The Correlation Between Histopathological and Ultrasound Findings Regarding Cesarean Section Scars—A Three-Year Survey Study
Highlights
- The predictive ultrasound parameters for the risk of rupture/dehiscence of the uterine scar showed varying cut-off values, ranging between 2.0 and 3.5 mm for the lower segment and up to 0.97 mm for the myometrium.
- This observation of inverse proportionality between the uterine thickness and the risk of rupture/dehiscence of the scar seems to be correlated with the histopathological features of the cesarean section scar.
Highlights
- The predictive ultrasound parameters for the risk of rupture/dehiscence of the uterine scar showed varying cut-off values, ranging between 2.0 and 3.5 mm for the lower segment and up to 0.97 mm for the myometrium.
- This observation of inverse proportionality between the uterine thickness and the risk of rupture/dehiscence of the scar seems to be correlated with the histopathological features of the cesarean section scar.
Abstract
Introduction
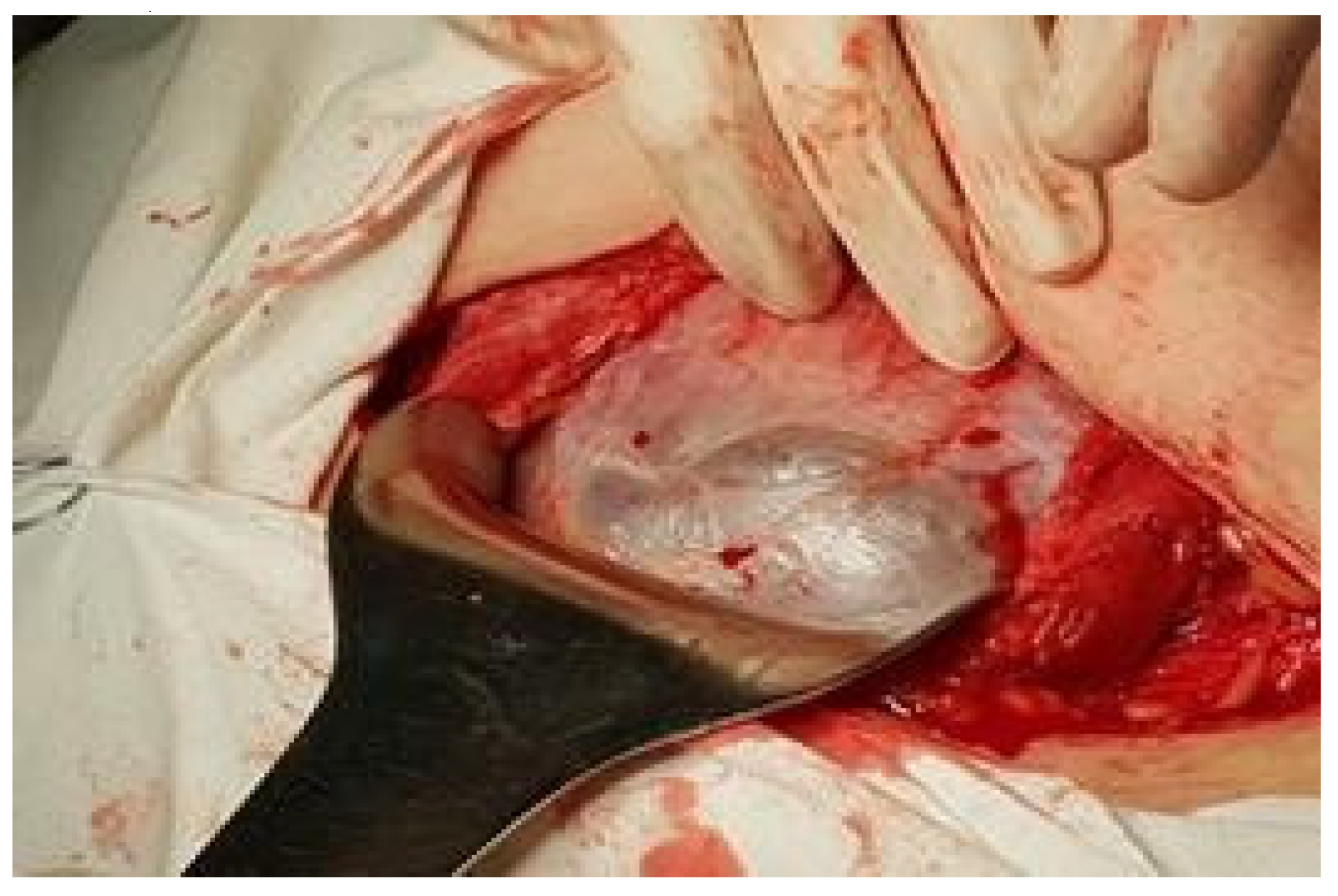
Materials and Methods
