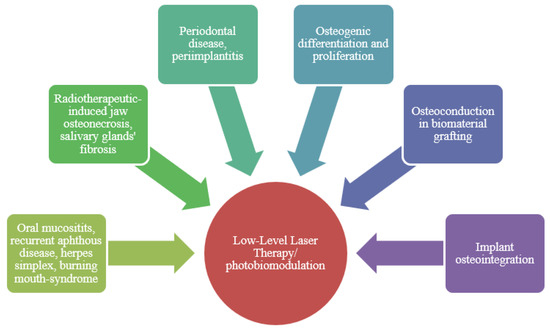
Figure 2.
LLLT clinical applications.
In the recurrent aphthous disease (affecting the non-keratinized mucosa of the oral cavity), the use of LLLT interferes in the inflammatory process by reducing the level of cytokines Interleukin 1b (IL-1b), Tumor Necrosis Factor- α (TNF-α) and Interferon (IFN). It notably reduces pain by modifying the neutral conduction through the release of enkephalins and endorphins, and moreover by making the ATP synthesis grow in neutral mitochondria by leading to the obstruction of stimuli. Therefore, the use of LLLT reduces the healing time in these patients [25]. Herpes simplex is another case in which the use of the LLLT significantly reduces pain compared to the treatment with acyclovir and it offers a full healing process of the wound in a shorter period of time [26].
The osteonecrosis of the maxilla, which is a consequence of the used drugs (bisphosphonates, monoclonal antibodies and radiotherapy) is a very known problem. The side effects of these drugs, especially bisphosphonates, last in time and influence the life quality of chronic patients. The LLLT combined with antibiotics and key-hole surgery with the use of piezo surgery, improves the conditions of jaw osteonecrosis by reducing clinical symptoms and by encouraging and enhancing the healing process, as well as by avoiding the relapse through neovascularization enhancement [27,28]. The radiotherapy of the salivary glands leads to dry mouth symptoms (burning mouth, mucositis, hyposialia, eating disorders) [29]. The use of LLLT in case of hyposalivation has been very efficient, enhancing saliva secretion and glandular cell regeneration, as well as the anti-oxidative effects of eliminating reactive oxygen species from soft tissues [16]. It was stated that LLLT reduces the symptoms of trigeminal neuralgia and does not cause side effects, therefore this method may be especially used in patients for whom drugs such as anti-seizure or painkillers have no efficacy [30].
The use of LLLT also spreads to the field of periodontology and implantology. LLLT also has good anti-inflammatory, analgesic and bio-stimulating effects, therefore being also used in periodontal therapies and in peri-implantitis. Naturally, some studies show positive results in the soft and hard tissues healing processes in these situations [31,32].
However, the effects of the use of laser after a long period of time does not have many advantages as traditional methods. The literature data show that the laser used on scaling and root planning (SRP), sulcular debridement (laser curettage) does not give better results and it has been considered unpredictable and indefinable on subgingival bacteria; the laser which has been considered efficient in vitro for the cleaning of roots from the precipitated calcium is the Er-YAG which is used in the treatment of hard tissues; therefore, this fact questions the use of the laser, since it is possible that it might damage the root during the operation and the doctor has a difficult control of the Er-YAG laser [32,33]. However, in case of fixture exposed out of the bone, this laser provides excellent results on the implant surface by reducing the porosity and micro-roughness. This particular feature helps reduce the bacterial adherence on the fixture surfaces after its exposure [33].
One of the application fields in which the use of laser is spreading is hard tissue regeneration (the results presented in Table 1). For many reasons, this field is in search of new technologies and materials which may improve the healing process, but, above all, it may regenerate both hard and soft tissues in cases of severe bone atrophy. For bone regeneration, the used materials must meet specific criteria: compatibility with the receiving organism, not to cause chronic inflammatory reactions; moreover, the biomaterials must have the property of osteoconduction, must provide an adequate environment for the development of cell processes, and at least, it must be osteoinductive. To be considered osteoinductive, a material must form ectopic bones by recruiting the progenitor cells in the implant site and must be able to induce the differentiation of the mesenchymal progenitor cells (MCS) [34,35,36].

