Abstract
Gastrointestinal metastases from cutaneous melanoma are rare and usually asymptomatic, with most patients not being clinically diagnosed throughout their lifetime. We report a case of how melanoma may metastasize insidiously in the small bowel. Unexplained iron deficiency anemia was assumed to be the result of underlying gastrointestinal bleeding. Therefore, the diagnosis of jejunal metastasis from cutaneous melanoma was suggested based on imaging findings and made through the histopathological examination. According to the international guidelines, the patient underwent the complete excision of the primary tumor and therapeutic lymph node dissection. Furthermore, an adjuvant treatment was required to reduce the risk of recurrence. Both immunotherapy and surgical therapy have been shown to be effective, providing long-term survival in this case.
Introduction
Intestinal melanoma is a rare form of cancer that can be either primary or metastatic. Primary small bowel melanoma is an extremely rare entity and, in most cases, the diagnosis is difficult to make [1].
However, researchers believe that all melanomas of the small bowel are metastases from unknown primary cutaneous melanoma, and consequently, primary melanomas of the small intestine are generally not separate entities [2].
Cutaneous, anal, or ocular melanomas can metastasize in different sites of the digestive tract and the most common one is the small bowel. Therefore, metastatic melanoma from the small intestine is very common and it can occur in 35% to 70% of the patients [3,4,5,6].
In most patients, the disease is undetectable in the early stages, and diagnosis being made late, when complications have already occurred. In some cases, the diagnosis is missed throughout lifetime [7,8].
This article reports the case of small bowel metastatic melanoma, where surgery and immunotherapy were effective treatments, offering the best chance for long-term survival. A brief discussion of the pertinent literature data was also made.
Case Presentation
A 52-year-old male patient was evaluated in December 2020 for severe anemia of unknown etiology. His medical background revealed an oncological history of left temporal ulcerated cutaneous melanoma dating from 2018, which was removed by means of wide surgical excision with sentinel lymph node biopsy (SLNB). The histopathology revealed a stage IIIC melanoma. The tumor infiltrated the subcutaneous fat (Clark's Level V), with ulceration, positive sentinel lymph node (pT4b, pN1 (SNL)), BRAF negative.
After the surgical intervention, taking into consideration the patient's request, it was decided to keep the patient under clinical observation. Six months later, on the imaging evaluation, the PET-CT revealed multiple hepatic, skin and lung metastases.
Thus, the tumor board decided to initiate Nivolumab (1 mg/kg) + Ipilimumab (3 mg/kg) every three weeks for 4 administrations and then Nivolumab 240 mg twice a month, as a maintenance treatment.
In December 2020, the laboratory examinations revealed that his hemoglobin level was 6.6 g/dL. Additional tests were performed to diagnose the specific cause of the patient's anemia. The lab values indicate iron deficiency anemia. We further examined the relationships between iron deficiency anemia and positive immunochemical fecal occult blood test (FOBT).
Neither upper nor lower gastrointestinal endoscopy revealed an actively bleeding source. Subsequently, a CT enterography was performed. The results showed a diffuse parietal thickening at the jejunal loops.
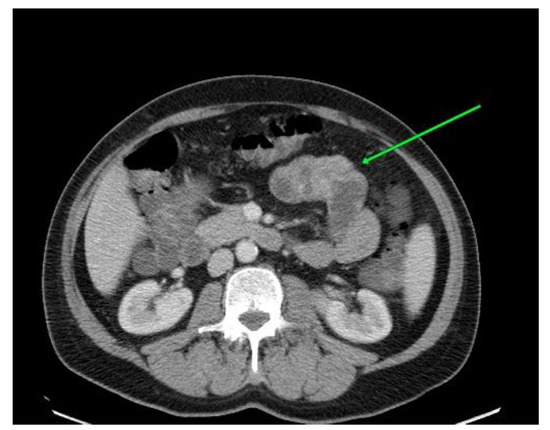
Figure 1.
Computed tomography revealed parietal thickening located in the middle and distal jejunum.
To complete the diagnosis, the patient underwent an entero-MRI which showed an intraluminal jejunal tumor and concentric wall thickening of the middle and distal jejunal loops.
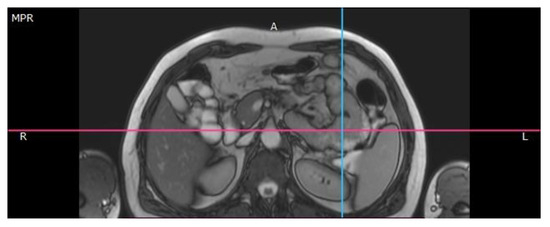
Figure 2.