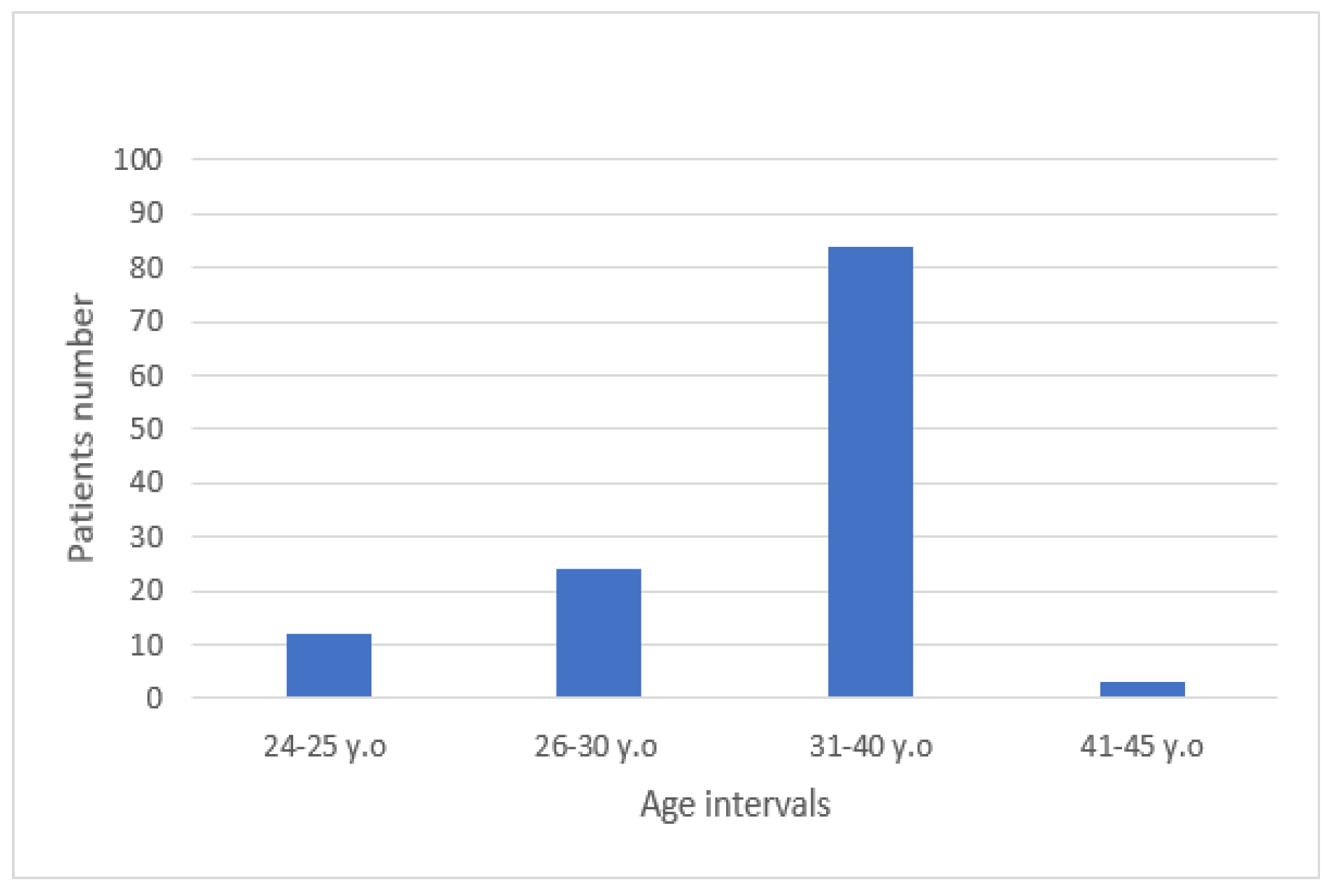
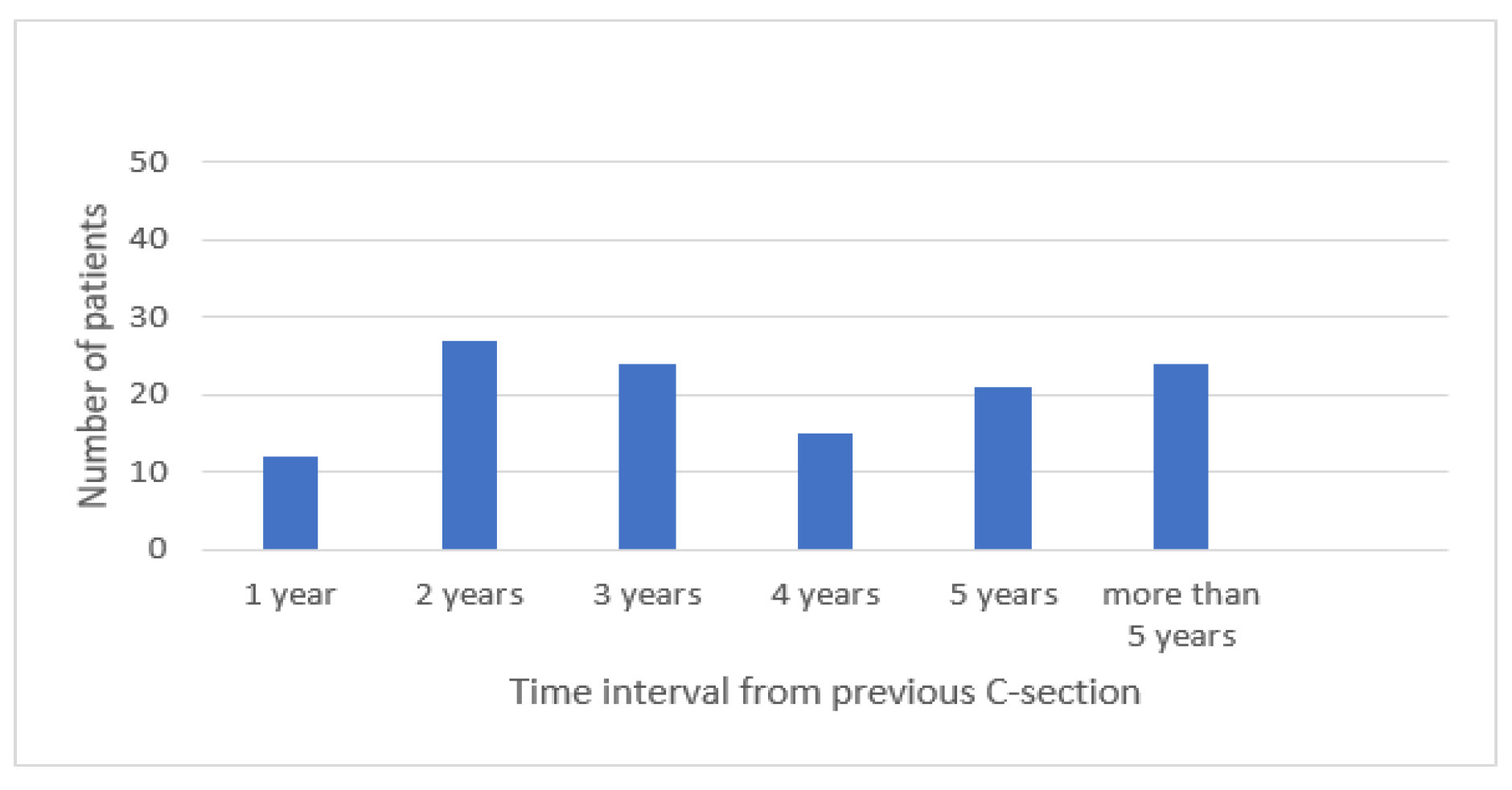
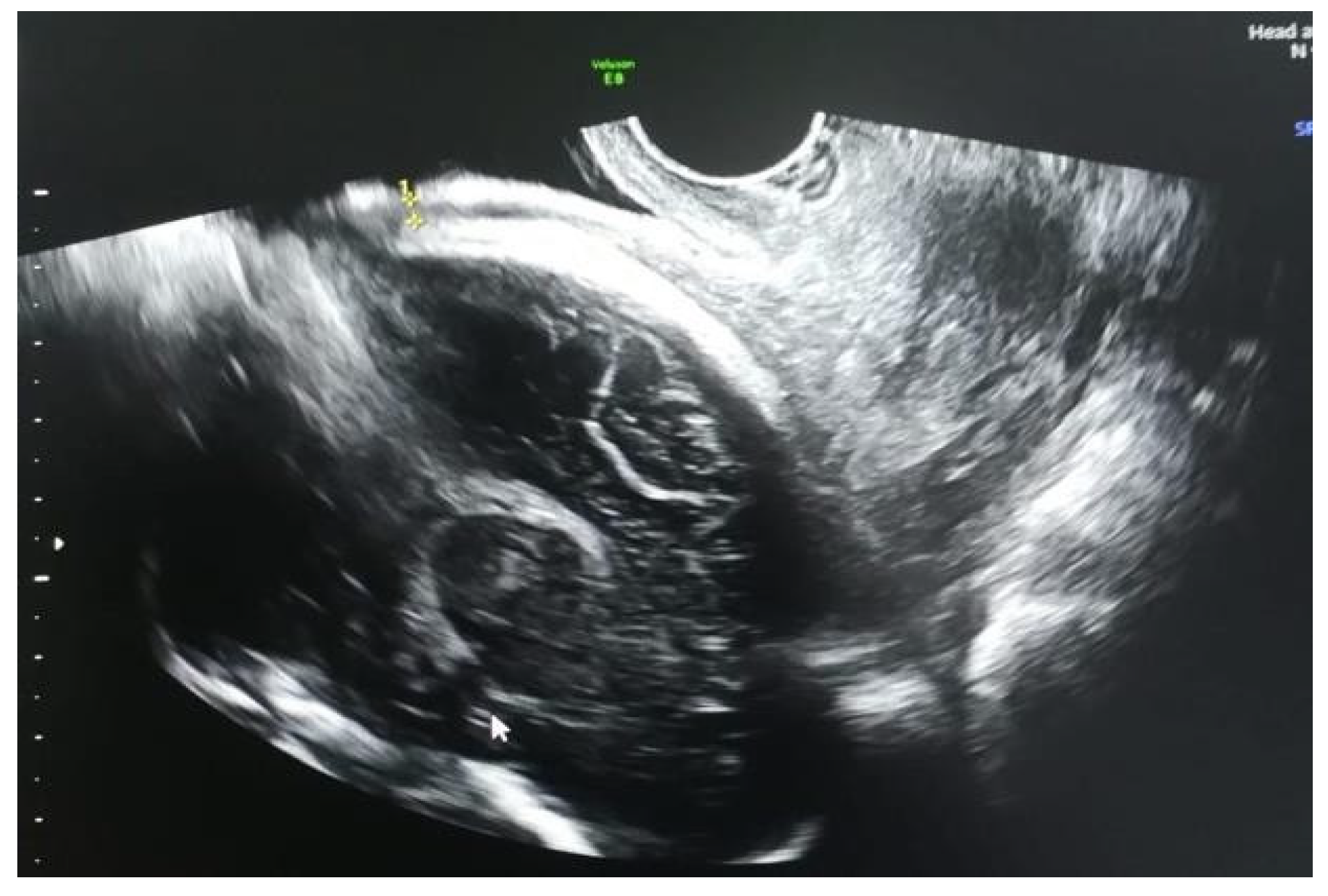
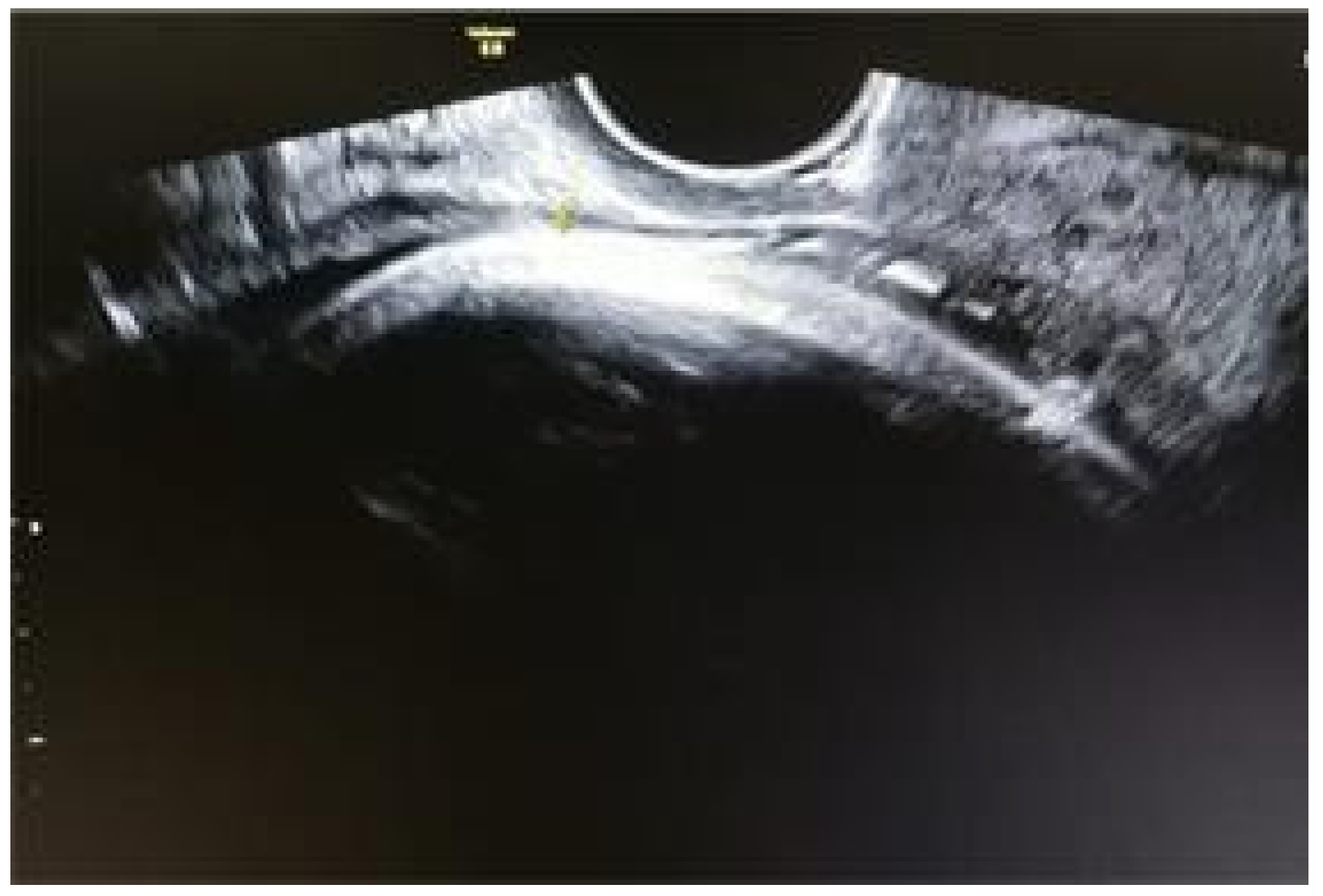
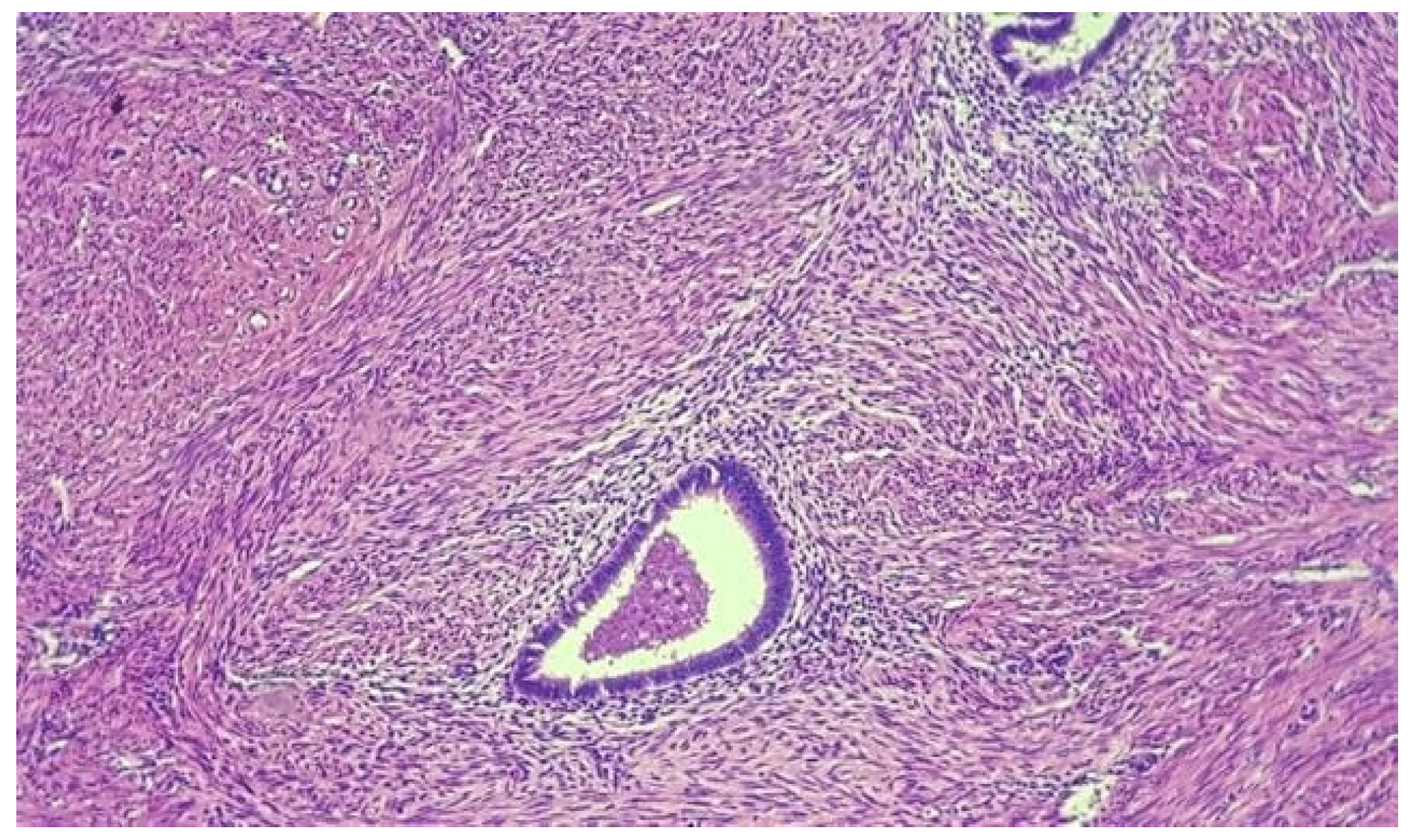
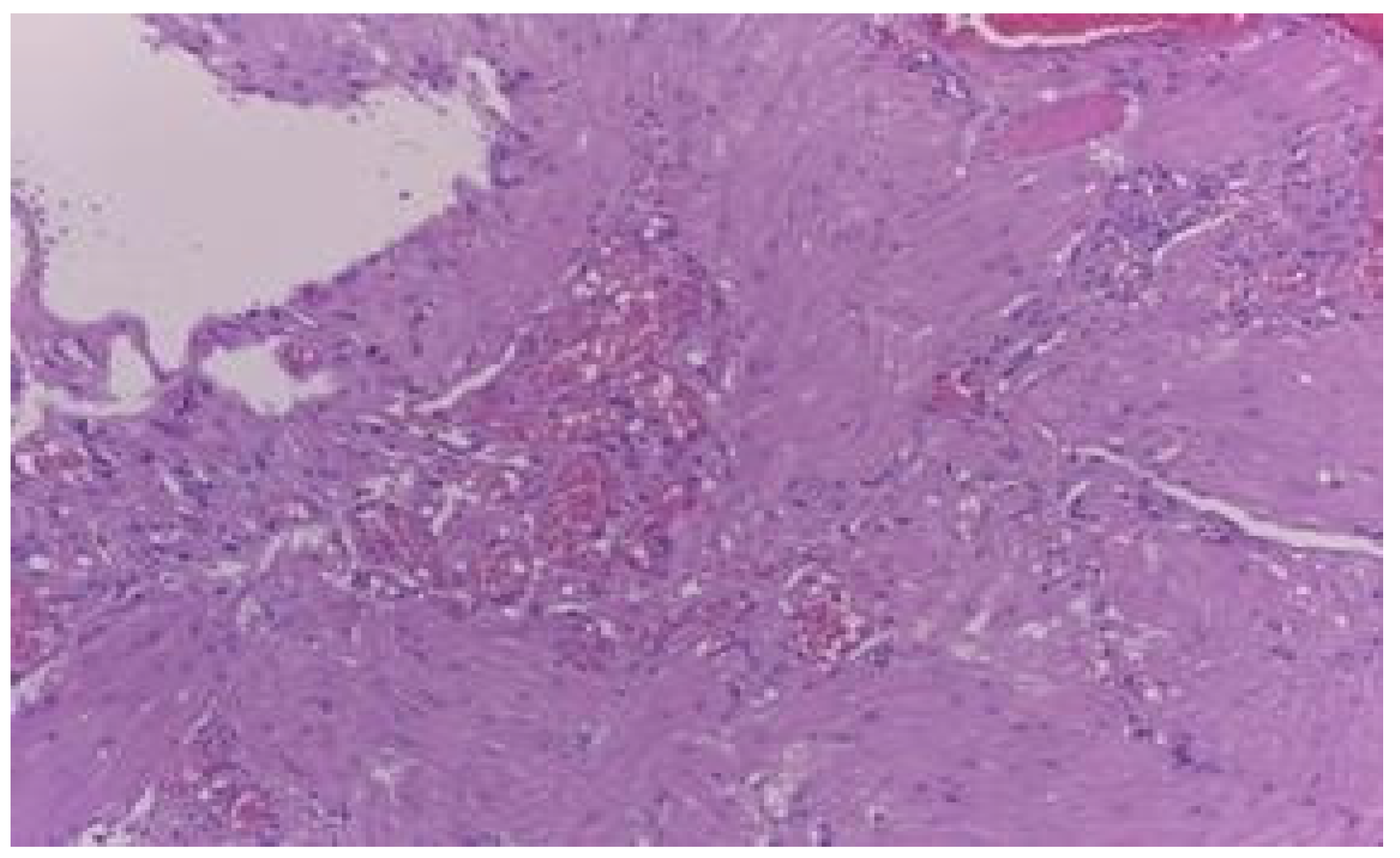
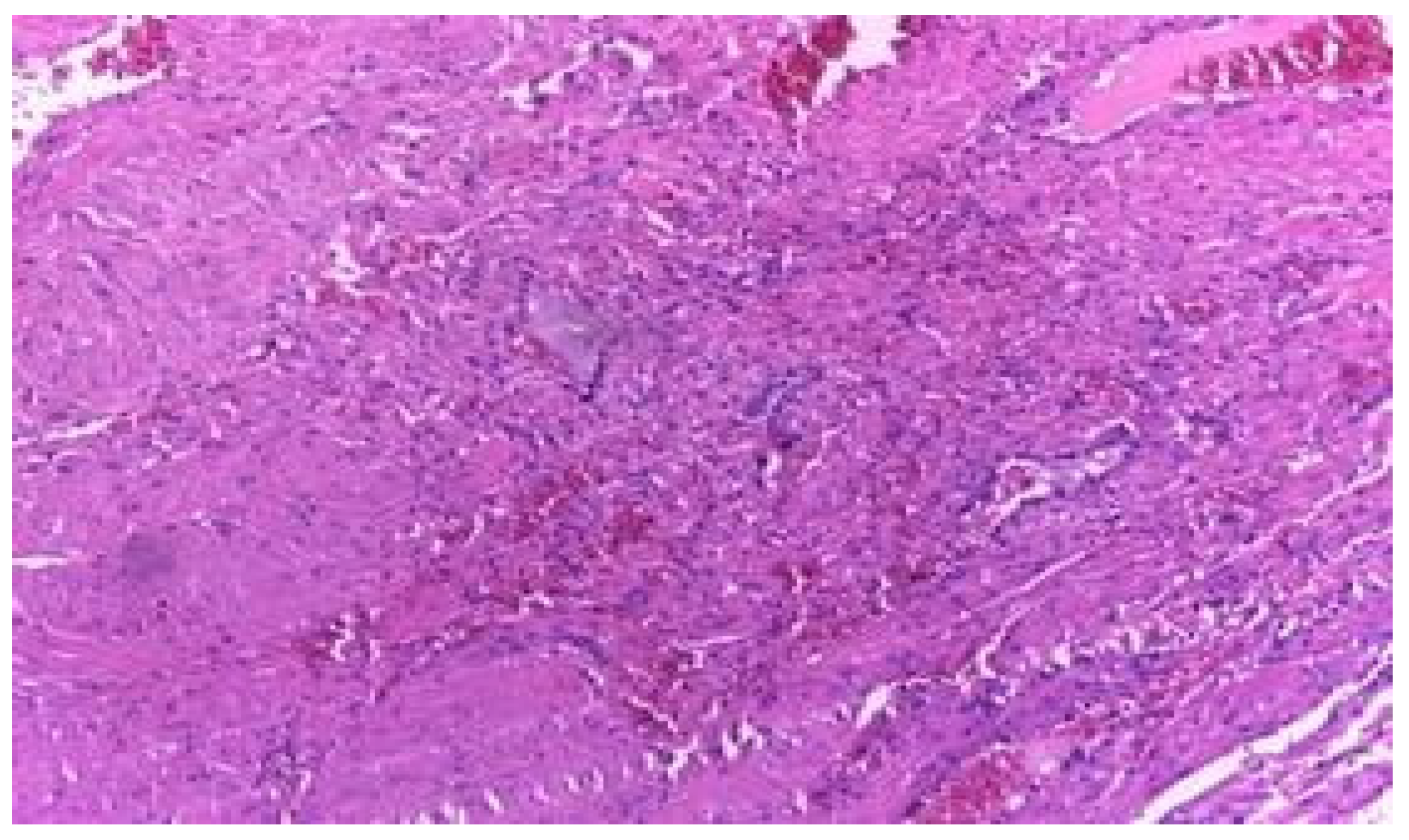
Results
Discussions
- o factors regarding hysterorrhaphy
- o factors related to lower segment formation and scar location
- o factors with possible negative impact on scar healing
- o other factors: maternal age, multiple pregnancies, vaginal births, placenta praevia, etc.
