Case Presentation
Patient H.F. aged 44 years, female, presented with the following complaints: pain in the right hypochondriac region, asthenia, unintentional weight loss (approximately 13 kilos in four months), loss of appetite, nausea and vomiting (2-3 episodes/day). She was referred to our hospital by a regional hospital for further investigation and biopsy of some hepatic lesions seen on an abdominal and pelvic CT scans. At the CT scan, multiple hypodense lesions in both hepatic lobes were described. In contrast-enhanced CT, the lesions appear as rapidly enhancing lesions visible on the arterial phase. This enhancement pattern is characteristic to hypervascular metastases such as those from neuroendocrine tumors, renal cell carcinoma, breast carcinoma, melanoma and thyroid carcinoma. In the right lobe, the lesion had a maximum diameter of 176/111 mm AP/LL, while, in the left lobe, the maximum diameter was up to 82 mm. There was also a small amount of ascites.
She had a history of tobacco use, but she had quit smoking eleven years ago. The patient was also suffering from arterial hypertension, for which she was under treatment with angiotensin converting enzyme inhibitors and diuretics. There was no relevant family history.
On the clinical examination, the patient was in a good general state, apyretic, with a blood pressure of 130/70 mmHg, a pulse of 80 bpm, and a SpO2 of 98%. Lung and cardiac auscultation revealed no pathological aspects and the abdomen was insensitive to superficial and deep palpation.
Laboratory investigation revealed: cholestasis with an elevated alkaline phosphatase (ALP) of 472 U/l (98-279), and a gamma-glutamyl transferase (GGT) of 305 U/l (7- 32), thrombocytosis PLT 486x10^3/ul (150-350), elevated erythrocyte sedimentation rate (ESR) 35 mm/1h (1-10). The transaminases were within normal limits, the surface antigen of the hepatitis B virus (HBsAg) was negative and the antibodies to the hepatitis C virus (anti-HCV antibodies) were also negative.
The presence of hepatic lesions was confirmed by abdominal ultrasonography and a needle biopsy of the liver was performed. Based on the clinical presentation, ultrasound and CT scan images, the hepatic lesions were suspected to be metastases, most probably, of a neuroendocrine tumor.
In anticipation of the histological results, investigations were conducted to search for a possible primary tumor. The upper endoscopy revealed acute erythematous pangastritis, while the lower endoscopy revealed grade II internal hemorrhoids, but no tumors. A thoracic CT scan was also performed, describing bone sclerotic lesions on the vertebral bodies (T6, T10, T11), sternum, scapula bilateral and the 6th left costal arch, and a mixed predominantly osteolytic bone lesion on the 7th right costal arch. Besides these bone lesions, a nodular densification in the right breast was also observed.
A mammography (
Figure 1) was performed, showing an irregular opacity with spiculiform contour in the supero-external dial of the right breast. The maximum diameter of the lesion was 18/16 mm, and multiple microcalcifications (over 30) were observed. The next step was the ultrasound guided biopsy of the breast. At this moment, the possibility of having two different tumors at the same time was also included in the differential diagnosis.
The histopathological exam of the breast biopsy (
Figure 2), classified the breast lesion, according to the WHO definition in 2012, as an invasive breast carcinoma with neuroendocrine features. The coloration for neuron specific enolase (NSE) and synaptophysin were positive, while the cells were negative for chromogranin and CD56. Moreover, estrogen receptors (ER) were present in 100% of the tumor cells, while progesterone receptors (PR) were 80% positive. The ki67 index of proliferation was 5%. The tumor was moderately differentiated, grade II Nottingham with a total score of six (tubule formation=3, nuclear pleomorphism=2, mitotic activity=1).
