A Perfusion Decellularization Heart Model—An Interesting Tool for Cell-Matrix Interaction Studies
Highlights
- After heart decellularization, there is the possibility of developing research in the field of biomaterials, tissue engineering and cardiac cell cultures.
- The decellularized extracellular matrix appears to be an attractive substrate for cells grown on these surfaces because they retain their stem cell characteristics for a long time.
Highlights
- After heart decellularization, there is the possibility of developing research in the field of biomaterials, tissue engineering and cardiac cell cultures.
- The decellularized extracellular matrix appears to be an attractive substrate for cells grown on these surfaces because they retain their stem cell characteristics for a long time.
Abstract
Introduction
Materials and Methods
Larger hearts (pig and human)
The rat heart
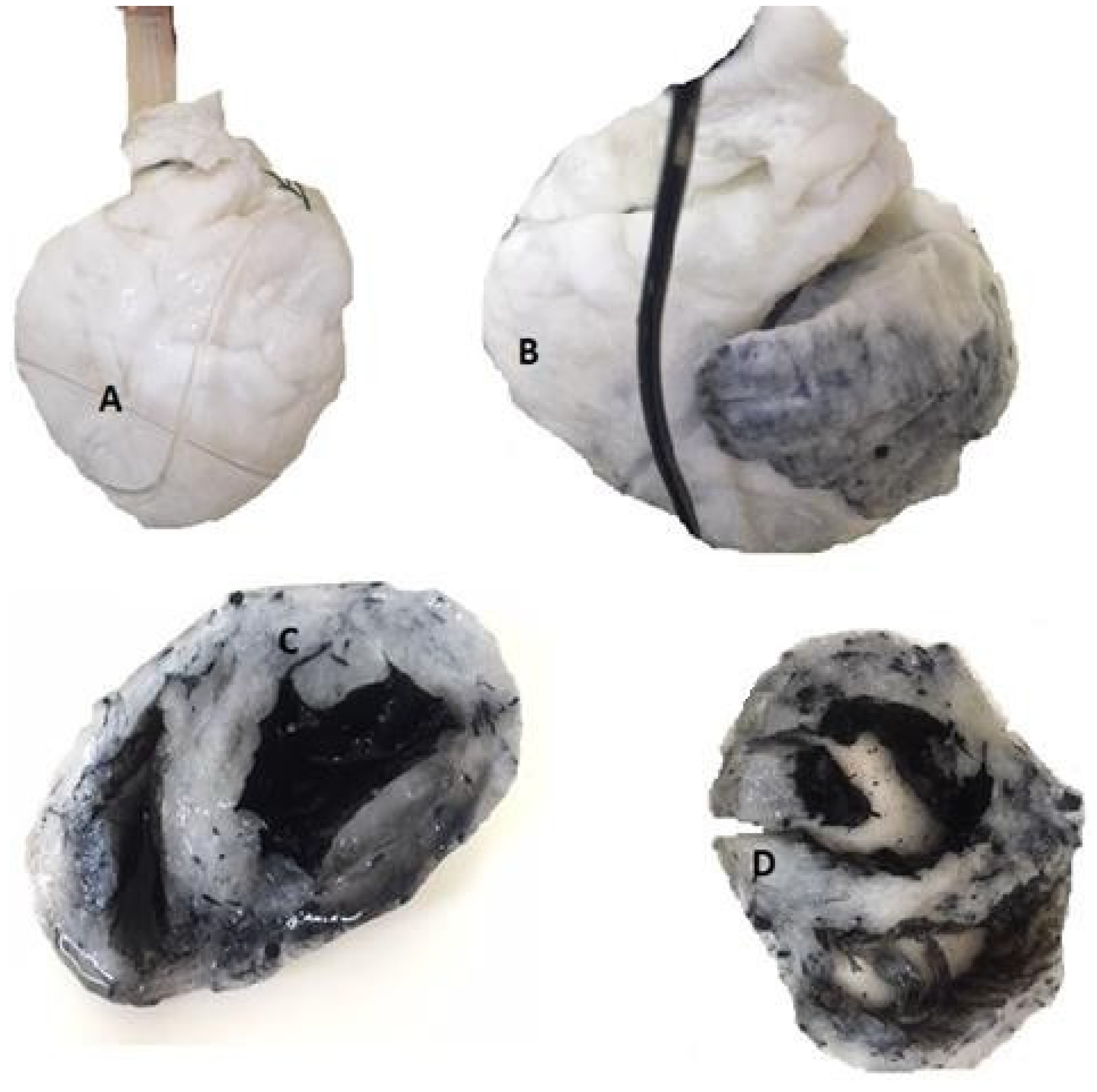
Results
Discussions
- -
- The quantity of cells
- -
- Density
- -
- Lipid content
- -
- Thickness of tissue.