Magnetic resonance imaging reveals jejunal tumor.
The 18F-fluorodeoxyglucose positron emission tomography revealed areas of increased uptake of fludeoxyglucose near the angle of Treitz. The lesion was poorly described due to enteric intussusception.
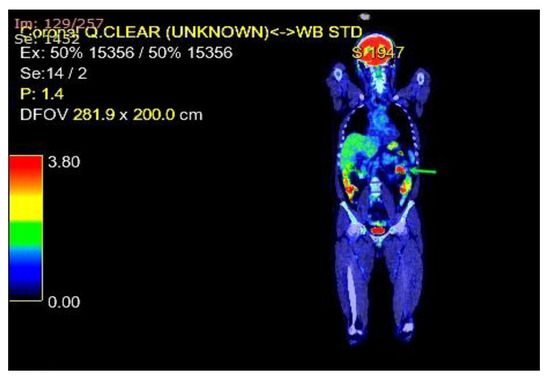
Figure 3.
A whole-body PET imaging with fluorodeoxyglucose revealed the intussusception of the small bowel caused by the jejunal metastasis.
Therefore, surgery with a biopsy of the lesion was mandatory to make an accurate diagnosis. The patient underwent successful laparotomy and extensive jejunal resection with end-to-end anastomosis and regional mesenteric lymphadenectomy. The recovery was successful after the major surgery without any complications.
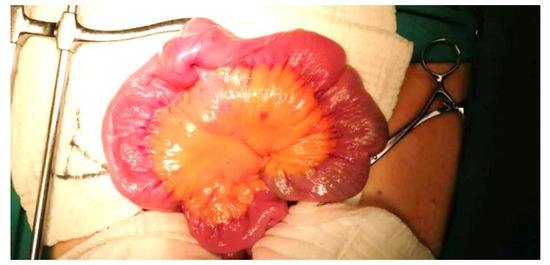
Figure 4.
Intraoperative imaging of multiple jejunal masses.
On the histopathological examination, the resected jejunal segment showed mixed epithelioid and spindle cell melanoma, revealing the diagnosis of metastatic malignant melanoma.
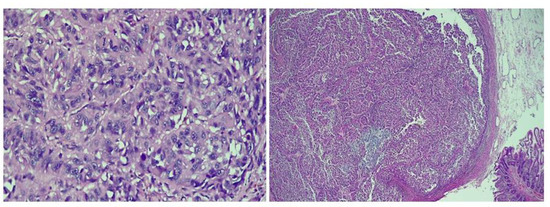
Figure 5.
The histopathological appearance of the jejunum [hematoxylin and eosin (H&E); 200×].
Considering these results, and the important clinical benefit of Nivolumab, the tumor board decided to continue the treatment with checkpoint inhibitors.
In May 2021, three months after the beginning of the treatment, the CT scan showed a stable disease, confirming that the combination therapy with Nivolumab and Ipilimumab, followed by Nivolumab for maintenance, can provide long-term benefits.
Discussion
Improvement in the management of metastatic melanoma has led to improved overall survival, therefore, we experience a substantially increased number of uncommon metastatic sites. The melanoma is the tumor that metastasizes most frequently to the gastrointestinal tract, followed by breast carcinoma and lung carcinoma.
The small intestine, especially the jejunum, is the most common site of gastrointestinal melanoma metastases, followed by the large intestine and the stomach [9].
The most common melanoma metastases are placed at the level of gastrointestinal tract (20-60%), brain (10- 40%), lung (18-40%), liver (10-20%) and bone (11-17%) [10,11,12,13,14,15,16,17].
Up to 2021, a number of 34 cases of jejunal metastases have been reported. There is a clear predisposition for the occurrence of intussusception upon diagnosis [18,19,20,21,22,23,24]. Less often, the patient is diagnosed with small bowel perforation [25,26,27,28,29], obstruction [30] occult gastrointestinal bleeding and anemia [31,32,33,34,35], or abdominal pain [36].
Three cases reported a primary malignant melanoma of the gallbladder with multiple gastrointestinal metastases including the jejunum [37,38,39], and one case presented jejunoileal metastases of malignant melanoma of the choroid [40].
The data support the idea that melanomas of the extremities are more likely to spread to the gastro-intestinal tract, more often than head and neck melanomas [41].