Conclusions
Conflict of interest disclosure
Compliance with ethical standards
References
- Poidevin, L.O. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961, 81, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Burger, N.F.; Darazs, B.; Boes, E.G. An echographic evaluation during the early puerperium of the uterine wound after caesarean section. J Clin Ultrasound. 1982, 10, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, S.J.; Hsieh, F.J. Observation of cesarean section scar by transvaginal ultrasonography. Ultrasound Med Biol. 1990, 16, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Osser, O.V.; Jokubkiene, L.; Valentin, L. Cesarean section scar defects: agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet Gynecol. 2010, 35, 75–83. [Google Scholar] [CrossRef]
- Bij de Vaate, A.J.; Brölmann, H.A.; van der Voet, L.F.; van der Slikke, J.W.; Veersema, S.; Huirne, J.A. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol. 2011, 37, 93–99. [Google Scholar] [CrossRef]
- Van der Voet, L.F.; Bij de Vaate, A.M.; Veersema, S.; Brölmann, H.A.; Huirne, J.A. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014, 121, 236–244. [Google Scholar] [CrossRef]
- Monteagudo, A.; Carreno, C.; Timor-Tritsch, I.E. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the "niche" in the scar. J Ultrasound Med. 2001, 20, 1105–1115. [Google Scholar] [CrossRef]
- Basic, E.; Basic-Cetkovic, V.; Kozaric, H.; Rama, A. Ultrasound evaluation of uterine scar after cesarean section. Acta Inform Med. 2012, 20, 149–153. [Google Scholar] [CrossRef]
- Bij de Vaate, A.J.; van der Voet, L.F.; Naji, O.; Witmer, M.; Veersema, S.; Brölmann, H.A.; Bourne, T.; Huirne, J.A. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014, 43, 372–382. [Google Scholar] [CrossRef]
- Vikhareva Osser, O.; Valentin, L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG. 2010, 117, 1119–1126. [Google Scholar] [CrossRef]
- Hayakawa, H.; Itakura, A.; Mitsui, T.; Okada, M.; Suzuki, M.; Tamakoshi, K.; Kikkawa, F. Methods for myometrium closure and other factors impacting effects on cesarean section scars of the uterine segment detected by the ultrasonography. Acta Obstet Gynecol Scand. 2006, 85, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, F.; G¨okdogan, A.; Kelekci, S.; Ayg¨un, M.; Savan, K. Incomplete healing of the uterine incision after caesarean section: Is it preventable? Eur J Obstet Gynecol Reprod Biol. 2006, 124, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ofili-Yebovi, D.; Ben-Nagi, J.; Sawyer, E.; Yazbek, J.; Lee, C.; Gonzalez, J.; Jurkovic, D. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol. 2008, 31, 72–77. [Google Scholar] [CrossRef]
- Mangla, D.; Singh, S.P.; Swasty, *!!! REPLACE !!!*; Chauhan, J. A study to determine scar integrity in pregnant women with previous lower segment caesarean section. Int J Reprod Contracept Obstet Gynecol. 2016, 5, 711–714. [Google Scholar] [CrossRef]
- Bujold, E.; Jastrow, N.; Simoneau, J.; Brunet, S.; Gauthier, R.J. Prediction of complete uterine rupture by sonographic evaluation of the lower uterine segment. Am J Obstet Gynecol. 2009, 201, 320.e1–6. [Google Scholar] [CrossRef]
- Jastrow, N.; Chaillet, N.; Roberge, S.; Morency, A.M.; Lacasse, Y.; Bujold, E. Sonographic lower uterine segment thickness and risk of uterine scar defect: a systematic review. J Obstet Gynaecol Can. 2010, 32, 321–327. [Google Scholar] [CrossRef]
- Rozenberg, P.; Goffinet, F.; Phillippe, H.J.; Nisand, I. Ultrasonographic measurement of lower uterine segment to assess risk of defects of scarred uterus. Lancet 1996, 347, 281–284. [Google Scholar] [CrossRef]
- Cheung, V.Y.; Constantinescu, O.C.; Ahluwalia, B.S. Sonographic evaluation of the lower uterine segment in patients with previous cesarean delivery. J Ultrasound Med. 2004, 23, 1441–1447. [Google Scholar] [CrossRef]
- Cheung, V.Y. Sonographic measurement of the lower uterine segment thickness in women with previous caesarean section. J Obstet Gynaecol Can. 2005, 27, 674–681. [Google Scholar] [CrossRef]
- Sen, S.; Malik, S.; Salhan, S. Ultrasonographic evaluation of lower uterine segment thickness in patients of previous cesarean section. Int J Gynaecol Obstet. 2004, 87, 215–219. [Google Scholar] [CrossRef]
- Stanescu, A.D.; Balalau, D.O.; Ples, L.; Paunica, S.; Balalau, C. Postpartum depression: Prevention and multimodal therapy. J Mind Med Sci. 2018, 5, 163–168. [Google Scholar] [CrossRef]