Table 1.
Literature review results regarding the LLLT influence on tissue regeneration.
Discussion
Pereira et al. used a low-level gallium-aluminum-arsenide diode laser to observe the effects of bone surrounding endosseous implants on the healing process. The study revealed that the LLLT influence the osseous integration through some modifications made on the bone-implant surface in comparison with the control group has shown no advantages for bone regeneration [50]. On the other hand, Grassi et al. confirm that the use of the low-level intensity laser stimulates the osteogenic proliferation and differentiation, and it also improves the cell adhesion to the implant surface [45]. Other studies have recognized the capacity of LLLT to stimulate the bone formation around the implant and therefore it improves the osseous integration [51,52].
Maluf et al. carried out their research by comparing two groups of mice in order to assess the mechanical resistance and the effects obtained after the use of LLLT. Between the two tested groups, one has received laser irradiation (LLLT) and the other one was the control group. This study concluded that in the group in which low level laser was used, it was harder to remove the implant from the bone than in the group which was not treated with laser. In this case, an engine dynamometer was also used in order to measure the torque necessary to detach the implant from the bone. The result of this study revealed that the group treated with the diode laser presented a resistance of more than 50% for the removal of the implant compared to the other group, which received no radiations. This study recognizes the effect of laser on the improvement of the adhesion between the bone and the implant [53].
Nowadays, one of the greatest defects in replacing bone materials is osteoinduction, since most of them only have osteoconduction. For this reason, Oliveira et al. tested the LLLT and its interaction with biomaterials. The biphasic hydroxyapatite/tricalcic phosphate and the bovine bone are biomaterials which have osteoconduction, however osteoinduction remains their weak point [54,55]. For 13 days, the tested groups were irradiated each 48 hours for 40 seconds per area. After 60 days, another operation was performed for the implant placement. The results after 15 and 45 days from the implant placement revealed great differences between the two groups, the control and the tested ones. The conclusion of this study was that the LLLT stimulates the osseous integration with a high exposure of osteocalcin (OCN) and bone morphogenic protein-2 (BMP-2), which are associated to the osteoblast activity. They also revealed that the necessary force to remove one implant in the group which received laser irradiation may be compared to the force necessary to remove the implant from the autologous bone [42]. This author had also stated these conclusions in another previous research, also by using the same biomaterials, namely bovine bone and biphasic hydroxyapatite/tricalcic phosphate. The low-level laser therapy is considered promising for osteoblast differentiation and growth stimulation. 90 days after the laser treatment, the groups that underwent laser irradiation had an increase of BMP-2 and alkaline phosphatase (ALP) and bone growth was also evidenced. These results show that LLLT influences the osteoconductive properties of these biomaterials by maintaining the bone volume of the graft [41].
Photobiomodulation was also tested by other authors for the irradiation of the implant site in which bovine bone was used for bone replacement. Immediately after surgery, laser therapy (LLLT) was undergone during which seven sessions of radiation were performed every 48 hours. The use of LLLT in these cases led to a higher osteoblast activity, an increased deposition of collagen, with a faster organization of the Harversian system as the regeneration of the cortical bone [43,56,57]. Another study selected the proteins derived from the dental enamel matrix derivate (EMD) to replace an intrabony defect and this treatment was combined with LLLT. The radiation started immediately after the operation. A diode laser 4J/cm2 was used in five days, on each side of the bone defect for five minutes. After 12 months, the results revealed that this combination improved the effect of the EMD on the intrabony defect [58].
Biostimulation with Light-emitting diode (LED) is another alternative way which led to promising results. The use of the blue LED light in the stimulation of the pre-osteoblasts offered similar results to the ones of the infrared lasers [46], thus encouraging the differentiation of osteogenes and inhibiting cell proliferation [59]. The LED has a non-coherent light if compared to the laser LLLT, which releases a coherent light, while the LED is easier to control, safer for our eyes and it has a lower cost. In bone defects in which biomaterials are employed, the use of the LED together with photosensitive substances led to better results for the osteointegration of implants, bone formation around the implants. In terms of time evolution, it can be compared to the autologous bone [60]. Despite the advantages provided by the LED, this method also has some limitations, since it cannot be used in cases in which a high density of power is required in a small area, therefore in this specific case laser is preferred (LLLT) [22].
