Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study
Highlights
- This study investigates the changes present in the fungal microbiota of diabetic patients with diabetic kidney disease, and existing correlations between treatment regimens and microbiota patterns.
- These preliminary results pave the way for additional studies on larger patient cohorts.
Highlights
- This study investigates the changes present in the fungal microbiota of diabetic patients with diabetic kidney disease, and existing correlations between treatment regimens and microbiota patterns.
- These preliminary results pave the way for additional studies on larger patient cohorts.
Abstract
Introduction
Materials and Methods
Patients
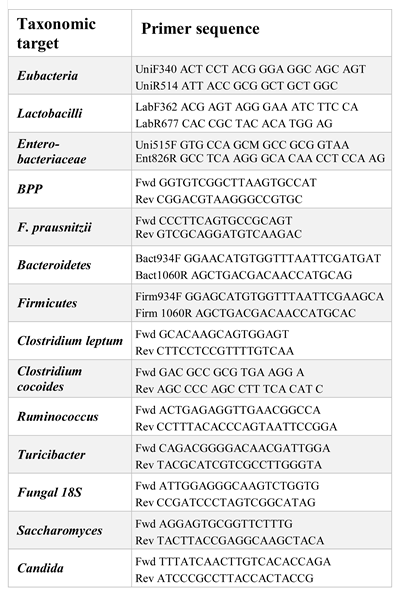
Microbiota analysis
