Abstract
Giant pancreatic pseudocysts are considered those cysts whose dimensions exceed 10 cm and are sporadically reported in the literature. Although there are multiple treatment modalities, there are currently no treatment guidelines or consensus on the best therapeutic approach for giant pancreatic pseudocysts. We report the case of a 32-year-old male patient with a giant pancreatic pseudocyst after an episode of acute pancreatitis, which was treated by internal surgical drainage through cyst-jejunal anastomosis. This surgical procedure was followed by the formation of a retroperitoneal abscess which was resolved by ultrasound-guided drainage. The subsequent evolution of the patient was favorable, without other complications. Given their complex anatomical relations, the treatment of giant pseudocysts requires strategies adapted to the local conditions. The optimal choice of the operative time and of the therapeutic strategy is based on clinical considerations and the effectiveness of the method used can be assessed by a long-term follow-up.
Introduction
The pancreatic pseudocyst is a fluid peripancreatic collection padded by a wall consisting of fibrous and granulation tissue with no epithelial lining, resulting from the disruption of the pancreatic ducts. Most often, these ductal disruptions occur after an inflammatory process of the pancreas or after trauma. Communication with the pancreatic ducts may be persistent or may be interrupted by local inflammatory processes [1,2].
Pseudocysts over 10 cm in diameter are considered "giants" and are rarely reported. Their approach requires adapted strategies given their complex anatomical relations [3,4,5].
There is currently no classification nor guidelines for the treatment of pancreatic pseudocysts that allow the optimal choice of the operative time and of the therapeutic strategy, so that their management is based on clinical considerations and the experience of the surgeons [6,7,8].
Case Presentation
A 32-year-old male with a history of heavy alcohol use is hospitalized for pain in the left hypochondrium and left abdominal flank, nausea and vomiting. These symptoms occurred at an interval of 8 weeks after an episode of acute pancreatitis and worsened progressively.
The physical examination reveals tenderness on palpation and a bulky tumor in the left upper abdominal quadrant. The patient had no fever and the results of the laboratory tests were within normal limits.
The abdominal ultrasound reveals a large homogeneous cystic lesion in the left hypochondrium, well delimited, with thin walls located anteriorly to the body and tail of the pancreas, and a polyp in the infundibular region of the gallbladder, measuring 1.07 cm (Figure 1 and Figure 2).
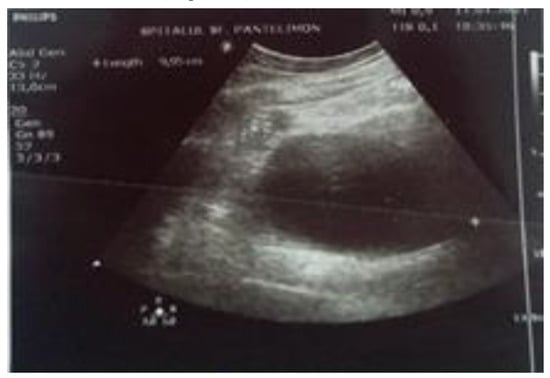
Figure 1.
The ultrasound appearance of the pseudocyst.
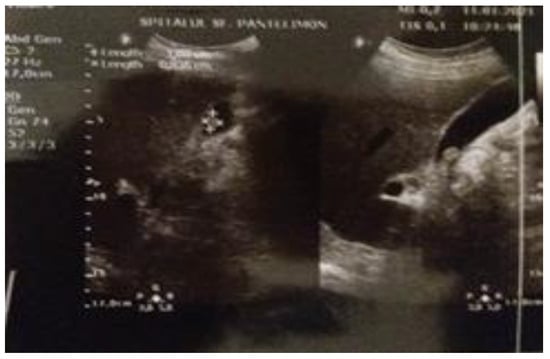
Figure 2.
The ultrasound image of the gallbladder polyp.
A contrast enhanced CT scan of the abdomen showed a bulky cystic formation at the level of the body and the tail of the pancreas, with homogeneous fluid content that extends caudally to the flank and left iliac fossa, measuring 60/120/ 220mm (Figure 3).
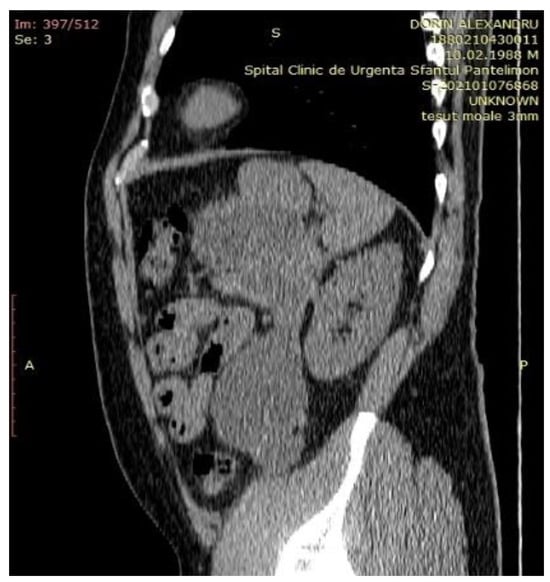
Figure 3.