Most patients with metastatic intestinal melanoma are clinically non-diagnosed throughout lifetime. However, the autopsy reports described small bowel metastases in approximately 60% of the patients with melanoma [42].
In cancer patients, anemia should always be investigated to rule out intestinal metastases. This is not true only for melanoma patients, but also for patients with other primary tumor types [43,44].
However, despite the tremendous developments in the imaging techniques, the early diagnosis of intestinal melanomas remains a true challenge.
Traditionally, the tumor assessment by means of computed tomography is useful, but it may present limited sensitivity [45,46].
Therefore, modern imaging techniques – including magnetic resonance imaging, positron emission tomography, and capsule or double-balloon endoscopy [47,48] enhance the diagnostic performance in the detection of melanoma metastases from the small intestine.
In our case, the histopathological results confirmed the clinical suspicion of metastatic intestinal melanoma. The technique of extensive jejunal resection was discussed elsewhere [49,50].
Upon the diagnosis in 2018, the patient presented with locally advanced melanoma and positive sentinel lymph nodes pT4b, pN1, stage IIIC of disease.
According to international guidelines, patients with stage IIIC melanoma should undergo the complete excision of the primary tumor and therapeutic lymph node dissection [51]. Furthermore, adjuvant treatment is required to reduce the risk of recurrence. Unfortunately, in our country, Interferon was the only reimbursed treatment in the adjuvant therapy of advanced melanoma. After the efficacy and toxicity of this treatment were discussed with the patient, he decided to remain under observation.
The landmark phase 3 CheckMate 067 trial highlights the clinical benefit of Nivolumab and Ipilimumab in advanced and metastatic melanoma [52].
The sustained benefits demonstrated in this study accelerated in 2015 the approval of the combination of Nivolumab and Ipilimumab for the frontline therapy in this cancer site.
The 6.5-year outcomes from the global Checkmate 067 trial demonstrated long-term survival, with 81% of the patients being alive, and 27.6 months of median treatment- free in the Nivolumab plus Ipilimumab arm [53].
At the end of 2020, after the metastases were found, the patient initiated the treatment with Nivolumab and Ipilimumab, and currently the disease is under control after 30 months of immunotherapy.
When maintenance therapies are stopped
International guidelines recommend continuing maintenance treatment with nivolumab until disease progression or unacceptable toxicity. However, some studies consider two years of maintenance therapy sufficient, provided that response, either partial or complete, is achieved [54].
In our case, the patient did not experience any immune- related toxicities, and we chose the continue the maintenance with nivolumab with the intent of achieving long-term control of the disease.
Highlights
- ✓ The small intestine, especially the jejunum, is the most common site of gastrointestinal melanoma metastases.
- ✓ The diagnosis of intestinal metastases can be missed throughout lifetime, and it is essential to focus on the clinical examination, and any abnormal blood loss should be further evaluated.
- ✓ The development of immune checkpoint inhibitors has changed the way melanoma is treated, and the association of Nivolumab and Ipilimumab is one of the most effective options for advanced and metastatic melanoma.
Conclusions
In conclusion, it seems that melanoma is more than a cutaneous cancer.