The statistical analysis
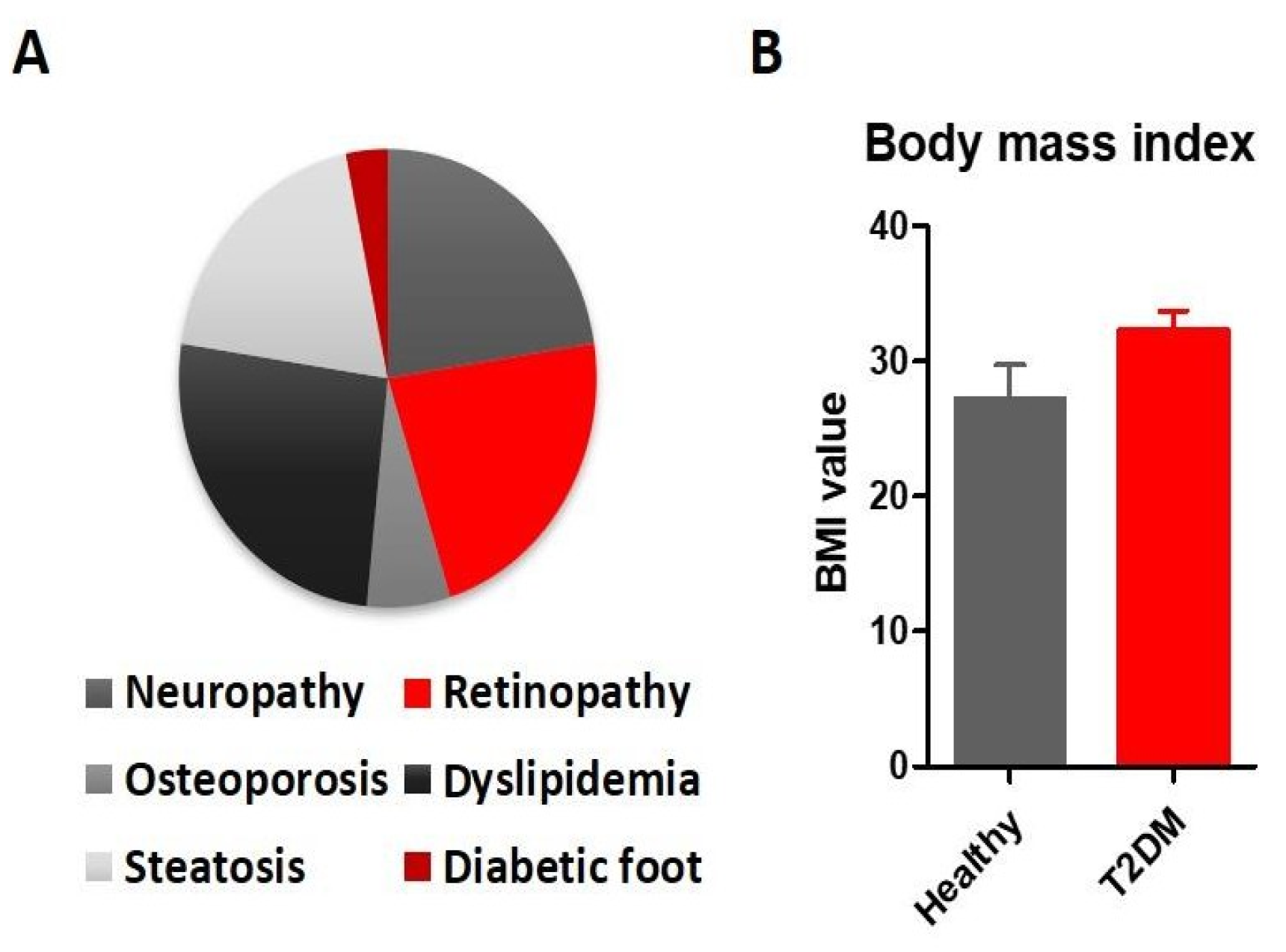
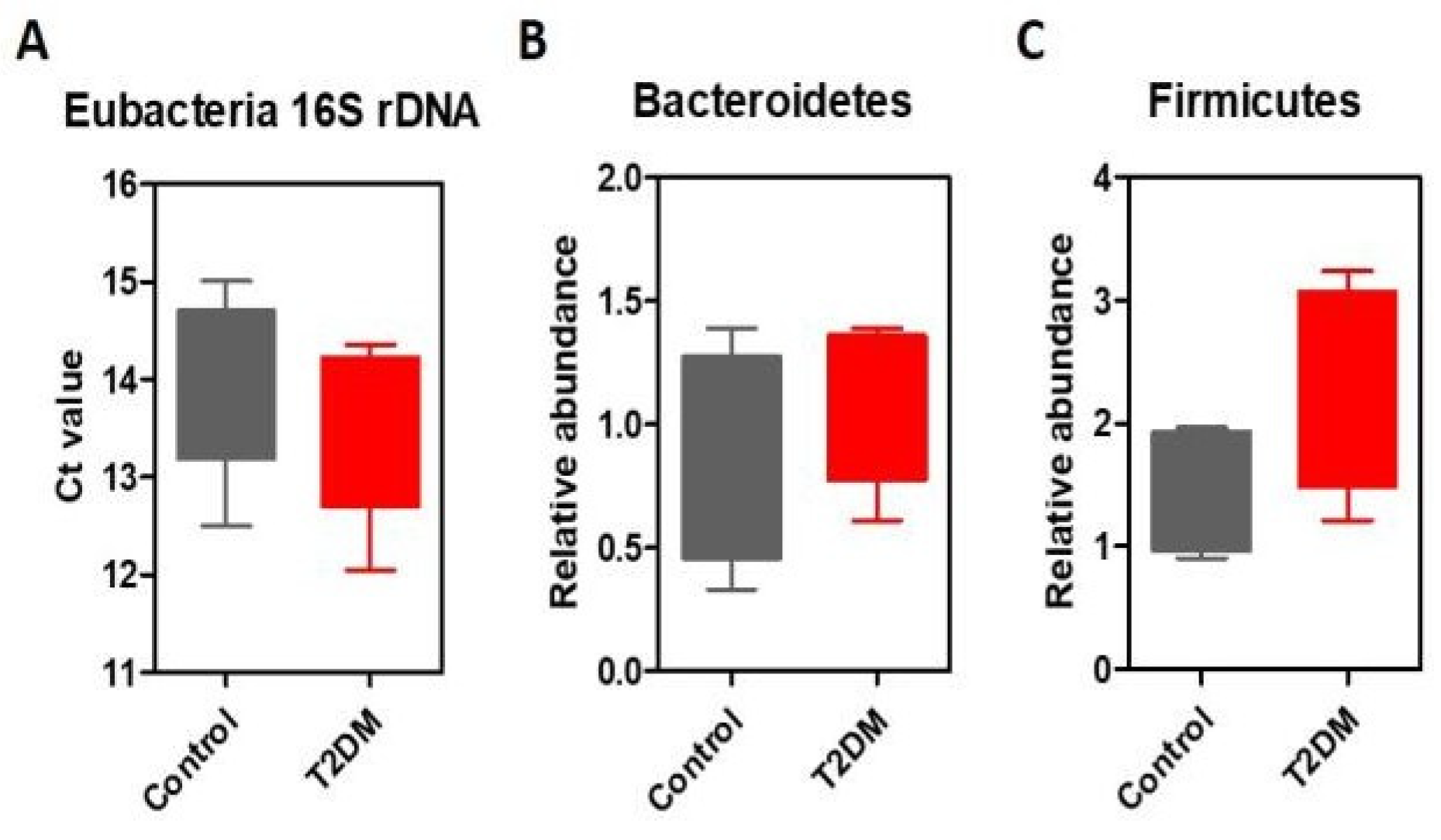
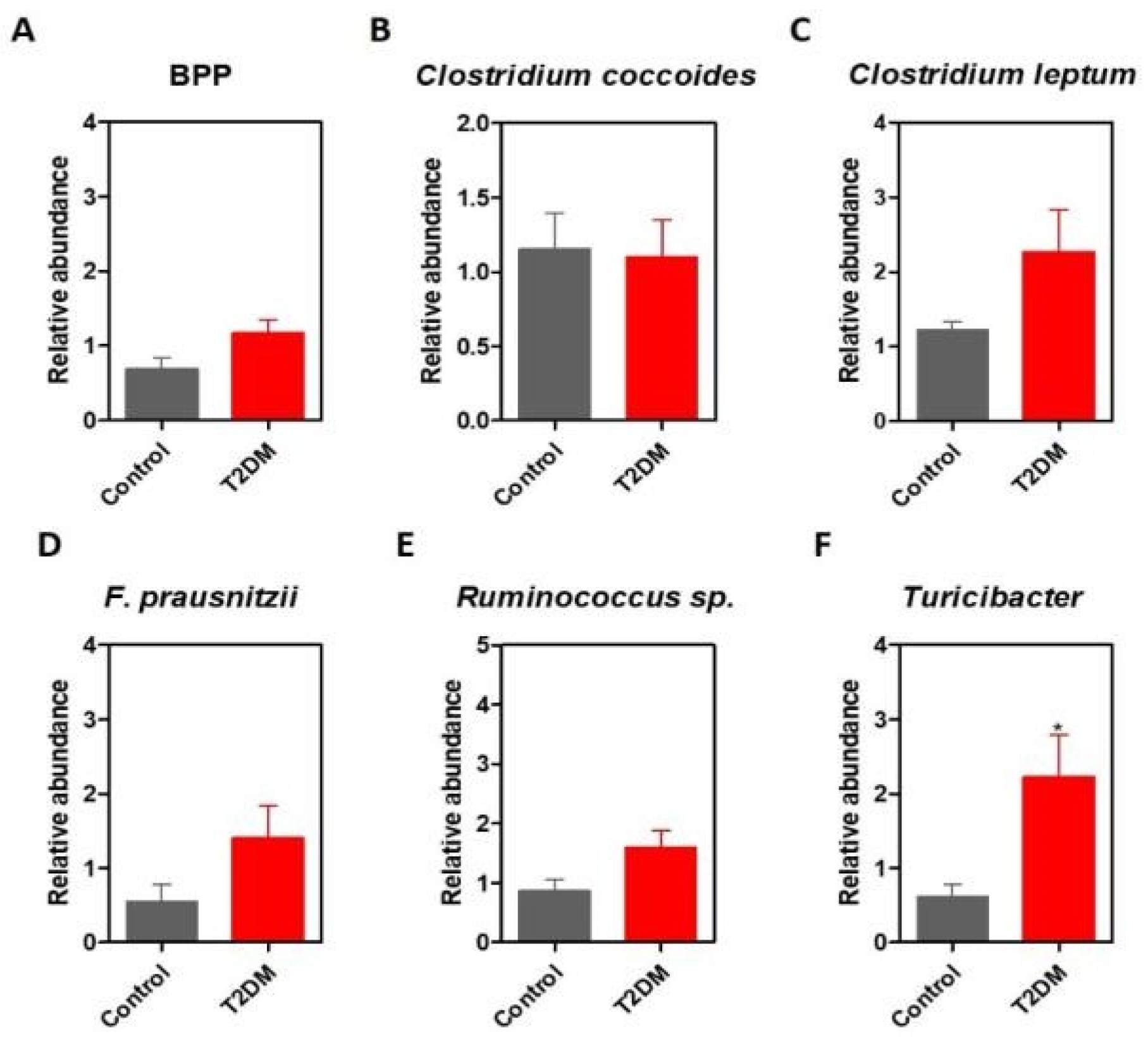
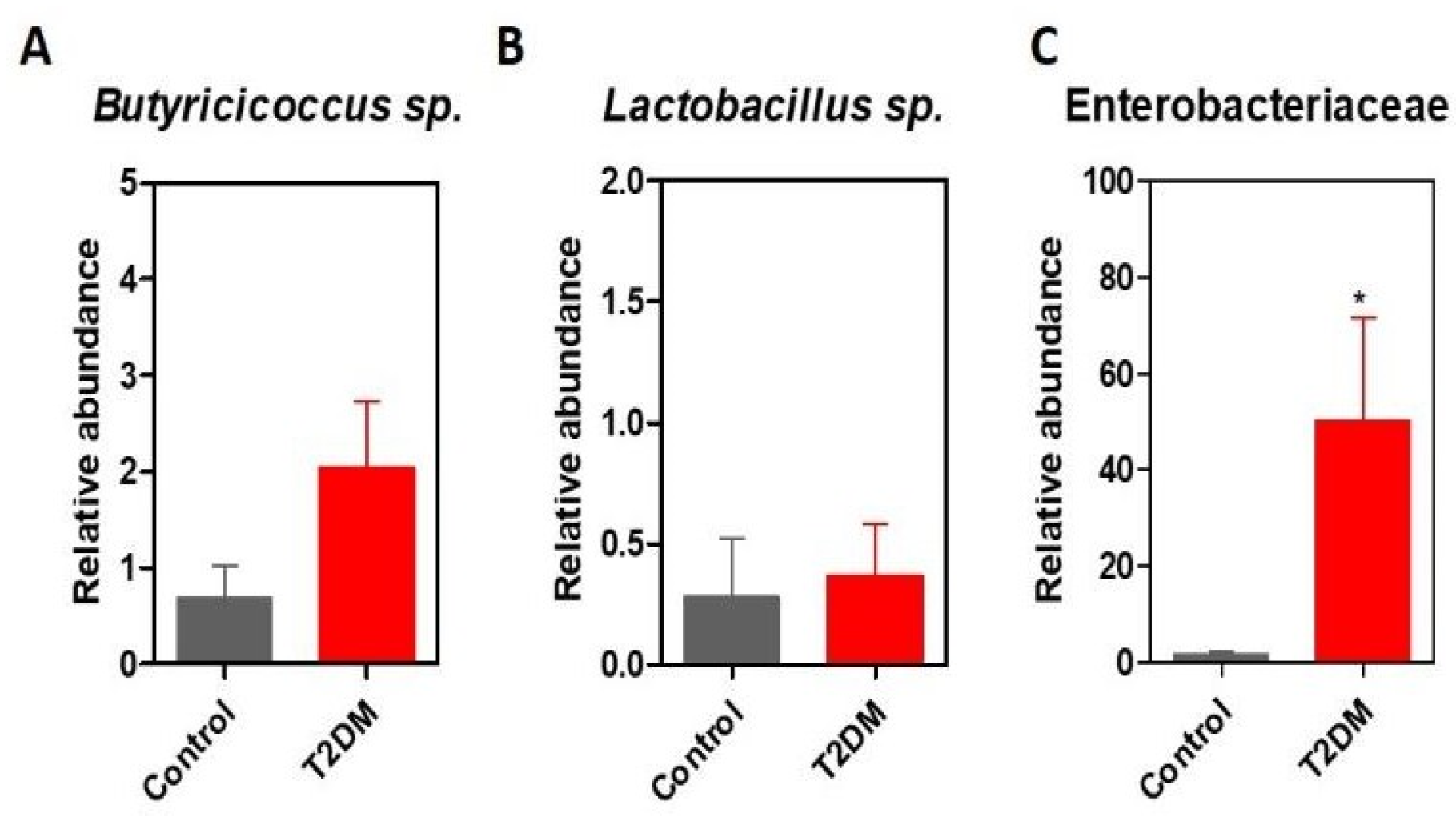
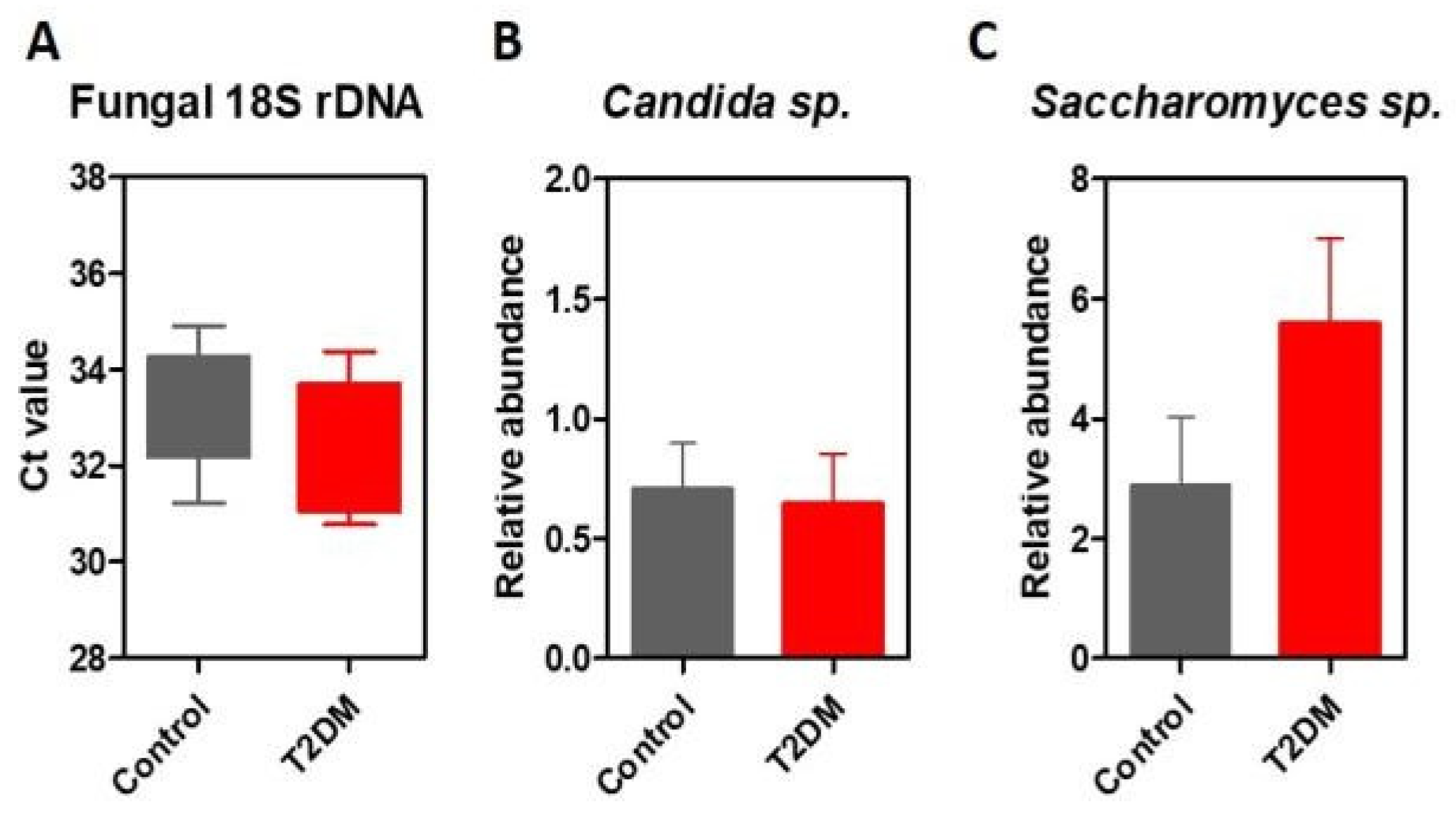
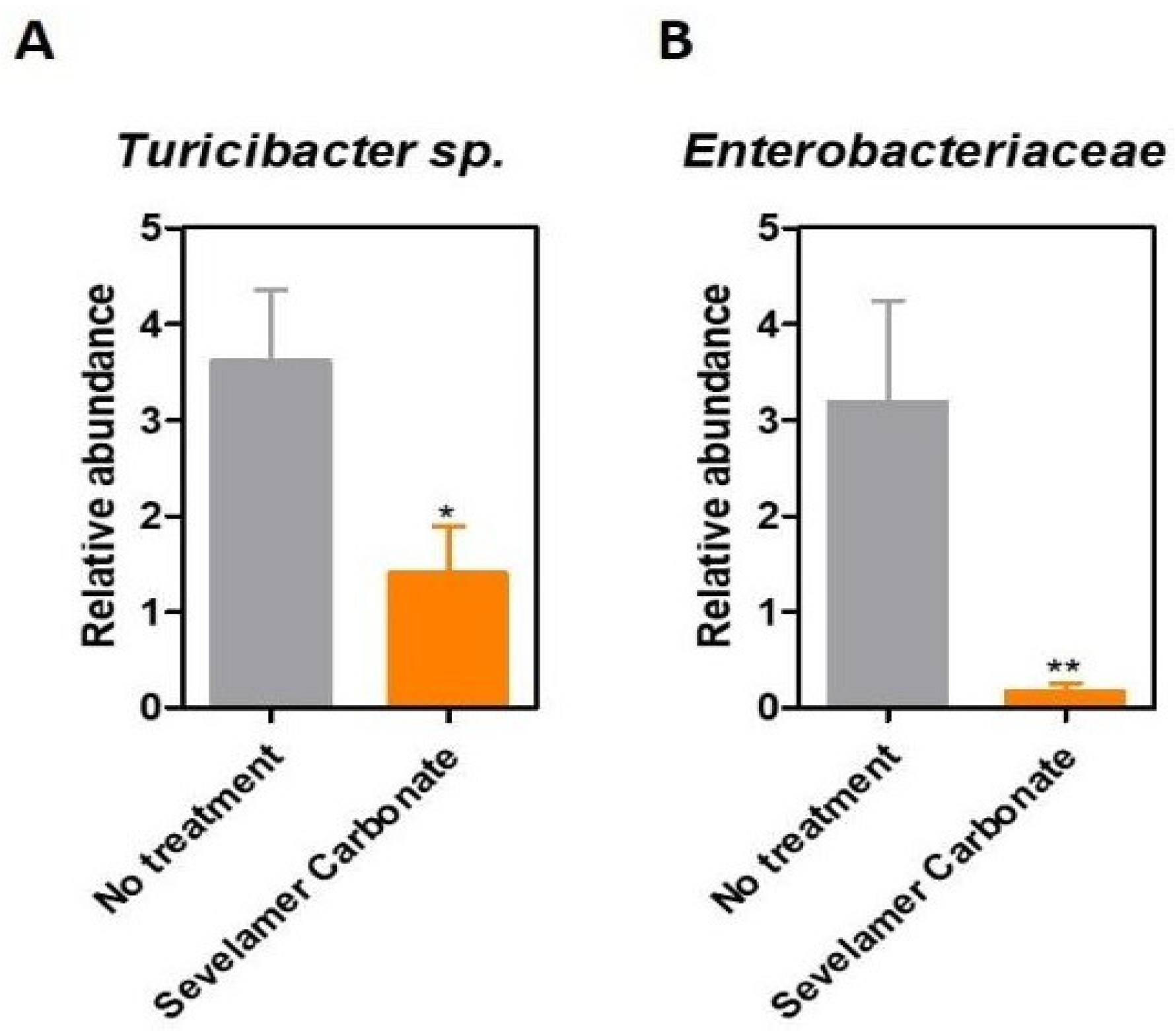
Results
Discussions
Conclusions
Acknowledgments
Conflicts of Interest disclosure
Compliance with ethical standards
References
- Astrup, A.F.N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev. 2001, 1, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ayadurai, S.; Hattingh, H.L.; Tee, L.B.; et al. A narrative review of diabetes intervention studies to explore diabetes care opportunities for pharmacists. J Diabetes Res. 2016, 2016, 5897452. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J Diabetes. 2016, 8, 336–344. [Google Scholar] [CrossRef]