A CT scan showing a large pancreatic pseudocyst in the lesser sac extended inferiorly to the left iliac fossa.
Surgery is decided upon and the pancreatic pseudocyst is opened at the level of the tail of the pancreas, crossing an avascular area of the transverse mesocolon. After emptying the pseudocyst content through internal drainage by a Roux- en-Y cyst-jejunal anastomosis, cholecystectomy is also performed (Figure 4).
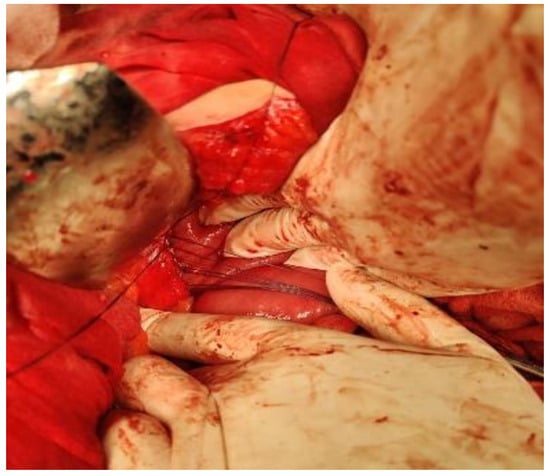
Figure 4.
Cyst-jejunal anastomosis.
The histopathological examination of the gallbladder indicated hypertrophic mucosa and foci of adenomyosis. The fragment withdrawn intraoperatively from the pseudocyst wall was described histopathologically as diffuse fibrosis, vascular congestion and mixed inflammatory infiltrate.
The postoperative evolution was favorable, with uneventful discharge on the 7th postoperative day, the patient being subsequently followed up in the outpatient department. After 21 days postoperatively, the patient is hospitalized for fever of 38.5ºC, pain in the left abdominal flank and altered general condition. The physical examination reveals pain in the left iliac fossa, where a tumor formation is perceived upon deep palpation.
The laboratory test results reveal leukocytosis levels of 21,300/µl and a C-reactive protein level of 293 mg/ l. The abdominal ultrasound reveals a non-homogeneous collection of (14.5/3 cm), located anteriorly to the left psoas muscle (Figure 5).
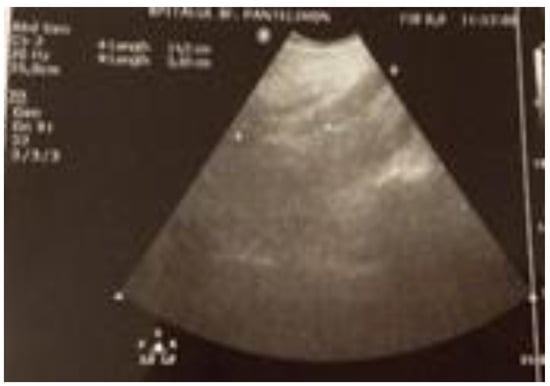
Figure 5.
The ultrasound image of the retroperitoneal abscess.
The CT examination shows a large collection located anteriorly to the left psoas muscle, with a non-homogeneous appearance containing gas (Figure 6).
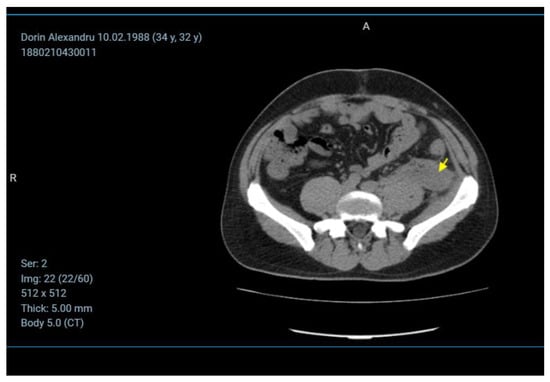
Figure 6.
The CT image of the retroperitoneal abscess.
Under ultrasound guidance, a 12 Fr tube is inserted into the cavity of the collection and the fetid pus is evacuated. The tube is maintained until the drainage becomes serous, and the ultrasound check confirms the disappearance of the retroperitoneal collection (Figure 7 and Figure 8).
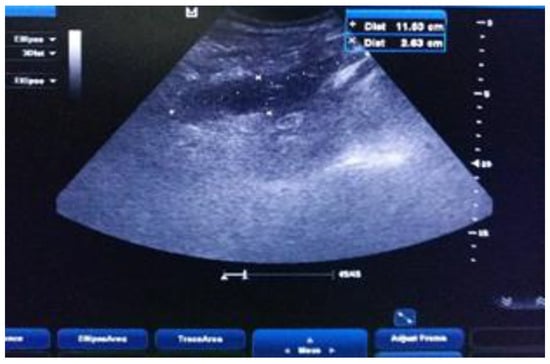
Figure 7.