Other clinical studies assessed the influence of LLLT in posttraumatic bony defects, as a single approach or associated to guided bone regeneration, bone marrow harvesting (in order to induce osteogenesis), synthetic osteogenic proteins grafting [37,38,39,44,61]. The results are promising, so that they state that LLLT has a direct influence on the quality of structured tissue. Still, the in vivo and ex vivo involved studies included small number of subjects in order to conclude on the direct relation and effect between LLLT and osteoregeneration and neovascularization.
In their in vitro research, Mergoni et al. used human osteoblasts through irradiation with laser 915 nm. This study concluded that this wavelength in the proliferation and differentiation of cells does not offer better effects if the group which was not irradiated is compared, but despite this, the use of LLLT encourages the formation of bone nodes (deposition of Ca2+) [47,48]. On the other hand, Jawad et al. concluded that cell proliferation increases when we increase the laser power and when we use a lower power osteoblast differentiation. When a diode laser 940nm is used, the cell proliferation of osteoblasts is lower; when the power of irradiation is of 100mW and when the irradiation with a power of 300mW is used, the osteoblast proliferation increases. The ALP and ONC expressions which influence the osteoblast differentiation were respectively lower with an irradiation of 300mW if compared to the one of 100mW [49]. Barbosa et al. carried out a research in which they compared an infrared laser and a red-light laser [62]. This study confirms that the use of the laser having the wavelength in the red spectrum does not always lead to good results, compared to the laser with infrared light. As for the healing process, it depends not only on the time, but also on the wave length. In association with low laser local treatment, there are other factors which should also be considered: the general health state of the patients, and the factors that could decrease the organisms’ regenerative capacity, such as inflammatory diseases, vicious habits (tobacco, alcohol consumption), chronic medication [63,64].
The same result was obtained by Queiroga et al., Renno et al. who stated that some specific combinations of doses with different wave lengths lead to several results on the different cell lines [47,65]. Therefore, the proliferation of osteosarcoma cells irradiated with an 830nm wavelength did not lead to cell increase. The results were different for the irradiated cells with laser with a wavelength of 670nm (me 5J/cm2) and 780nm (me 1.5 dhe 10J/cm2), in this case the proliferation was notably increased. On the other hand, the cell line of osteoblasts had no cell proliferation for the wave length of 780nm and irradiation (1.5 dhe 10J/cm2), but and an increase was noticed for the wave length of 830nm and irradiation (10J/cm2).
Tani et al. tested three different wave lengths, LED (450nm), red light (635nm) and infra-red (808nm). The aim was to assess the effect of these wave lengths on cell proliferation, differentiation and adhesion of the human mesenchymal stromal pre-osteoblast cells (hMSC). As opposed to the LED irradiation, the red light as well as the infrared light formed depositions of Ca2+, namely bone nodes. The wavelength of 635nm which is in the spectrum of red light for the hMSC cells was able to encourage the osteogenic proliferation, differentiation and adhesion. Although this study shares the use of laser of 635nm wave length as an efficient treatment for bone stimulation and regeneration, it confirms that the doses of irradiation must be verified, since the irradiation with low doses creates the stimulation of cell signals and the irradiation with high doses may lead to the inhibition of biological stimuli [66]. Conversely, Ghidini et al. revealed that the red wave length (645nm) is absorbed by the tissues more in-depth [67]. The results collected in the present paper show that there is still confusion in the protocols to follow during laser bio-stimulation. Some conclusions drawn from the bio-stimulation effects clearly show that this therapy is effective in regeneration and, for this reason, a protocol about the lines and the doses prescribed and recommended by the World Association for Laser Therapy (WALT) is required [68].
Highlights
- ✓
- Low-Level Laser Therapy improves the osseous integration of biomaterials and their properties.
- ✓
- Low-Level Laser Therapy significantly increases cellular adhesion on implant surfaces, having a positive impact on the enduring quality of grafting materials.