- Kok, N.; Wiersma, I.C.; Opmeer, B.C.; de Graaf, I.M.; Mol, B.W.; Pajkrt, E. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2014, 42, 132–139. [Google Scholar] [CrossRef]
- Kinjo, T.; Masamoto, H.; Mekaru, K.; Taira, Y.; Chinen, Y.; Nitta, H.; Aoki, Y. Measurements of the Lower Uterine Segment at Term in Women with Previous Cesarean Delivery. Open Journal of Obstetrics and Gynecology. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Balalau, D.O.; Sima, R.M.; Bacalbașa, N.; Pleș, L.; Stănescu, A.D. Emergency peripartum hysterectomy, physical and mental consequences: a 6-year study. J Mind Med Sci. 2016, 3, 65–70. [Google Scholar] [CrossRef]
- Naji, O.; Daemen, A.; Smith, A.; Abdallah, Y.; Saso, S.; Stalder, C.; Sayasneh, A.; McIndoe, A.; Ghaem-Maghami, S.; Timmerman, D.; Bourne, T. Bourne Changes in Cesarean section scar dimensions during pregnancy: a prospective longitudinal study. Ultrasound Obstet Gynecol. 2013, 41, 556–562. [Google Scholar] [CrossRef]
- Bălălău, D.O.; Bacalbașa, N.; Stănescu, A.D. Cesarean scar defects and placental abnormalities – a 3-year survey study. J Mind Med Sci. 2017, 4, 156–162. [Google Scholar] [CrossRef]
- Siegel, I. Scars of the pregnant and nonpregnant uterus Histologic comparison of scars two weeks postoperatively. Am J Obstet Gynecol. 1952, 64, 301–308. [Google Scholar] [CrossRef]
- Wojdecki, J.; Grynsztajn, A. Scar formation in the uterus after cesarean section. Am J Obstet Gynecol. 1970, 107, 322–324. [Google Scholar] [CrossRef]
- Morris, H. Surgical pathology of the lower uterine segment caesarean section scar: is the scar a source of clinical symptoms? Int J Gynecol Pathol. 1995, 14, 16–20. [Google Scholar] [CrossRef]
- Balalau, D.O.; Sima, R.M.; Bacalbasa, N.; Banu, P.; Bălălău, C.; Ples, L.; Stanescu, A.D. High-grade cervical dysplasia in pregnancy – psychological and medical challenges. J Mind Med Sci. 2017, 4, 24–30. [Google Scholar] [CrossRef]
- Boda, D.; Negrei, C.; Nicolescu, F.; Balalau, C. Assessment of some oxidative stress parameters in methotrexate treated psoriasis patients. Farmacia 2014, 62, 704–710. [Google Scholar]
- Pantea-Stoian, A.; Pituru, S.M.; Hainarosie, R.; et al. Testosterone therapy, new opportunities in diabetes mellitus. Farmacia 2018, 66, 1–7. [Google Scholar]
- Ben-Nagi, J.; Walker, A.; Jurkovic, D.; Yazbek, J.; Aplin, J.D. Effects of cesarean delivery on the endometrium. Int J Gynaecol Obstet. 2009, 106, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Flo, K.; Widnes, C.; Vartun, A.; Acharya, G. Blood flow to the scarred gravid uterus at 22-24 weeks of gestation. BJOC 2014, 121, 210–215. [Google Scholar] [CrossRef]


© 2019 by the author. 2019 Oana D. Bălălău, Nicolae Bacalbașa, Cristian Bălălău, Carolina Negrei, Bianca Gălățeanu, Octav Ghinghină, Cristina Răduță, Liana Pleș, Anca D. Stănescu, Vasile A. Dumitru
Share and Cite
Bălălău, O.D.; Bacalbașa, N.; Bălălău, C.; Negrei, C.; Gălățeanu, B.; Ghinghină, O.; Răduță, C.; Pleș, L.; Stănescu, A.D.; Dumitru, V.A. The Correlation Between Histopathological and Ultrasound Findings Regarding Cesarean Section Scars—A Three-Year Survey Study. J. Mind Med. Sci. 2019, 6, 143-149. https://doi.org/10.22543/7674.61.P143149
Bălălău OD, Bacalbașa N, Bălălău C, Negrei C, Gălățeanu B, Ghinghină O, Răduță C, Pleș L, Stănescu AD, Dumitru VA. The Correlation Between Histopathological and Ultrasound Findings Regarding Cesarean Section Scars—A Three-Year Survey Study. Journal of Mind and Medical Sciences. 2019; 6(1):143-149. https://doi.org/10.22543/7674.61.P143149
Chicago/Turabian StyleBălălău, Oana D., Nicolae Bacalbașa, Cristian Bălălău, Carolina Negrei, Bianca Gălățeanu, Octav Ghinghină, Cristina Răduță, Liana Pleș, Anca D. Stănescu, and Vasile A. Dumitru. 2019. "The Correlation Between Histopathological and Ultrasound Findings Regarding Cesarean Section Scars—A Three-Year Survey Study" Journal of Mind and Medical Sciences 6, no. 1: 143-149. https://doi.org/10.22543/7674.61.P143149
APA StyleBălălău, O. D., Bacalbașa, N., Bălălău, C., Negrei, C., Gălățeanu, B., Ghinghină, O., Răduță, C., Pleș, L., Stănescu, A. D., & Dumitru, V. A. (2019). The Correlation Between Histopathological and Ultrasound Findings Regarding Cesarean Section Scars—A Three-Year Survey Study. Journal of Mind and Medical Sciences, 6(1), 143-149. https://doi.org/10.22543/7674.61.P143149