The ultrasound-guided drainage of the abscess.
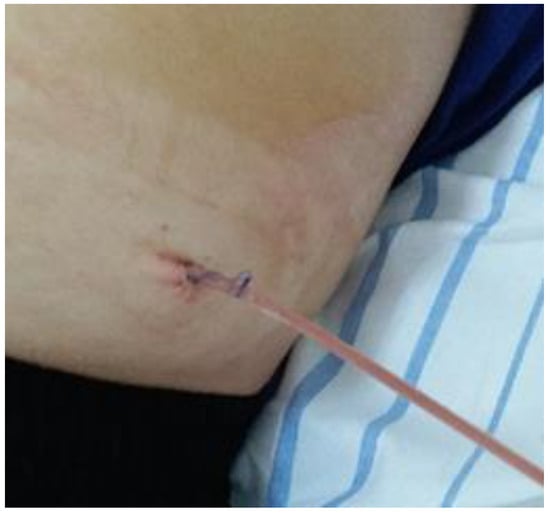
Figure 8.
The drainage of the retroperitoneal abscess.
The patient is discharged on the 5th postoperative day, without fever, with laboratory tests within normal limits. Subsequently, he is followed up and evaluated in the outpatient department. Repeated evaluations confirm the favorable evolution until up to 3 months postoperatively.
Discussion
Epidemiology
The etiology of pancreatic pseudocyst is closely related to the causes which produce the disruption of the pancreatic ducts. The causes that lead to the formation of pseudocysts are chronic and acute pancreatitis or pancreatic trauma. Almost 70% of the pancreatic pseudocysts are associated with alcohol consumption and pancreatitis induced by it [1,2,3].
Alcohol abuse is the leading cause of acute and chronic pancreatitis, hence the different incidences of pancreatic pseudocysts reported worldwide. Pseudocysts associated with alcohol-induced pancreatitis amount to 70% in the USA and South Africa, while in France they represent 94% of the cases. Chronic pancreatitis as a result of alcoholism complicated with pseudocysts amount to 71% in the UK and 85% in Finland [4,5,6].
Gallstones-induced pancreatitis is the cause of 6% of the pseudocysts, while closed abdominal trauma or pancreatic lesions during surgery, such as gastric surgery, cause about 6% of the pseudocysts [7,8].
Other causes of acute pancreatitis leading to acute pseudocysts are ERCP and pancreatic cancer [9,10].
Diagnosis
- Symptoms
The clinical manifestations of pseudocysts cover a very wide spectrum. Recent episodes of acute pancreatitis, closed abdominal trauma, pancreatic surgery, or chronic ethanol use associated with chronic pancreatitis may be identified in the patients' histories [11].
Small pseudocysts are usually asymptomatic. Symptoms are caused by complications associated with pseudocysts. Abdominal pain is caused by the expansion of the pseudocyst. Symptoms like vomiting, early satiety or jaundice could also occur. Gastrointestinal bleeding is caused by vascular occlusion/ thrombosis or arterial pseudoaneurysm rupture into the pancreatic duct (hemosuccus pancreaticus). The infection of the pseudocyst may cause fever and clinical manifestations of sepsis [12,13].
- Imaging
The imaging methods are the most effective diagnostic tools for pancreatic pseudocysts, but in the absence of documented pancreatitis, a cystic lesion in the pancreas must be interpreted as a cystic tumor until proven otherwise [14].
Transabdominal ultrasound is the most accessible and low-cost imaging investigation available for the diagnosis of pancreatic pseudocysts. Its sensitivity rates are influenced by the surgeons’ experience and range between 75% and 90% [15].
Pancreatic pseudocysts occur as a round or oval echoic structure bounded by a smooth wall. In the presence of necrotic debris and the occurrence of hemorrhage or infection, pseudocysts can appear with internal echoes [16,17].
A CT scan has a sensitivity of 90% to 100% in the diagnosis of a pseudocyst. A rounded, thick-walled fluid-filled mass adjacent to the pancreas in the clinical context of pancreatitis is highly suggestive for the pseudocyst. The surrounding anatomy and additional pathologies are highlighted by CT scans. The main drawback of CT scans is the difficulty in distinguishing between pseudocysts and cystic neoplasms [18].
These drawbacks are overcome by using endoscopic ultrasound which provides high quality images, allowing the differentiation between cystic neoplasm and pancreatic pseudocysts. Endoscopic ultrasounds can also be used to perform fine needle aspiration for the laboratory evaluation of the cystic fluid and to guide endoscopic drainage [19,20].
Magnetic resonance imaging (MRI) is a sensitive diagnostic method for pancreatic pseudocysts, but not routinely used because CT scans offer all of the diagnostic information that is required for the treatment. MRI is superior to CT scans in detecting bleeding and debris within the fluid collections and in depicting choledocholithiasis [21].