Conclusions
The biomodulation or LLLT based on the scientific results shown in this review may be considered as an efficient therapy for hard and soft tissues regeneration. The results show that it notably improves the osteointegration and the properties of biomaterials, if combined. Nowadays, there is still no standard protocol of work approved for the use of laser LLLT in bone regeneration, therefore, the research must continue in this direction since it might lead to promising results. In vivo studies are focused on animal models and there is still not strongly enough evidence regarding human subjects and laser biomodulation. The use of LLLT requires that the users of laser medical devices be prepared both theoretically and regarding specific protocols.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Acknowledgments
For this study, we would like to express our gratitude to the Dipartimento di Medicina Interdisciplinare (D.I.M) of the University of Medicine of Bari "Aldo Moro", Bari, Italy, and to the Dentistry Department of Tirana, Albania, which have formed this International research group.
Acronyms and abbreviations
| LLLT | Low-Level Laser Therapy |
| LASER | Light Amplification by Stimulated Emission of Radiation |
| ATP | Adenosine-Triphosphate |
| OM | oral mucositis |
| PBM | photobiomodulation |
| IL-1b | Interleukin 1b |
| TNF-α | Tumor Necrosis Factor |
| IFN | Interferon |
| OCN | Osteocalcin |
| BMP-2 | Bone Morphogenic Protein 2 |
| EMD | Enamel Matrix Derivate |
| LED | Light-emitting Diode |
| hMSC | Human Mesenchymal Stromal Cells |
| WALT | World Association for Laser Therapy |
References
- Scarano, A.; Lorusso, F.; Inchingolo, F.; Postiglione, F.; Petrini, M. The Effects of Erbium-Doped Yttrium Aluminum Garnet Laser (Er: YAG) Irradiation on Sandblasted and Acid-Etched (SLA) Titanium, an In Vitro Study. Materials (Basel). 2020, 13, 4174. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; Silvestris, F.; Ballini, A.; Inchingolo, F.; Dipalma, G.; Scacco, S.; Tetè, S. Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J Biol Regul Homeost Agents. 2011, 25, 603–614. [Google Scholar]
- Tarullo, A.; Laino, L.; Tarullo, A.; Inchingolo, F.; Flace, P.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Cagiano, R. Use of a diode laser in an excisional biopsy of two spoonlike neoformations on the tongue tip. Acta Biomed. 2011, 82, 63–68. [Google Scholar] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Comparison between traditional surgery, CO2 and Nd:Yag laser treatment for generalized gingival hyperplasia in Sturge-Weber syndrome: a retrospective study. J Investig Clin Dent. 2010, 1, 85–89. [Google Scholar] [CrossRef]
- Ardeleanu, V.; Andronache, L.F.; Gherghiceanu, F.; Paunica, S.; Balalau, C.; Stoian, A.P. Treatment of lipomas and diffuse lipomatosis with NDYAG 1064 NM laser and their impact on the quality of life. J Mind Med Sci. 2020, 7, 16–22. [Google Scholar] [CrossRef]
- De Benedittis, M.; Petruzzi, M.; Pastore, L.; Inchingolo, F.; Serpico, R. Nd:YAG laser for gingivectomy in Sturge-Weber syndrome. J Oral Maxillofac Surg. 2007, 65, 314–316. [Google Scholar] [CrossRef]
- Scarano, A.; Petrini, M.; Inchingolo, F.; Lorusso, F.; Amuso, D. A new technique for the treatment of nasal telangiectasia using atmospheric plasma (voltaic arc dermabrasion): Postoperative pain assessment by thermal infrared imaging. J Cosmet Dermatol. 2020, 19, 2912–2918. [Google Scholar] [CrossRef]