Melanoma can develop anywhere on the body and decision making in this cancer site should always be made in multidisciplinary teams. Moreover, it is essential to focus on clinical examination, and any abnormal blood loss to be further evaluated. If diagnosed in the early stages, melanoma can be successfully treated with surgery alone. But stage III melanoma has an increased risk of loco- regional recurrence and distant metastases. In these cases, the benefit of adjuvant immunotherapy is certain. However, even if patients have not had the opportunity of adjuvant treatment, combination therapy in the metastatic setting is also a valid and effective alternative.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Gill, S.S.; Heuman, D.M.; Mihas, A.A. Small Intestinal Neoplasms. J. Clin. Gastroenterol. 2001, 33, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Albahra, M.; Nzeako, U.C.; Sobin, L.H. Malignant melanomas in the small intestine: a study of 103 patients. . 1996, 91, 1001–6. [Google Scholar]
- Crippa, S.; Bovo, G.; Romano, F.; Mussi, C.; Uggeri, F. Melanoma metastatic to the gallbladder and small bowel: report of a case and review of the literature. Melanoma Res. 2004, 14, 427–430. [Google Scholar] [CrossRef]
- Patel, J.; Didolkar, M.; Pickren, J.; Moore, R. Metastatic pattern of malignant melanoma. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.; Bataille, V.; Krivokapic, Z. Melanoma of the small intestine. Lancet Oncol. 2009, 10, 516–521. [Google Scholar] [CrossRef] [PubMed]
- A Kotteas, E.; Adamopoulos, A.; Drogitis, P.D.; Zalonis, A.; Giannopoulos, K.V.; Karapanagiotou, E.M.; Saif, M.W.; Syrigos, K.N. Gastrointestinal bleeding as initial presentation of melanoma of unknown primary origin: report of a case and review of the literature. . 2009, 23, 487–9. [Google Scholar]
- Bender, G.N.; Maglinte, D.D.T.; McLarney, J.H.; Rex, D.; Kelvin, F.M. Malignant melanoma: Patterns of metastasis to the small bowel, reliability of imaging studies, and clinical relevance. Am. J. Gastroenterol. 2001, 96, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Retsas, S.; Christofyllakis, C. Melanoma involving the gastrointestinal tract. Anticancer Res. 2001, 21, 1503–7. [Google Scholar]
- Bento, L.H.; Minata, M.K.; Batista, C.P.; Martins, B.d.C.; Tolentino, L.H.L.; Scomparim, R.C.; Kawaguti, F.S.; de Oliveira, C.C.G.; de Lima, M.S.; Geiger, S.N.; et al. Clinical and endoscopic aspects of metastases to the gastrointestinal tract. Endoscopy 2019, 51, 646–652. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro-Oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef]
- Suceveanu, A.-I.; Stoian, A. Interferon-free therapy is not a trigger for hepatocellular carcinoma in patients with chronic infection with hepatitis C virus. Farmacia 2018, 66, 904–908. [Google Scholar] [CrossRef]
- Washington, K.; McDonagh, D. Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol. 1995, 8, 427–33. [Google Scholar] [PubMed]
- Orlov-Slavu, C.; Parosanu, A.; Olaru, M.; Serban, D.; Paunica, I.; Nitipir, C. How opportune is multigene testing in metastatic colorectal cancer? A review. J. Mind Med Sci. 2021, 8, 215–220. [Google Scholar] [CrossRef]
- Tarini, E.Z.; Özardali, H.I. Immunohistochemical expression of progesterone receptor and C-erb-B2 in cervical squamous cell carcinoma and epithelial dysplasia. J. Clin. Investig. Surg. 2021, 6, 43–47. [Google Scholar] [CrossRef]
- Petersen, R.P.; Hanish, S.I.; Haney, J.C.; Miller, C.C.; Burfeind, W.R.; Tyler, D.S.; Seigler, H.F.; Wolfe, W.; D’amico, T.A.; Harpole, D.H. Improved survival with pulmonary metastasectomy: An analysis of 1720 patients with pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 2007, 133, 104–110.e2. [Google Scholar] [CrossRef] [PubMed]
- Rose DM, Essner R, Hughes TM, Tang PC, Bilchik A, Wanek LA, Thompson JF, Morton DL. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001, 136, 950–955. [Google Scholar] [CrossRef]