- Rusu, A.; Bala, C.G.; Craciun, A.E.; et al. HbA1c levels are associated with severity of hypoxemia and not with apnea hypopnea index in patients with type 2 diabetes: Results from a cross-sectional study. Journal of Diabetes. 2017, 9, 555–561. [Google Scholar] [CrossRef]
- Bala, C.; Craciun, A.E.; Hancu, N. Updating the concept of metabolically healthy obesity. Acta Endocrinologica-Bucharest. 2016, 12, 197–205. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care. 2010, 33, 2277–2284. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010, 5, e9085. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012, 490, 55–60. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes. Isbn. 2016, 978, 88. [Google Scholar]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; et al. Diversity of the human intestinal microbial flora. Science. 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sakamoto, M.B.Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002, 46, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.T.R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Env Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef]
- Lopez, C.A.; Miller, B.M.; Rivera-Chavez, F.; et al. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science. 2016, 353, 1249–1253. [Google Scholar] [CrossRef]
- Winter, S.E.; Lopez, C.A.; Bäumler, A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011, 6, e25792. [Google Scholar] [CrossRef]
- Paunica, M.; Gheorghiu, R.; Curaj, A.; Holeab, C. Foresight for restructuring R&D systems. Amfiteatru economic 2009, 11, 201–210. [Google Scholar]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.J.M. Gut microbiota in human adults with type 2 diabetes differs from non- diabetic adults. PLoS One. 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Chelariu, M.; Grosu, M.; Gheorghe, I.; Gradisteanu, G.; Picu, A.; Petcu, L.; et al. Host metabolic syndrome can disrupt the intestinal microbiota and promote the acquisition of resistance and virulence genes in Enterobacteriaceae stains. Rom Biotechnol Lett. 2017, 22, 12643–12650. [Google Scholar]
- Suceveanu, A.I.; Pantea Stoian, A.; Parepa, R.I.; Voinea, C.; Hainarosie, R.; Manuc, D.; et al. Gut microbiota patterns in obese and type 2 diabetes. (T2D) patients from Romanian black sea coast region. Rev Chim. 2018, 69, 2260–2267. [Google Scholar] [CrossRef]