The evolution of pancreatic pseudocysts
Peripancreatic fluid collections occur after episodes of acute pancreatitis and may progress to the formation of pseudocysts or may be resorbed. In more than 60% of the cases, pancreatic pseudocysts are resorbed spontaneously, so in the initial stages, the attitude is to observe them closely. Usually, fluid collections are resorbed within about 4 weeks, this time limit being the separation between fluid collections and pancreatic pseudocysts. According to most authors, the time limit for defining a pseudocyst is 6 weeks, especially when the surgical treatment of the pseudocyst is considered [22,23].
The treatment is imposed by the appearance of complications - bleeding, infection, digestive tract obstruction, and portal hypertension. Spontaneous splenic rupture and cutaneous fistulation of the pancreatic pseudocyst are exceptional complications [24].
The classification of pancreatic pseudocysts
Several criteria for classifying pancreatic pseudocysts have been suggested in time.
In 1961, Sarles suggested a classification of pseudocysts based on the type of pancreatitis that caused their occurrence. Thus, two categories of pseudocysts are distinguished. The first category includes those resulting from the necrosis of the pancreatic parenchyma with the extravasation of pancreatic juice as a consequence of an episode of acute pancreatitis. These types of pseudocysts are called “postnecrotic”. The second category of pseudocysts are the so-called "by retention" and occur in chronic pancreatitis as a result of pancreatic juice extravasation into the peripancreatic tissues caused by the disruption of the pancreatic ducts behind an obstacle such as stones, plugs, or strictures. This classification rules out pseudocysts occurring after acute-to-chronic pancreatitis [25].
The latter category of pseudocysts is included in the classification of D’Egidio and Schein, which takes into account both the type of underling pancreatitis and the communication of the pseudocyst with the pancreatic ducts. According to this classification, type I, "postnecrotic" pseudocysts, appear after an episode of acute pancreatitis, the anatomy of the ducts being normal. In rare situations, these pseudocysts have a communication with the pancreatic ducts. Type II pseudocysts include those that appear after an episode of acute-to-chronic pancreatitis and in which there is a communication with the pancreatic ducts. Type III comprises pseudocysts that appear in chronic pancreatitis associating a ductal obstruction and in which communication with the pancreatic ducts is always present [26].
Nealon and Walser propose a classification that takes into account the anatomy of the pancreatic ducts and the communication with the pancreatic pseudocyst based on endoscopic retrograde cholangiopancreatography. This classification subdivides pancreatic pseudocysts into 7 types. According to the authors' experience, patients with no communication between the duct and the cyst and normal duct anatomy are best suited for percutaneous drainage, while the other categories benefit from surgical treatment [27].
Pan et al. propose a classification of pancreatic pseudocysts that can assist in the selection of the optimal therapy. Based on a retrospective study on 893 patients, the authors propose a classification that takes into account the clinical manifestations, the size and the location of pseudocysts, in addition to the communication with the pancreatic ducts. Among the possible therapeutic methods, endoscopic drainage is recommended by the authors as the first option when conditions permit that the distance between the pseudocyst and the gastrointestinal wall be less than 1 cm. Surgical internal drainage is indicated in situations when the endoscopic treatment is not applicable, having a success rate of up to 99%. Distal pancreatectomy is recommended in patients with pseudocysts located in the pancreatic tail and when splenic vein involvement or upper gastrointestinal bleeding also occur. The high rate of complications (30.8%) and a frequent need for open surgery (38.5%) is reported in this study for the percutaneous drainage of the pseudocysts. The authors recommend this procedure for ruptured cysts and symptomatic or infected mature cysts in patients who are not eligible for the surgical treatment [28].
Treatment methods
- Percutaneous drainage under imaging guidance
It is a simple, inexpensive and low trauma intervention that can be used even when the pseudocyst wall is not matured. The route of drainage can be retroperitoneal or transperitoneal, i.e. transgastric or transduodenal. Drainage guidance can be performed fluoroscopically, ultrasonographically or, most commonly, upon computed tomography. The choice of patients who can benefit from this procedure must be made based on certain criteria in order to avoid failure or complications. The best results are obtained in cases of infected pseudocysts, expanding immature cysts larger than 5 cm and in patients who are not eligible for the surgical treatment. Continuous percutaneous drainage is more effective than simple percutaneous aspiration [29,30,31].
This method is not an option for retention pseudocysts in chronic pancreatitis, where due to communication with the pancreatic ducts, percutaneous drainage is inefficient.
The complications associated with this type of drainage are infection, bleeding, pancreatic fistula, and often the drainage tube blockage due to necrotic tissue [32].
- Endoscopic drainage
Endoscopic transmural drainage is a feasible option when the pseudocyst is in apposition with the gastric or duodenal wall. Luminal compression can be obvious on upper endoscopy and in order to avoid of any intervening vessels and to ascertain the proximity of the cystic wall, an endoscopic ultrasound guided puncture of the pseudocyst is performed. Transmural stents are left in place pending the complete resolution of the pancreatic pseudocyst, which is monitored by imaging investigations at 4-week intervals [33].