- Gómez-León, N.; Pacheco-Barcia, V.; Ballesteros, A.I.; Fraga, J.; Colomer, R.; Friera, A. Skeletal muscle and solitary bone metastases from malignant melanoma: multimodality imaging and oncological outcome. Melanoma Res. 2018, 28, 562–570. [Google Scholar] [CrossRef]
- Đokić, M.; Badovinac, D.; Petrič, M.; Trotovšek, B. An unusual presentation of metastatic malignant melanoma causing jejuno-jejunal intussusception: a case report. J. Med Case Rep. 2018, 12, 337. [Google Scholar] [CrossRef]
- Mucci, T.; Long, W.; Witkiewicz, A.; Mastrangelo, M.J.; Rosato, E.L.; Berger, A.C. Metastatic Melanoma Causing Jejunal Intussusception. J. Gastrointest. Surg. 2007, 11, 1755–1757. [Google Scholar] [CrossRef]
- Butt, H.W.; Soin, S.; Ali, F.; Wojtkowski, A. Jejunojejunal intussusception secondary to metastatic uveal melanoma after 11 years of remission. BMJ Case Rep. 2019, 12, e229535. [Google Scholar] [CrossRef]
- Resta, G. Jejuno-jejunal invagination due to intestinal melanoma. World J. Gastroenterol. 2007, 13, 310–2. [Google Scholar] [CrossRef]
- Harvey, K.P.; Lin, Y.-H.A.; Albert, M.R. Laparoscopic Resection of Metastatic Mucosal Melanoma Causing Jejunal Intussusception. Surg. Laparosc. Endosc. Percutaneous Tech. 2010, 20, e66–e68. [Google Scholar] [CrossRef]
- Suárez, A.G.; Ganfornina, F.S.; Ruiz, J.L.; Romero, F.B. Intususcepción yeyunal por metástasis de melanoma cutáneo. Cirugia Espanola 2008, 84, 174–175. [Google Scholar] [CrossRef]
- Muiños-Ruano, L.; Llaneza-Folgueras, A.; Rizzo-Ramos, A.; Menéndez-Dizy, C. Invaginación intestinal en el adulto secundaria a metástasis de melanoma cutáneo. Actas Dermo-Sifiliograficas 2012, 103, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Ozdemir, S.; Gecim, E.; Erden, E.; Icli, F. Intestinal malignant melanoma presenting with small bowel invagination: A case report. Turk. J. Gastroenterol. 2010, 21, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Bilello, J.F.; Peterson, W.M. Retrograde Jejunojejunal Intussusception Secondary to Metastatic Melanoma. Mayo Clin. Proc. 2005, 80, 1098. [Google Scholar] [CrossRef]
- Strobel, K. Dünndarminvagination bei intestinal metastasierendem malignen Melanom [Small intestine invagination in metastatic intestinal malignant melanoma]. Rofo-Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Bild. Verfahr. 2001, 173, 768–769. [Google Scholar] [CrossRef] [PubMed]
- H, D.U.S.; Irene, T.; Gj, V.E.; Jclm, V.R.; Bac, S.H.; Mattijs, D.V. Small bowel perforation caused by advanced melanoma. Tumori. 2014, 100, 140e–3e. [Google Scholar] [CrossRef]
- Alwhouhayb, M.; Mathur, P.; Al Bayaty, M. Metastatic melanoma presenting as a perforated small bowel. Turk J Gastroenterol. 2006, 17, 223–5. [Google Scholar]
- Khaleghian, R. The Small Bowel Enema in the Management of Small Bowel Obstruction. Australas. Radiol. 1983, 27, 154–159. [Google Scholar] [CrossRef]
- Pirisi, M.; Bal, S.K.; Czarnowski, C. A woman with anemia and a small-bowel obstruction. Can. Med Assoc. J. 2004, 170, 788–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quentin, V.; Porneuf, M.; Girardot, P.; Le Bris, M.; Nouel, O. Mélanome malin révelé par des métastases jéjunales hémorragiques [Malignant melanoma first noticed because of hemorrhagic jejunal metastasis]. Gastroenterol Clin Biol. 2009, 33, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Félix SA, Escobedo Palau JA, Marigil Gómez MA, Ducons García J. Metástasis intestinal de melanoma como causa de anemia de origen incierto [Intestinal metastasis of melanoma as the cause of anemia of uncertain origin]. Rev Esp Enferm Dig. 1992, 82, 205–206. [Google Scholar]
- Wulf V, Schröder HJ. Dünndarmmetastase eines malignen Melanoms als seltene Ursache einer intestinalen Blutung [Metastasis to the small intestine of malignant melanoma as a rare cause of intestinal hemorrhage]. Zentralbl Chir. 1994, 119, 515–516. [Google Scholar]
- Wong, L.S.; Boughli, I.; Odogwu, S.; Roberts, P.N. Metastatic melanoma of the small bowel as a cause of occult intestinal bleeding. J R Coll Surg Edinb. 1999, 44, 341–3. [Google Scholar]