 |
© 2019 by the author. 2019 Gratiela P. Gradisteanu, Roxana A. Stoica, Laura Petcu, Ariana Picu, Adrian P. Suceveanu, Teodor Salmen, Diana S. Stefan, Cristian Serafinceanu, Mariana C. Chifiriuc, Anca P. Stoian
Share and Cite
Gradisteanu, G.P.; Stoica, R.A.; Petcu, L.; Picu, A.; Suceveanu, A.P.; Salmen, T.; Stefan, D.S.; Serafinceanu, C.; Chifiriuc, M.C.; Stoian, A.P. Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study. J. Mind Med. Sci. 2019, 6, 130-136. https://doi.org/10.22543/7674.61.P130136
Gradisteanu GP, Stoica RA, Petcu L, Picu A, Suceveanu AP, Salmen T, Stefan DS, Serafinceanu C, Chifiriuc MC, Stoian AP. Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study. Journal of Mind and Medical Sciences. 2019; 6(1):130-136. https://doi.org/10.22543/7674.61.P130136
Chicago/Turabian StyleGradisteanu, Gratiela P., Roxana A. Stoica, Laura Petcu, Ariana Picu, Adrian P. Suceveanu, Teodor Salmen, Diana S. Stefan, Cristian Serafinceanu, Mariana C. Chifiriuc, and Anca P. Stoian. 2019. "Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study" Journal of Mind and Medical Sciences 6, no. 1: 130-136. https://doi.org/10.22543/7674.61.P130136
APA StyleGradisteanu, G. P., Stoica, R. A., Petcu, L., Picu, A., Suceveanu, A. P., Salmen, T., Stefan, D. S., Serafinceanu, C., Chifiriuc, M. C., & Stoian, A. P. (2019). Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study. Journal of Mind and Medical Sciences, 6(1), 130-136. https://doi.org/10.22543/7674.61.P130136