- Marina, C.N.; Mutu, D.E.; Raducu, L.; Scaunasu, R.V.; Jecan, C.R. Reconstruction of the periorbital region defects following excision of basal cell carcinomas. J Clin Invest Surg. 2020, 5, 51–55. [Google Scholar] [CrossRef]
- Ianosi, S.; Neagoe, D.; Branisteanu, D.E.; Popescu, M.; Calina, D.; Zlatian, O.; Docea, A.O.; Marinas, M.C.; Iordache, A.M.; Mitruț, P.; Ianosi, G. Comparative efficacy of oral contraceptive versus local treatment versus intense pulsed light combined with vacuum in endocrine acne in women. J Biol Regul Homeost Agents. 2018, 32, 711–718. [Google Scholar]
- Inchingolo, F.; Tarullo, A.; Cagiano, R.; Resta, G.; Dipalma, G.; Inchingolo, A.M.; Tarullo, A.; Scacco, S.; Marrelli, M.; Corti, L.; Tatullo, M. Successful use of a topical mixture with ozolipoile in the treatment of actinic ulcers. Clin Cosmet Investig Dermatol. 2015, 8, 147–150. [Google Scholar] [CrossRef]
- Indre, M.G.; Sampelean, D.; Taru, V.; Cozma, A.; Sampelean, D.; Milaciu, M.V.; Orasan, O.H. Non-dental oral cavity findings in gastroesophageal reflux disease: a systematic review and metaanalysis. J Mind Med Sci. 2021, 8, 60–70. [Google Scholar] [CrossRef]
- Chiniforush, N.; Pourhajibagher, M.; Parker, S.; Benedicenti, S.; Bahador, A.; Salagean, T.; Bordea, I.R. The Effect of Antimicrobial Photodynamic Therapy Using Chlorophyllin-Phycocyanin Mixture on Enterococcus faecalis: The Influence of Different Light Sources. Appl Sci. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Dalvi, S.; Benedicenti, S.; Sălăgean, T.; Bordea, I.R.; Hanna, R. Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials. Pharmaceutics. 2021, 13, 836. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagnosis Photodyn Ther. 2020, 29, 101611. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers (Basel). 2020, 12, 1949. [Google Scholar] [CrossRef]
- Popa, D.; Bordea, I.R.; Burde, A.V.; Crisan, B.; Campian, R.S.; Constantiniuc, M. Surface modification of zirconia after laser irradiation. Optoelectronics and Advanced Materials – Rapid Communications. 2016, 10, 785–788. [Google Scholar]
- Bordea, I.R.; Lucaciu, P.O.; Crisan, B.; Mirza, C.M.; Popa, D.; Mesaros, A.S.; Pelekanos, S.; Campian, R.S. The influence of chromophore presence in an experimental bleaching gel on laser assisted tooth whitening efficiency. Studia Universitatis Babes-Bolyai Chemia. 2016, 61, 215–223. [Google Scholar]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; Pham, V.H.; Dipalma, G.; Ballini, A. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines. 2020, 8, 115. [Google Scholar] [CrossRef]
- Pawelczyk-Madalińska, M.; Benedicenti, S.; Sălăgean, T.; Bordea, I.R.; Hanna, R. Impact of Adjunctive Diode Laser Application to Non-Surgical Periodontal Therapy on Clinical, Microbiological and Immunological Outcomes in Management of Chronic Periodontitis: A Systematic Review of Human Randomized Controlled Clinical Trials. J Inflamm Res. 2021, 14, 2515–2545. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, S.; Klämpfl, F.; Schmidt, M.; Zeilhofer, H.F. Lasers in Oral and Maxillofacial Surgery; Springer International Publishing: Cham, 2020; pp. 45–57. ISBN 978-3-030-29604-9. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A systematic review and meta-analysis of the effect of low-level laser therapy (LLLT) on chemotherapy-induced oral mucositis in pediatric and young patients. Eur J Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, K.A.M.; Vahdatinia, F.; Jamalpour, M.R.; Vescovi, P.; Fornaini, C.; Merigo, E.; Fekrazad, R. Photobiomodulation in Oral Medicine. Photobiomodul Photomed Laser Surg. 2019, 37, 837–861. [Google Scholar] [CrossRef]