Recently, a new device has emerged, which, unlike the double-pigtail stents (DPS), has a minimal risk of migration and allows a wider communication between the pseudocyst and the digestive lumen. This lumen-apposing metal stent (LAMS) has a biflanged shape that allows for tissue apposition, but it is associated with a higher bleeding rate when compared to the DPS [34,35].
Endoscopic transpapillary drainage can be considered if the pseudocyst is not located in the tail of the pancreas and communicates with the pancreatic duct [36].
After the pancreatic duct sphincterotomy, a plastic stent of 5–7Fr is placed into the pseudocyst cavity. These stents are exchanged every 6–8 weeks until the pseudocyst regression is obtained [37].
Teoh et al. conclude that endoscopic cyst-gastrostomy provides overall success for selected patients, but has a lower primary success rate compared to laparoscopic and open pancreatic cyst-gastrostomy [38].
According to some authors, the endoscopic treatment can be considered the first-line treatment approach in pancreatic pseudocysts, being a procedure that can be repeated and having a shorter length of hospital stay and lower hospital cost compared to the surgical group [39].
- Surgical drainage
In 1898, Werner Körte established a turning point in the treatment of pancreatic pseudocysts by differentiating them from other pancreatic cystic lesions as a distinct pathological entity [40].
The internal surgical drainage of the pancreatic pseudocyst is the oldest method of treatment used. In 1911, Louis Ombrédanne performed the first internal drainage of a pancreatic pseudocyst by means of a cystoduodenostomy [41].
The first pancreatic cystogastrostomy was performed by Jedlicka in 1921. Ten years later, Jurasz performed the same posterior gastric wall anastomosis with the pseudocyst through an anterior gastrotomy. The latter procedure has the advantage of avoiding dissection through inflamed tissues and would become the most commonly used surgical method for pseudocysts in contact with the posterior wall of the stomach [42,43].
In 1946, Köning first performed the anastomosis of a pseudocyst with a Roux-en-Y loop. This technical option is to be chosen in pseudocysts that are not close to the stomach [44].
Ye Jun et al. published a clinical study on 208 patients who underwent cystogastrostomy and Roux-en-Y-type cystojejunostomy in the treatment of pancreatic pseudocysts, analyzing the risk factors for recurrence and complications. The results show that there are no significant differences between the two internal drainage procedures in terms of cure rate, reoperation rate, and mortality at approximately 43 months after the procedure, although theoretically, there is a slightly increased risk of related complications for Roux-en-Y-type cystojejunostomy. The average size of pseudocysts in their study was 10.1-10.7 ± 42.0 cm. Regarding the criteria for choosing the drainage procedure, the choice was made intraoperatively by an experienced surgeon with long-term experience in pancreatic surgery [45].
Internal drainage has the best results for symptomatic pseudocysts in terms of permanent resolution 91-97%, with mortality rates of 0-13% and morbidity rates of 10- 30% [46].
According to Melman, the open approach drainage method has the highest overall success rate, defined as cyst resolution, of over 90%, compared to endoscopic or laparoscopic internal drainage [47].
Complications related to internal drainage are secondary hemorrhage, infection, recurrence, amounting to 16% morbidity and 2.5% mortality rates [48].
Over the last two decades, laparoscopic internal drainage, following the principles of the techniques described for open surgery, has become a feasible technique with promising results [49].
Pancreatic resections for pseudocyst have indications in rarer circumstances, such as biliary or duodenal obstruction, multiple cysts or gastrointestinal hemorrhage [50].
Regarding the size of the pseudocyst, cases of giant pseudocysts are sporadically reported in the literature, and the results obtained by different treatment methods are often contradictory. In a series of fifty-two patients with pancreatic pseudocysts of various sizes, Johnson observed that the postoperative complication rate was directly proportional with the size of the pseudocyst. In this series, four of the pseudocysts were over 15 cm and were treated by cystogastrostomy and, out of these, three had life-threatening postoperative complications. The authors attribute these complications to the incomplete emptying of the cyst and conclude that cystogastrostomy may not be appropriate for the treatment of giant pancreatic pseudocysts [51].
Wang et al. report the case of a 65-year-old man presenting a pancreatic pseudocyst, measuring 25.7 cm×15.3 cm×10.9 cm, which was drained through an open cystogastrostomy with uneventful postoperative course [52].
A good postoperative outcome is reported by Golash after a cystogastrostomy for a giant pseudocyst of the pancreas was performed laparoscopically [53].
In a series of ten patients with giant pseudocysts after acute pancreatitis, Oria et al. first performed a video-assisted pancreatic necrosectomy and then used the opening in the pseudocyst wall to perform an anastomosis with a Roux-en-Y limb of jejunum. The authors consider that by doing so, postoperative retroperitoneal complications are avoided [54].
Udeshika et al. report the case of a 27-year-old male presenting a 30-cm pancreatic pseudocyst successfully treated by means of endoscopic ultrasound (EUS) guided drainage using stents and pigtails. The authors recommend this approach as a possible initial method in the management of giant pseudocysts and follow up with repeated endoscopy and indicating surgery in case of failure of this procedure [55].