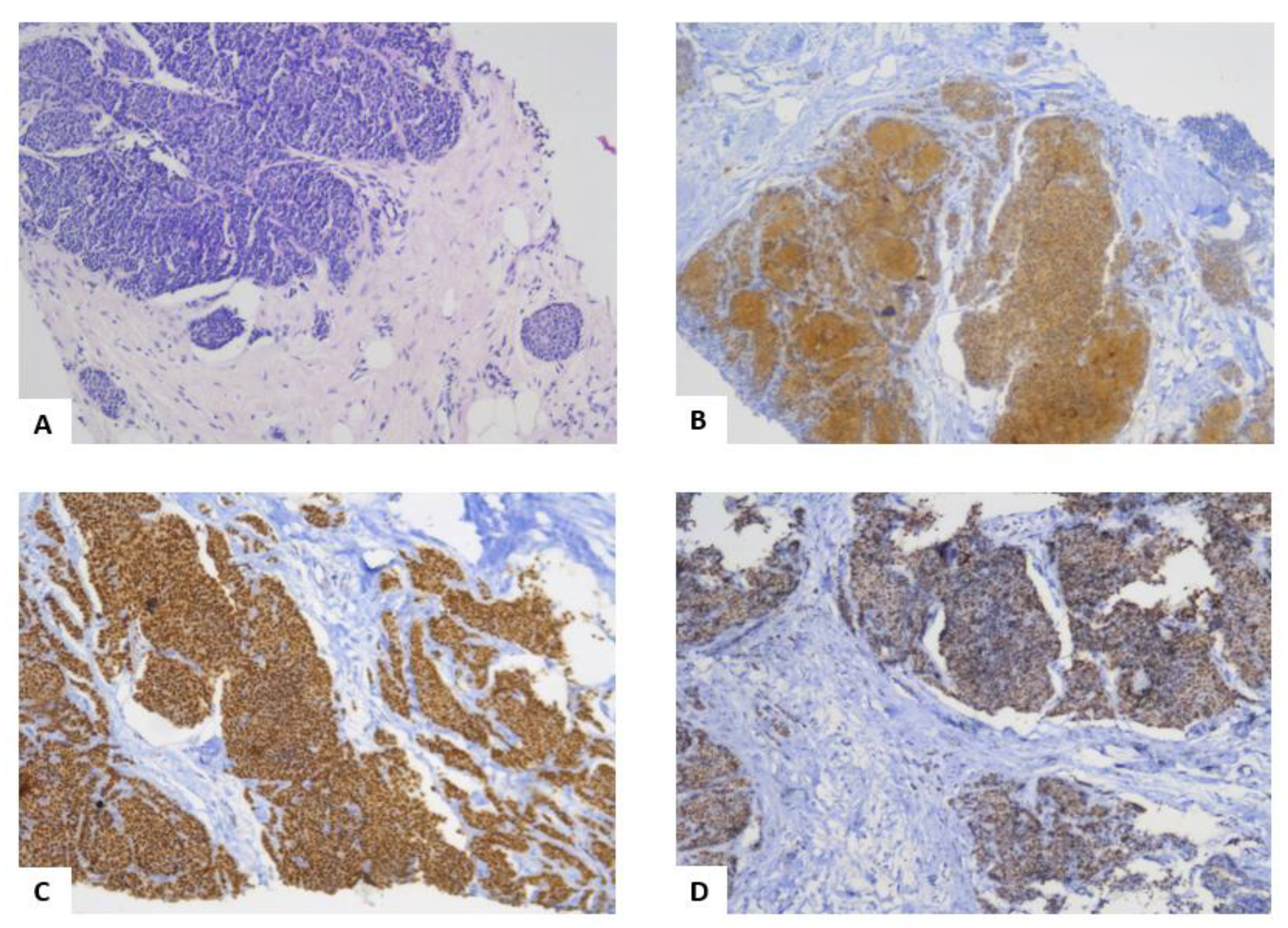
The liver biopsy (
Figure 3) confirmed the diagnosis by describing the aspect of metastases originating most probably from a mammary carcinoma. This was supported by the presence of ER in the liver biopsy. Also, by comparing the breast biopsy (
Figure 2A) and the liver biopsy (
Figure 3A) in HE staining, it was observed that the tumor cells were similar.
The final diagnosis was: Invasive breast carcinoma with neuroendocrine features with liver and bone metastases. Grade II hypertension with moderate to high risk. Acute erythematous pangastritis. Grade II internal hemorrhoids. The patient was referred to the oncologist.