- -
- The lysis of the cell membrane
- -
- The separation of cellular components from the extracellular matrix
- -
- The solubilization of cytosolic and nuclear cellular components
- -
- The removal of cellular debris from the tissue.
- Physical factors
- Chemical factors
- -
- Non-ionic detergents
- -
- Ionic detergents
- -
- Zwitterion detergents
- Enzymatic factors
Conclusions
Conflict of interest disclosure
Compliance with ethical standards
References
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in Normal Skin and in Pathological Processes. N Am J Med Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Keskin, D.; Kalluri, R. Interaction between the extracellular matrix and lymphatics - consequences for lymphangiogenesis and lymphatic function. Matrix Biol. 2010, 29, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Fomovsky, G.M.; Thomopoulos, S.; Holmes, J.W. Contribution of Extracellular Matrix to the Mechanical Properties of the Heart. J Mol Cell Cardiol. 2010, 48, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Kapelko, V.I. Extracellular matrix alterations in cardiomyopathy: The possible crucial role in the dilative form. Exp Clin Cardiol. 2001, 6, 41–49. [Google Scholar] [PubMed]
- Eckhouse, S.R.; Spinale, F.G. Changes in the Myocardial Interstitium and Contribution to the Progression of Heart Failure. Heart Fail Clin. 2012, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Medugorac, I.; Jacob, R. Characterisation of left ventricular collagen in the rat. Cardiovasc Res. 1983, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Janicki, J.S.; Shroff, S.G.; Pick, R.; Chen, R.M.; Bashey, R.I. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988, 62, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.; Weber, K.T.; Eghbali, M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990, 67, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Halper, J.; Kjaer, M. Basic Components of Connective Tissues and Extracellular Matrix: Elastin, Fibrillin, Fibulins, Fibrinogen, Fibronectin, Laminin, Tenascins and Thrombospondins. In Progress in Heritable Soft Connective Tissue Diseases. Advances in Experimental Medicine and Biology; Halper, J., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 802. [Google Scholar]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-based Biomaterial Scaffolds and the Host Response. Biomaterials. 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Hauch, K.D.; Laflamme, M.A. Cardiac Applications for Human Pluripotent Stem Cells. Curr Pharm Des. 2009, 15, 2791–2806. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; To, F.; Butler, J.R.; Claude, A.; McLaughlin, R.M.; Williams, L.N.; de Jongh Curry, A.L.; Liao, J. Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir. 2013, 29, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Integrins. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26867/.
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials. 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-based Biomaterial Scaffolds and the Host Response. Biomaterials. 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget 2016, 7, 58671–58683. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular Matrix and Heart Development. Birth Defects Res A Clin Mol Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.C.; Stanescu, A.M.A.; Pantea, S.A.; et al. Hyperkalemia and Cardiovascular Diseases: New Molecules for the Treatment. Revista de Chimie 2018, 69, 1367–1370. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the author. 2019 Mihai Meșină, Ion Mîndrilă, Cristian Meșină, Cosmin Vasile Obleagă, Octavian Istrătoaie
Share and Cite
Meșină, M.; Mîndrilă, I.; Meșină, C.; Obleagă, C.V.; Istrătoaie, O. A Perfusion Decellularization Heart Model—An Interesting Tool for Cell-Matrix Interaction Studies. J. Mind Med. Sci. 2019, 6, 137-142. https://doi.org/10.22543/7674.61.P137142
Meșină M, Mîndrilă I, Meșină C, Obleagă CV, Istrătoaie O. A Perfusion Decellularization Heart Model—An Interesting Tool for Cell-Matrix Interaction Studies. Journal of Mind and Medical Sciences. 2019; 6(1):137-142. https://doi.org/10.22543/7674.61.P137142
Chicago/Turabian StyleMeșină, Mihai, Ion Mîndrilă, Cristian Meșină, Cosmin Vasile Obleagă, and Octavian Istrătoaie. 2019. "A Perfusion Decellularization Heart Model—An Interesting Tool for Cell-Matrix Interaction Studies" Journal of Mind and Medical Sciences 6, no. 1: 137-142. https://doi.org/10.22543/7674.61.P137142
APA StyleMeșină, M., Mîndrilă, I., Meșină, C., Obleagă, C. V., & Istrătoaie, O. (2019). A Perfusion Decellularization Heart Model—An Interesting Tool for Cell-Matrix Interaction Studies. Journal of Mind and Medical Sciences, 6(1), 137-142. https://doi.org/10.22543/7674.61.P137142