Conclusions
Cases of giant pancreatic pseudocysts are rarely reported in the literature. Currently, there is no consensus on the choice of the optimal treatment method or guidelines and no studies to assess the associated risk factors, recurrence rate or complications associated with the treatment of giant pseudocysts compared to small pseudocysts.
Although there are multiple methods of treatment, it is difficult to choose the most appropriate one, given the large size and complex anatomical relations of giant pseudocysts. Most often, the treatment of choice is made based on the experience of the surgeon and the technical equipment available in the hospital and the therapeutic approach type will be adapted to the conditions. The effectiveness of the method used can be assessed by a long-term follow-up.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Walt, A.J.; Bouwman, D.L.; Weaver, D.W.; Sachs, R.J. The Impact of Technology on the Management of Pancreatic Pseudocyst. Fifth annual Samuel Jason Mixter Lecture. Arch. Surg. 1990, 125, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Ratnayake, C.B.; Saikia, S.; Nayar, M.; Oppong, K.; French, J.J.; Windsor, J.A.; Pandanaboyana, S. Endoscopic transmural drainage is associated with improved outcomes in disconnected pancreatic duct syndrome: a systematic review and meta-analysis. BMC Gastroenterol. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Machicado, J.D.; Dudekula, A.; Tang, G.; Xu, H.; Wu, B.U.; Forsmark, C.E.; Yadav, D. Period prevalence of chronic pancreatitis diagnosis from 2001–2013 in the commercially insured population of the United States. Pancreatology 2019, 19, 813–818. [Google Scholar] [CrossRef]
- Usatoff, V.; Brancatisano, R.; Williamson, R.C.N. Operative treatment of pseudocysts in patients with chronic pancreatitis. Br. J. Surg. 2000, 87, 1494–1499. [Google Scholar] [CrossRef]
- Siegel, J.B.; Mukherjee, R.; Lancaster, W.P.; Morgan, K.A. Distal Pancreatectomy for Pancreatitis in the Modern Era. J. Surg. Res. 2022, 275, 29–34. [Google Scholar] [CrossRef]
- Facciorusso, A.; Amato, A.; Crinò, S.F.; Sinagra, E.; Maida, M.; Fugazza, A.; Binda, C.; Coluccio, C.; Repici, A.; Anderloni, A.; et al. Definition of a hospital volume threshold to optimize outcomes after drainage of pancreatic fluid collections with lumen-apposing metal stents: a nationwide cohort study. Gastrointest. Endosc. 2021, 95, 1158–1172. [Google Scholar] [CrossRef]
- Habashi, S.; Draganov, P.V. Pancreatic pseudocyst. World J. Gastroenterol. 2009, 15, 38–47. [Google Scholar] [CrossRef]
- Sedlack, R.; Affi, A.; Vazquez-Sequeiros, E.; Norton, I.D.; Clain, J.E.; Wiersema, M.J. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest. Endosc. 2002, 56, 543–547. [Google Scholar] [CrossRef]
- E Morgan, D.; Baron, T.H.; Smith, J.K.; Robbin, M.L.; Kenney, P.J. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology 1997, 203, 773–778. [Google Scholar] [CrossRef]
- Bradley, E.L.; Gonzalez, A.C.; Clements, J.L., Jr. Acute pancreatic pseudocysts: incidence and implications. Ann Surg. 1976, 184, 734–737. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, C.J. Management of complications of acute pancreatitis. Curr. Opin. Gastroenterol. 2018, 34, 336–342. [Google Scholar] [CrossRef]
- Palmela, C.; Barjas, E.; Grenho, J.; Ferreira, R.; Santos, A.A.; Maio, R.; Cravo, M. Two Very Rare Complications of Pancreatic Pseudocysts in one Patient: What are the Odds? Clin Surg. 2017, 2, 1595. [Google Scholar]
- van der Gaag, N.A.; van Gulik, T.M.; Busch, O.R.C.; Sprangers, M.A.; Bruno, M.J.; Zevenbergen, C.; Gouma, D.J.; Boermeester, M.A. Functional and Medical Outcomes After Tailored Surgery for Pain Due to Chronic Pancreatitis. Ann. Surg. 2012, 255, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Naveena, R. Clinical spectrum of obstructive jaundice: a descriptive crosssectional study. J Clin Invest Surg. 2021, 6, 53–57. [Google Scholar] [CrossRef]
- Velikova, M.; Galunska, B.; Dimitrova, R.; Stoyanov, Z. Alcohol consumption and cognitive aging: can it be beneficial? J. Mind Med Sci. 2021, 8, 5–16. [Google Scholar] [CrossRef]
- Köhler, H.; Schafmayer, A.; E Lüdtke, F.; Lepsien, G.; Peiper, H.-J. Surgical treatment of pancreatic pseudocysts. Br. J. Surg. 1987, 74, 813–815. [Google Scholar] [CrossRef]
- Dumitriu, B.; Valcea, S.; Andrei, G.; Beuran, M. The impact of patient-dependent risk factors on morbidity and mortality following gastric surgery for malignancies. J. Mind Med Sci. 2021, 8, 267–272. [Google Scholar] [CrossRef]
- Gaikwad, V.S.; Kisku, S.M.C.; Kurian, J.J.; Jacob, T.J.K.; Mathai, J. Laparoscopic Cystogastrostomy in Children with Pancreatic Pseudocysts: A Preliminary Experience of Eight Cases. J. Indian Assoc. Pediatr. Surg. 2022, 27, 77–82. [Google Scholar] [CrossRef]