Discussions
In this case, the patient was diagnosed with invasive breast carcinoma with neuroendocrine features, according to the WHO classification in 2012, based on the immunohistochemical staining for NSE and the aspect of tumor cells. The various definitions of this type of cancer are worth being discussed, as there are few articles on this topic, and the definitions have changed several times in the last years, and this may affect the way these studies are interpreted [
3,
4].
The lack of uniformity in the definition and classification of neuroendocrine carcinomas hampers an exact estimate of the prevalence of these tumors, ranging from 0.1% to 15% depending on the series. This may also explain the controversial data on the prognostic implication of neuroendocrine differentiation in breast cancer [
3]. In 2003, neuroendocrine carcinoma (NEC) of the breast was endorsed as a distinct entity in the third edition of the WHO Classification of Tumors series. Neuroendocrine tumors (NETs) of the breast were defined as tumors of epithelial origin, with morphology similar to that of gastrointestinal and pulmonary NETs, expressing neuroendocrine immunohistochemical markers (synaptophysin, chromogranin A) in at least 50% of the total invasive tumor cell population [
4].
In 2012, in the fourth-edition volume WHO classification of tumors of the breast, NECs were included under the category “carcinomas with neuroendocrine features”, and they were defined as tumors exhibiting morphological features similar to those of the NETs of the gastrointestinal tract and of the lung, expressing neuroendocrine markers to any extent [
4]. The revised 2012 WHO classification includes three categories of NETs of the breast: (1) NETs well differentiated, which resembles carcinoid tumors and includes low and intermediate grade tumors; (2) NEC poorly differentiated (small cell carcinoma), which has the same features as a primary small cell carcinoma of the lung; (3) invasive breast carcinoma with neuroendocrine features, including no special type (NST), as well as special types such as solid papillary carcinoma and the hypercellular subtype of mucinous carcinoma [
4,
5,
6,
7].
Although the majority of the articles in the literature refer to the definition from 2012, it is important to mention that, in 2019, the International Agency for Research on Cancer (IARC) and the WHO adopted the term “neuroendocrine neoplasm (NEN)” as a term encompassing all tumor classes with predominant neuroendocrine differentiation, including both well- differentiated and poorly differentiated forms. The morphology and the expression of the markers of neuroendocrine differentiation were recognized as the key features defining these neoplasms at any specific anatomical site. A uniform classification framework for NENs at all anatomical locations was proposed in order to reduce the inconsistencies and contradictions among the various systems currently in use [
4,
5,
6,
7].
Regarding the clinical presentation, neuroendocrine tumors of the breast occur predominately in white postmenopausal women in the sixth to seventh decade of life [
2,
5]. There are no notable or specific differences in presentation from other high-grade breast carcinomas and the clinical syndromes related to hormone production are extremely rare. Serological tests may detect circulating neuroendocrine markers such as chromogranin A [
1,
2]. "Carcinoid syndrome" is the term applied to a group of symptoms mediated by various humoral factors elaborated by some well-differentiated NETs which synthesize, store, and release a variety of polypeptides, biogenic amines, and prostaglandins. Carcinoid syndrome is most common in the setting of disseminated disease, particularly liver metastases, but it can occur in apparently locoregional disease [
8]. The liver inactivates bioactive products secreted into the portal circulation. This may explain why patients with gastrointestinal NETs most often develop carcinoid syndrome if they suffer from hepatic metastases, resulting in the secretion of tumor products into the systemic circulation [
9]. In the large majority of cases, carcinoid syndrome is associated with metastatic tumors originating in the midgut (jejunum, ileum, and cecum); however, the expression is variable in individual patients. Less often, carcinoid syndrome is caused by a NET arising in the lung or in the distal colon or rectum and extremely rarely it is caused by NET arising in other sites such as the breasts. In
Table 1, the most important clinical manifestations of carcinoid syndrome are highlighted [
10].
In this case, the presentation was also not specific and was due mainly to pain in the right hypochondriac region, caused by the infiltration of the liver. What is notable in our case, is that the age of onset was 44 years and the menopause was not installed.