- Vale, F.A.; Moreira, M.S.; de Almeida, F.C.; Ramalho, K.M. Low-level laser therapy in the treatment of recurrent aphthous ulcers: a systematic review. ScientificWorldJournal. 2015, 2015, 150412. [Google Scholar] [CrossRef]
- Honarmand, M.; Farhadmollashahi, L.; Vosoughirahbar, E. Comparing the effect of diode laser against acyclovir cream for the treatment of herpes labialis. J Clin Exp Dent. 2017, 9, e729–e732. [Google Scholar] [CrossRef][Green Version]
- Weber, J.B.; Camilotti, R.S.; Ponte, M.E. Efficacy of laser therapy in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ): a systematic review. Lasers Med Sci. 2016, 31, 1261–1272. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; Barbato, E.; Romeo, U. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte-and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J Clin Med. 2020, 9, 3505. [Google Scholar] [CrossRef]
- Wibawa, A.; Sucharitakul, J.; Dansirikul, R.; Pisarnturakit, P.P.; Bhuridej, P.; Arirachakaran, P.; et al. Low-Level Laser Therapy to the Major Salivary Glands Increases Salivary Flow and MUC5B Protein Secretion in Diabetic Patients with Hyposalivation: A Preliminary Study. Makara J Health Res. 2018, 22, 14–21. [Google Scholar] [CrossRef]
- Falaki, F.; Nejat, A.H.; Dalirsani, Z. The Effect of Low-level Laser Therapy on Trigeminal Neuralgia: A Review of Literature. J Dent Res Dent Clin Dent Prospects. 2014, 8, 1–5. [Google Scholar] [CrossRef]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and Implant Dentistry: Part 1. Photobiomodul Photomed Laser Surg. 2019, 37, 739–765. [Google Scholar] [CrossRef]
- American Academy of Periodontology statement on the efficacy of lasers in the non-surgical treatment of inflammatory periodontal disease. J Periodontol. 2011, 82, 513–514. [CrossRef] [PubMed]
- Popescu, B.; Oașă, I.D.; Bertesteanu, S.V.; Balalau, C.; Manole, F.; Domuta, M.; Oancea, A.L. Strategies to improve activity and results of the head and neck tumor board. J Clin Invest Surg. 2020, 5, 9–12. [Google Scholar] [CrossRef]
- Munerato, M.S.; Biguetti, C.C.; Parra da Silva, R.B.; Rodrigues da Silva, A.C.; Zucon Bacelar, A.C.; Lima da Silva, J.; Rondina Couto, M.C.; Húngaro Duarte, M.A.; Santiago-Junior, J.F.; Bossini, P.S.; Matsumoto, M.A. Inflammatory response and macrophage polarization using different physicochemical biomaterials for oral and maxillofacial reconstruction. Mater Sci Eng C Mater Biol Appl. 2020, 107, 110229. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001, 10 (Suppl 2), S96–101. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Nagata, M.J.; Santinoni, C.S.; Pola, N.M.; de Campos, N.; Messora, M.R.; Bomfim, S.R.; Ervolino, E.; Fucini, S.E.; Faleiros, P.L.; Garcia, V.G.; Bosco, A.F. Bone marrow aspirate combined with low-level laser therapy: a new therapeutic approach to enhance bone healing. J Photochem Photobiol B. 2013, 121, 6–14. [Google Scholar] [CrossRef]
- Garcia, V.G.; Sahyon, A.S.; Longo, M.; Fernandes, L.A.; Gualberto Junior, E.C.; Novaes, V.C.; Ervolino, E.; de Almeida, J.M.; Theodoro, L.H. Effect of LLLT on autogenous bone grafts in the repair of critical size defects in the calvaria of immunosuppressed rats. J Craniomaxillofac Surg. 2014, 42, 1196–1202. [Google Scholar] [CrossRef]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef]
- Cunha, M.J.; Esper, L.A.; Sbrana, M.C.; de Oliveira, P.G.; do Valle, A.L.; de Almeida, A.L. Effect of low-level laser on bone defects treated with bovine or autogenous bone grafts: in vivo study in rat calvaria. Biomed Res Int. 2014, 2014, 104230. [Google Scholar] [CrossRef]