- Shah, H.; Shah, R.; Sanghani, H.; Lakhani, N. Health related quality of life (HRQoL) and its associated surgical factors in diabetes foot ulcer patients. J. Clin. Investig. Surg. 2020, 5, 83–90. [Google Scholar] [CrossRef]
- Zorilă, A.L.; Zorilă, M.V.; Marinaş, M.C.; Ţolescu, R.Ş.; Zorilă, G.L.; Florou, C.; Neamţu, M.C.; Knieling, A.; Busuioc, C.J. Evaluation of brain injuries in children deceased due to head trauma. Rom J Morphol Embryol. 2017, 58, 1417–1428. [Google Scholar]
- Motofei, I.G. Biology of Cancer; From Cellular Cancerogenesis to Supracellular Evolution of Malignant Phenotype. Cancer Investig. 2018, 36, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Hainarosie, R.; Pietrosanu, C.; Rusescu, A.; Andronache, L.; Paunica, S.; Balalau, C.; Pituru, T. Modern concepts in non-surgical esthetics; a review. J. Mind Med Sci. 2019, 6, 190–195. [Google Scholar] [CrossRef]
- Uysal, E.; Dokur, M.; Maralcan, G. Abdominal surgery in autoimmune and autoimmune-related diseases: A review. J. Clin. Investig. Surg. 2021, 6, 76–87. [Google Scholar] [CrossRef]
- Motofei, I.G. Nobel Prize for immune checkpoint inhibitors, understanding the immunological switching between immunosuppression and autoimmunity. Expert Opin. Drug Saf. 2021, 21, 599–612. [Google Scholar] [CrossRef]
- Sarles, H.; Muratore, R.; Sarles, J.C. Etude anatomique des pancreatites chroniques de l’adulte. Sem Hop. 1961, 37, 1507–1522. [Google Scholar] [PubMed]
- D'Egidio, A.; Schein, M. Pancreatic pseudocysts: A proposed classification and its management implications. Br. J. Surg. 1991, 78, 981–984. [Google Scholar] [CrossRef]
- Nealon, W.H.; Walser, E. Main Pancreatic Ductal Anatomy Can Direct Choice of Modality for Treating Pancreatic Pseudocysts (Surgery Versus Percutaneous Drainage). Ann. Surg. 2002, 235, 751–758. [Google Scholar] [CrossRef]
- Pan, G.; Wan, M.H.; Xie, K.-L.; Li, W.; Hu, W.-M.; Liu, X.-B.; Tang, W.-F.; Wu, H. Classification and Management of Pancreatic Pseudocysts. Medicine 2015, 94, e960. [Google Scholar] [CrossRef] [PubMed]
- Loveday, B.P.T.; Mittal, A.; Phillips, A.; Windsor, J.A. Minimally Invasive Management of Pancreatic Abscess, Pseudocyst, and Necrosis: A Systematic Review of Current Guidelines. World J. Surg. 2008, 32, 2383–2394. [Google Scholar] [CrossRef]
- Tan, J.H.; Chin, W.; Shaikh, A.L.; Zheng, S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp. Ther. Med. 2020, 21, 1–1. [Google Scholar] [CrossRef]
- Adams, D.B.; Anderson, M.C. Percutaneous Catheter Drainage Compared With Internal Drainage in the Management of Pancreatic Pseudocyst. Ann. Surg. 1992, 215, 571–576; discussion 576–8. [Google Scholar] [CrossRef] [PubMed]
- Vansonnenberg, E.; Wittich, G.R.; Casola, G.; Brannigan, T.C.; Karnel, F.; E Stabile, B.; Varney, R.R.; Christensen, R.R. Percutaneous drainage of infected and noninfected pancreatic pseudocysts: experience in 101 cases. Radiology 1989, 170, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Nabi, Z.; Basha, J.; Reddy, D.N. Endoscopic management of pancreatic fluid collections-revisited. World J. Gastroenterol. 2017, 23, 2660–2672. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut 2016, 66, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Alzeerelhouseini, H.I.A.; Elqadi, M.; Elqadi, M.N.; Abukhalaf, S.A.; Ashhab, H.A. Endoscopic Drainage of Giant Pancreatic Pseudocysts Using Both Lumen-Apposing Metal Stent and Plastic Stent: A Report of Two Cases and Review of the Current Literature. Case Rep. Gastrointest. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Rosso, E.; Alexakis, N.; Ghaneh, P.; Lombard, M.; Smart, H.L.; Evans, J.; Neoptolemos, J.P. Pancreatic Pseudocyst in Chronic Pancreatitis: Endoscopic and Surgical Treatment. Dig. Surg. 2003, 20, 397–406. [Google Scholar] [CrossRef]
- Catalano, M.F.; Geenen, J.E.; Schmalz, M.J.; Johnson, G.; Dean, R.S.; Hogan, W.J. Treatment of pancreatic pseudocysts with ductal communication by transpapillary pancreatic duct endoprosthesis. Gastrointest. Endosc. 1995, 42, 214–218. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Dhir, V.; Jin, Z.-D.; Kida, M.; Seo, D.W.; Ho, K.Y. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J. Gastrointest. Endosc. 2016, 8, 310–318. [Google Scholar] [CrossRef]
- Hao, W.M.; Chen, Y.; Jiang, Y.; Yang, A. Endoscopic Versus Laparoscopic Treatment for Pancreatic Pseudocysts: A Systematic Review and Meta-analysis. Pancreas 2021, 50, 788–795. [Google Scholar] [CrossRef]
- Körte, W. Die chirurgischen krankheiten und die verletzungen des pankreas. Stuttgart Enke, 1898.