The main risk factors for NEC of the breast are currently believed to be the same as for non-neuroendocrine breast cancer, such as age and family history. Moreover, the risk of the disease may also be increased by early menarche, late menopause as well as significant exposure to estrogen, typical of patients undergoing hormone replacement therapy or taking oral contraceptives [
11]. Some evidence suggests a link between high prolactin level and breast cancer development; however, it is unclear whether breast NEC may be associated with hyperprolactinemia. Zang et al. have recently published two cases of breast NEC associated with hyperprolactinemia, one patient suffering from mental disorder under antipsychotic drugs, and another one diagnosed in late pregnancy [
12].
As well as the clinical presentation, the radiologic characteristics are also unspecific and similar to the other malignant breast lesions. In a mini review article, Gallo et al. summarized the imaging characteristics of breast NEC, reported in case reports or small series: the most common mammographic appearance is a hyperdense, irregularly shaped solitary mass; margins are more commonly reported as indistinct, micro-lobulated or speculated. In most cases, calcifications are absent [
12,
13,
14]. Taking into account the most common mammographic appearance described by Gallo et al., it is important to remember that multiple microcalcifications were observed in this case.
There are no data from prospective clinical trials on the optimal management of NETs of the breast, and these tumors are usually treated with the same strategy used for other types of invasive breast carcinoma. Therefore, outside of the context of the exceedingly rare NEC of the breast, neuroendocrine differentiations in breast neoplasms are not regarded as specific therapeutic implications [
4]. Surgery is the mainstay of the treatment for early NEC of the breast. Adjuvant radiation and systemic therapy must be decided in a personalizing view. Regimens including anthracyclines and/or taxanes are preferable when the indication for chemotherapy exists. Also, patients with positive hormone receptors are likely candidates for adjuvant endocrine therapy [
15].
Although breast NEC does not have a specific targeted therapy, several new targeted therapies based on specific biomarkers have recently been investigated in the NEC of the lung and in other types of breast carcinomas, which may provide guidance to their feasibility in breast NEC. According to an analysis performed by S.Vranic et al., several potential targets for novel therapies in breast NEC were identified, including farletuzumab and mirvetuximab soravtansine (FOLR1), sacituzumab govitecan (TROP-2), and HDAC inhibitors (H3K36Me3). For example, the expression of TROP-2 protein was found in 21% of the cases, suggesting that a small proportion of NEBCs may be sensitive to target therapy with sacituzumab govitecan. In some cases, CCND1 gene amplification may indicate the usefulness of investigational therapies [
16,
17]. All currently approved biomarkers of response to immune checkpoint inhibitors, have proven negative so far, thus suggesting that patients with neuroendocrine breast carcinoma are unlikely to benefit from immunotherapy [
18]. The reported results should serve as an early indication of potential clinical relevance in selected patients with breast NEC.
The tumor stage and the histological grade, which encompass mitotic counts, are used as the main prognostic parameters. The prognostic relevance of neuroendocrine differentiation in breast carcinoma is still under debate, because of the lack of specific criteria for its definition; therefore, several studies have been published with mixed results [
4]. These conflicting results might be explained by the different inclusion criteria based on whether 2003 or 2012 WHO definitions were applied to identify NEC, by the limited number of cases reported in each series and also by the analysis performed considering NEC as a whole, without analyzing the outcomes according to the different histologic subtypes [
19,
20]. According to a study published by Yang et al., within the same clinical stage or grade, neuroendocrine tumors and neuroendocrine carcinoma of the breast had worse disease-specific survival (DSS) and overall survival (OS) than corresponding stage or grade of invasive ductal carcinomas of no special type (IDCs-NST). In univariate and multivariate survival analyses, NENs of the breast had significantly worse DSS and OS than IDCs-NST [
21]. Even if this is a case of advanced breast carcinoma, with liver and bone metastases and a grade II Nottingham, the neuroendocrine component of the tumor made it difficult to make statements about the prognosis and the efficacy of the treatment.
A limitation of this case report is the lack of information regarding the management of the case in the oncology department and the response to the treatment. As a strength point, this case report presents the discovery of a rare tumor, in a young patient without other important risk factors or comorbidities. It is worth mentioning that one of the challenges of this case was its management, both medically and with regard to the doctor-patient relationship, because of the necessity to provide information to the patient on a rare pathology whose treatment and prognosis are not well known.