- de Oliveira, G.J.P.L.; Aroni, M.A.T.; Medeiros, M.C.; Marcantonio, E., Jr.; Marcantonio, R.A.C. Effect of low-level laser therapy on the healing of sites grafted with coagulum, deproteinized bovine bone, and biphasic ceramic made of hydroxyapatite and β-tricalcium phosphate. In vivo study in rats. Lasers Surg Med. 2018. [Google Scholar] [CrossRef]
- de Oliveira, G.J.P.L.; Aroni, M.A.T.; Pinotti, F.E.; Marcantonio, E., Jr.; Marcantonio, R.A.C. Low-level laser therapy (LLLT) in sites grafted with osteoconductive bone substitutes improves osseointegration. Lasers Med Sci. 2020, 35, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Gerbi, M.E.; Pinheiro, A.L.; Marzola, C.; Limeira Júnior Fde, A.; Ramalho, L.M.; Ponzi, E.A.; Soares, A.O.; Carvalho, L.C.; Lima, H.V.; Gonçalves, T.O. Assessment of bone repair associated with the use of organic bovine bone and membrane irradiated at 830 nm. Photomed Laser Surg. 2005, 23, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Renno, A.C.; McDonnell, P.A.; Crovace, M.C.; Zanotto, E.D.; Laakso, L. Effect of 830 nm laser phototherapy on osteoblasts grown in vitro on Biosilicate scaffolds. Photomed Laser Surg. 2010, 28, 131–133. [Google Scholar] [CrossRef]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; Silvestris, F.; Ballini, A.; Inchingolo, F.; Dipalma, G.; Scacco, S.; Tetè, S. Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J Biol Regul Homeost Agents. 2011, 25, 603–614. [Google Scholar]
- Pagin, M.T.; de Oliveira, F.A.; Oliveira, R.C.; Sant’Ana, A.C.; de Rezende, M.L.; Greghi, S.L.; Damante, C.A. Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med Sci. 2014, 29, 55–59. [Google Scholar] [CrossRef]
- Queiroga, A.S.; Sousa, F.B.; Araújo, J.M.S.; Santos, S.D.; Sousa, C.D.F.S.; Quintans, T.C.; Almeida, T.P.; Nonaka, C.F.W.; Batista, L.V.; Limeira Junior, F.A. Evaluation of bone repair in the femur of rats submitted to laser therapy in different wavelengths: An image segmentation method of analysis. Laser Methods in Chemistry, Biology, and Medicine. 2008, 18, 1087–1091. [Google Scholar] [CrossRef]
- Mergoni, G.; Vescovi, P.; Belletti, S.; Uggeri, J.; Nammour, S.; and Gatti, R. Effects of 915 nm laser irradiation on human osteoblasts: a preliminary in vitro study. Lasers Med Sci. 2018, 33, 1189–1195. [Google Scholar] [CrossRef]
- Jawad, M.M.; Husein, A.; Azlina, A.; Alam, M.K.; Hassan, R.; Shaari, R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J Biomed Opt. 2013, 18, 128001. [Google Scholar] [CrossRef]
- Pereira, C.L.; Sallum, E.A.; Nociti, F.H., Jr.; Moreira, R.W. The effect of low-intensity laser therapy on bone healing around titanium implants: a histometric study in rabbits. Int J Oral Maxillofac Implants. 2009, 24, 47–51. [Google Scholar]
- Mayer, L.; Gomes, F.V.; Carlsson, L.; Gerhardt-Oliveira, M. Histologic and Resonance Frequency Analysis of Peri-Implant Bone Healing After Low-Level Laser Therapy: An In Vivo Study. Int J Oral Maxillofac Implants. 2015, 30, 1028–1035. [Google Scholar] [CrossRef]
- Rajaei Jafarabadi, M.; Rouhi, G.; Kaka, G.; Sadraie, S.H.; Arum, J. The effects of photobiomodulation and low-amplitude high-frequency vibration on bone healing process: a comparative study. Lasers Med Sci. 2016, 31, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Maluf, A.P.; Maluf, R.P.; Brito Cda, R.; França, F.M.; de Brito, R.B., Jr. Mechanical evaluation of the influence of low-level laser therapy in secondary stability of implants in mice shinbones. Lasers Med Sci. 2010, 25, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Traini, T.; Scarano, A.; Degidi, M.; Perrotti, V.; Piccirilli, M.; Piattelli, A. Maxillary sinus augmentation with Bio-Oss particles: a light, scanning, and transmission electron microscopy study in man. J Biomed Mater Res B Appl Biomater. 2005, 74, 448–457. [Google Scholar] [CrossRef]