- Datner, E.M.; English, J.C., 3rd. Abdominal Pain in a Patient with Melanoma. Dermatol. Surg. 1998, 24, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-K.; Su, F.; Ma, W.-J.; Hu, H.-J.; Yang, Q.; Liu, F.; Li, Q.-S.; Li, F.-Y. Primary malignant melanoma of the gallbladder with multiple metastases. Medicine 2017, 96, e8793–e8793. [Google Scholar] [CrossRef]
- Hatanaka, N.; Miyata, M.; Kamiike, W.; Okumura, K.; Hashimoto, T.; Yamaguchi, T.; Kishino, Y.; Sakurai, M.; Matsuda, H. Radical resection of primary malignant melanoma of the gallbladder with multiple metastases: Report of a case. Surg. Today 1993, 23, 1023–1026. [Google Scholar] [CrossRef]
- Guerini A, Gottardi D, Galassi A, Armani A, Biondani L, Candiani V, Venza E. Malignant melanoma of the gallbladder. Case report and review of the literature. Arch Anat Cytol Pathol. 1990, 38, 168–170. [Google Scholar]
- Chagnon AM, Moro MA, Blanchard J, Borelli JP, Gueyffier C. A propos d'un cas de métastases duodénales et jéjuno-iléales d'un mélanome malin de la choroïde [A case of duodenal and jejunoileal metastases of malignant melanoma of the choroid]. Bull Soc Ophtalmol Fr. 1983, 83, 181–184. [Google Scholar]
- Asad-Ur-Rahman, F.; Abbass, A.; Majeed, U.; Navaneethan, U. Melanoma Metastasizing to the Small Intestine: A Case Report Illustrating Symptomatic and Asymptomatic Involvement. Cureus 2016, 8, e608. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, S.; Cazan, A.-M.; Rogozea, L.; Grigorescu, D.O. Original targeted therapy for the management of the burnout syndrome in nurses: an innovative approach and a new opportunity in the context of predictive, preventive and personalized medicine. EPMA J. 2020, 11, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Smarandache, A.; Omer, I.; Ciuhu, A.-N.; Rahnea-Nita, R.-A.; Grigorean, V.-T.; Andronache, L.; Stoian, A.-R. How does a medical team in the Oncology Department react to the Covid-19 pandemic? J. Mind Med Sci. 2021, 8, 286–291. [Google Scholar] [CrossRef]
- Alecu L, Tulin A, Ursut B, Ursut B, Oproiu A, Obrocea F, Ionescu M. Tumoră gastrointestinală stromală cu localizare hepatică primară unică--caz clinic [Gastrointestinal stromal tumor with primary hepatic unique location--clinical case]. Chirurgia (Bucur). 2011, 106, 677–681. [Google Scholar]
- Curcio, C.M.; Feinstein, R.S.; Humphrey, R.L.; Jones, B.; Siegelman, S.S. Computed Tomography of Entero-Enteric Intussusception. J. Comput. Assist. Tomogr. 1982, 6, 969–974. [Google Scholar] [CrossRef]
- Knox, A.M.; Donovan, D.A.; Wilkinson, M.R. The CT Appearances of Jejunojejunal Intussusception. Australas. Radiol. 1990, 34, 264–265. [Google Scholar] [CrossRef]
- Atiq, O.; Khan, A.S.; A Abrams, G. Metastatic amelanotic melanoma of the jejunum diagnosed on capsule endoscopy. Gastroenterol Hepatol (N Y). 2012, 8, 691–3. [Google Scholar]
- Siao, D.; Chang, M.; George, P.; Bharadhwaj, G. Melanoma Metastatic to the Jejunum Diagnosed by Double-Balloon Enteroscopy. ACG Case Rep. J. 2019, 6, e00109. [Google Scholar] [CrossRef]
- Alecu, L.; Tulin, A.; Enciu, O.; Bărbulescu, M.; Ursuţ, B.; Obrocea, F. Gastrointestinal Stromal Tumors - Diagnosis and Surgical Treatment. Chirurgia (Bucur). 2015, 110, 525–9. [Google Scholar]
- Dumitriu, B.; Valcea, S.; Andrei, G.; Beuran, M. Evaluation of anemia as a postoperative risk factor in the evolution of patients with gastric resection for malignancies. J. Clin. Investig. Surg. 2021, 6, 136–140. [Google Scholar] [CrossRef]
- Kudchadkar, R.R.; Michielin, O.; van Akkooi, A.C.J. Practice-Changing Developments in Stage III Melanoma: Surgery, Adjuvant Targeted Therapy, and Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39 (Suppl. 15), 9506–9506. [Google Scholar]
- Jansen, Y.; Rozeman, E.; Mason, R.; Goldinger, S.; Foppen, M.G.; Hoejberg, L.; Schmidt, H.; van Thienen, J.; Haanen, J.; Tiainen, L.; et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann. Oncol. 2019, 30, 1154–1161. [Google Scholar] [CrossRef]
© 2022 by the author. 2022 Cornelia Nitipir, Andreea Parosanu, Cristina Orlov-Slavu, Cătălin Piriianu, Radu Vrabie, Iulian Slavu, Valentin Calu.