- Navarro, S. El arte de la cirugía pancreática. Pasado, presente y futuro. Gastroenterol. Y Hepatol. 2017, 40, 648.e1–648.e11. [Google Scholar] [CrossRef]
- Jedlicka, R. Eine neue operationsmethode der pancrascysten (Pancreatogastrostomie). Zentralbl Chir. 1923, 50, 132. [Google Scholar]
- Adloff, M.; Schloegel, M.; Ollier, J.C.; Cuvelier, G. [Pancreatojejunostomy or pancreatogastrostomy after cephalic pancreatoduodenectomy]. Chirurgie 1992, 118, 63–70. [Google Scholar]
- Brown, L.; Loveday, B. Acute pancreatitis in Australian adults: can administrative data help with healthcare planning? ANZ J. Surg. 2022, 92, 8–9. [Google Scholar] [CrossRef]
- Ye, J.; Wang, L.; Lu, S.; Yang, D.; Hu, W.; Lu, H.; Zhang, Y. Clinical study on cystogastrostomy and Roux-en-Y-type cystojejunostomy in the treatment of pancreatic pseudocyst. Medicine 2021, 100, e25029. [Google Scholar] [CrossRef] [PubMed]
- Chan Núnez, C.; Jiménez González, A. Tratamiento quirúrgico de los pseudoquistes pancreáiticos [Surgical treatment of pancreatic pseudocyst]. Rev Gastroenterol Mex. 2004, 69 (Suppl. 3), 119–120. [Google Scholar]
- Melman, L.; Azar, R.; Beddow, K.; Brunt, L.M.; Halpin, V.J.; Eagon, J.C.; Frisella, M.M.; Edmundowicz, S.; Jonnalagadda, S.; Matthews, B.D. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg. Endosc. 2008, 23, 267–271. [Google Scholar] [CrossRef]
- Motofei, I.G. Biology of cancer; from cellular and molecular mechanisms to developmental processes and adaptation. Semin. Cancer Biol. 2021, 86, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Kaistha, S.; Nandi, B.; Kumar, A. Laparoscopic surgery in pancreatic diseases: Pushing the boundaries. Med J. Armed Forces India 2018, 75, 361–369. [Google Scholar] [CrossRef]
- A Grace, P.; Williamson, R.C.N. Modern management of pancreatic pseudocysts. Br. J. Surg. 1993, 80, 573–581. [Google Scholar] [CrossRef]
- Johnson, L.B.; Rattner, D.W.; Warshaw, A.L. The effect of size of giant pancreatic pseudocysts on the outcome of internal drainage procedures. Surg Gynecol Obstet. 1991, 173, 171–174. [Google Scholar]
- Wang, G.C.; Misra, S. A giant pancreatic pseudocyst treated by cystogastrostomy. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Golash, V.; Cutress, R. Laparaoscopic cytogastrostomy for a giant pseudocyst of pancreas. Surgeon 2005, 3, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Oría, A.; Ocampo, C.; Zandalazini, H.; Chiappetta, L.; Morán, C. Internal Drainage of Giant Acute Pseudocysts. Arch. Surg. 2000, 135, 136–140; discussion 141. [Google Scholar] [CrossRef] [PubMed]
- Udeshika, W.A.E.; Herath, H.M.M.T.B.; Dassanayake, S.U.B.; Pahalagamage, S.P.; Kulatunga, A. A case report of giant pancreatic pseudocyst following acute pancreatitis: experience with endoscopic internal drainage. BMC Res. Notes 2018, 11, 262. [Google Scholar] [CrossRef]
© 2022 by the author. 2022 Petrișor Banu, Bogdan Socea, Daniela Gabriela Balan, Vladimir Sandu, Tiberiu Onicel, Adrian Silaghi, Ioana Paunica, Vlad Denis Constantin.