- Ursu, R.G.; Luca, C.M.; Luca, A.S.; Toader, E.; Simion, L.; Iancu, L.S. Laboratory Diagnosis for Optimize Therapy of B Hepatitis Virus Infection by Using Biochemical and Molecular Biology Methods. Rev Chim. (Bucharest) 2016, 67, 2614–2617. [Google Scholar]
- Márquez Martínez, M.E.; Pinheiro, A.L.; Ramalho, L.M. Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med Sci. 2008, 23, 313–317. [Google Scholar] [CrossRef]
- Ozcelik, O.; Cenk Haytac, M.; Seydaoglu, G. Enamel matrix derivative and low-level laser therapy in the treatment of intra-bony defects: a randomized placebo-controlled clinical trial. J Clin Periodontol. 2008, 35, 147–156. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, Y.; Zhou, X.; Yang, Y.; Wang, Y. Irradiation by blue light-emitting diode enhances osteogenic differentiation in gingival mesenchymal stem cells in vitro. Lasers Med Sci. 2019, 34, 1473–1481. [Google Scholar] [CrossRef]
- Faria, P.E.; Felipucci, D.N.; Simioni, A.R.; Primo, F.L.; Tedesco, A.C.; Salata, L.A. Effects of Photodynamic Process (PDP) in Implant Osseointegration: A Histologic and Histometric Study in Dogs. Clin Implant Dent Relat Res. 2015, 17, 879–890. [Google Scholar] [CrossRef]
- Gerbi, M.E.M.M.; Miranda, J.M.; Arruda, J.A.A.; Moreno, L.M.M.; Carneiro, V.S.M.; Brasilino, N.C.; Menezes, R.F.; Brugnera Junior, A.; Pinheiro, A.L.B. Photobiomodulation Therapy in Bone Repair Associated with Bone Morphogenetic Proteins and Guided Bone Regeneration: A Histomorphometric Study. Photomed Laser Surg. 2018, 36, 581–588. [Google Scholar] [CrossRef]
- Barbosa, D.; de Souza, R.A.; Xavier, M.; da Silva, F.F.; Arisawa, E.A.; Villaverde, A.G. Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci. 2013, 28, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Motofei, I.G. Biology of cancer; from cellular and molecular mechanisms to developmental processes and adaptation. Semin Cancer Biol 2021, S1044-579X(21)00253-4. [Google Scholar] [CrossRef]
- Maierean, A.; Ciumarnean, L.; Alexescu, T.G.; Domokos, B.; Rajnoveanu, R.; Arghir, O.; Todea, D.; Buzoianu, A.D.; Dogaru, G.; Bordea, R.I. Complementary therapeutic approaches in asthma. Balneo Research Journal. 2019, 10, 204–212. [Google Scholar] [CrossRef]
- Renno, A.C.; McDonnell, P.A.; Parizotto, N.A.; Laakso, E.L. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int J Mol Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, G.; Setti, G.; Sala, A.; Giovannacci, I.; Veneri, F.; Greco Lucchina, A.; Sala, R.; Vescovi, P. Absorption and diffusion of a 645 nm diode laser beam in the bone. An ex vivo study. J Biol Regul Homeost Agents. 2019, 33 (Suppl. 2), 137–141. [Google Scholar]
- Bjordal, J.M. Low level laser therapy (LLLT) and World Association for Laser Therapy (WALT) dosage recommendations. Photomed Laser Surg. 2012, 30, 61–62. [Google Scholar] [CrossRef]
© 2022 by the author. 2022 Anida-Maria Babtan, Aranka Ilea, Claudia Nicoleta Feurdean, Sabino Ceci, Berate Pula, Sebastian Candrea, Daniela Azzollini, Fabio Piras, Luigi Curatoli, Alberto Corriero, Assunta Patano, Francesco Valente, Maria Elena Maggiore, Antonio Mancini, Delia Giovanniello, Ludovica Nucci, Rossella Elia, Adina Sirbu, Nausica Bianca Petrescu, Codruta Mirica, Andrea Galderisi, Filippo Cardarelli