Natural Alternatives for Pain Relief: A Study on Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata
Abstract
1. Introduction
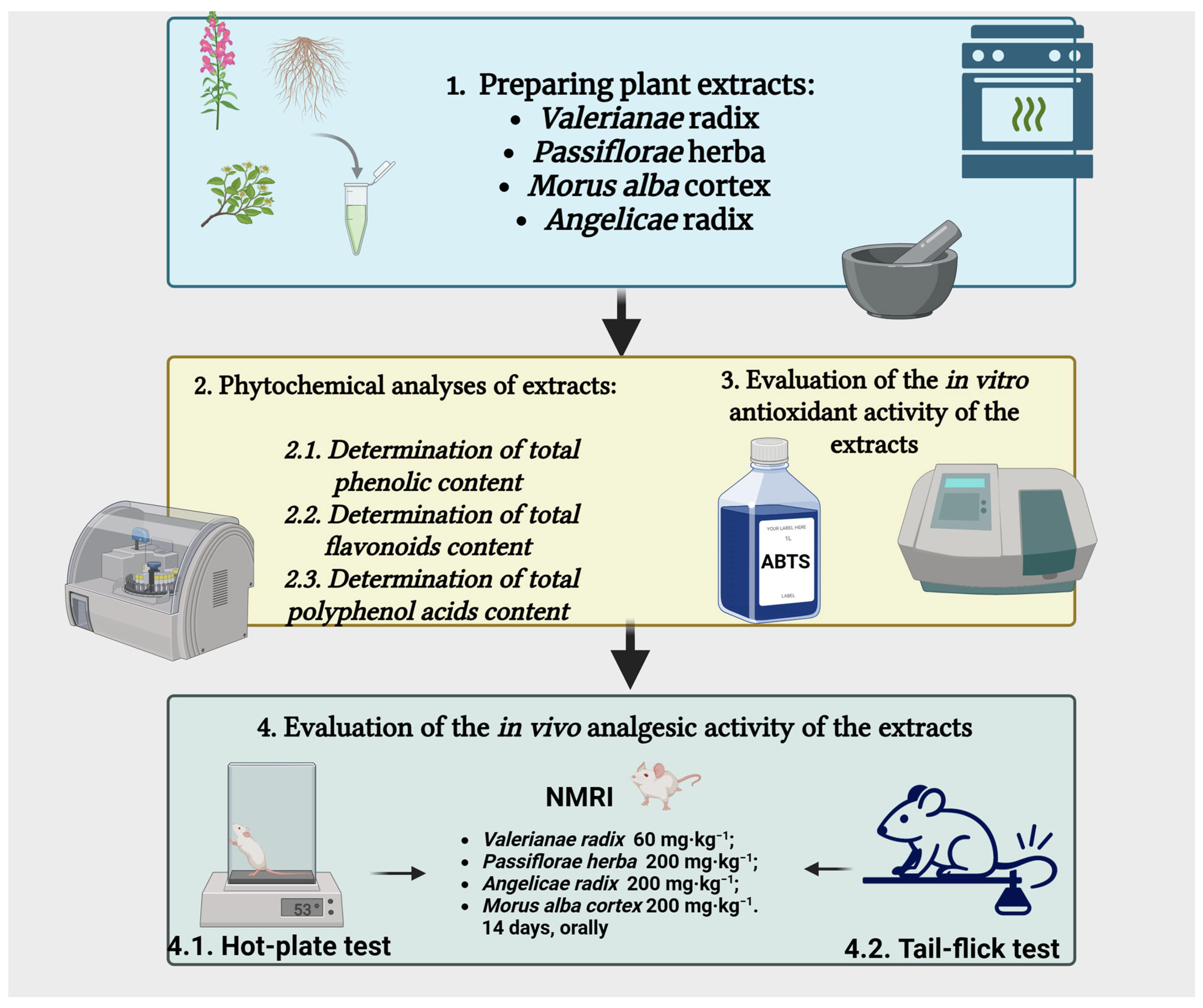
2. Materials and Methods
2.1. Plant Materials and Extracts
2.2. Phytochemical Analyses of Dry Plant Extracts
2.2.1. Determination of Total Phenolic Content (TP)
2.2.2. Determination of Total Flavonoid Content (TF)
2.2.3. Determination of Total Polyphenol Acids Content (TPA)
2.3. Evaluation of Antioxidant Activity In Vitro
2.4. Evaluation of Pain Sensitivity in Vivo
2.4.1. Animals
2.4.2. Experimental Groups
2.4.3. Hot-Plate Test
2.4.4. Tail-Flick Test
2.4.5. Statistical Analysis
3. Results
3.1. Phytochemical Analyses of Dry Plant Extracts
3.2. Evaluation of Antioxidant Activity in Vitro
3.3. Tests for the Evaluation of Pain Sensitivity
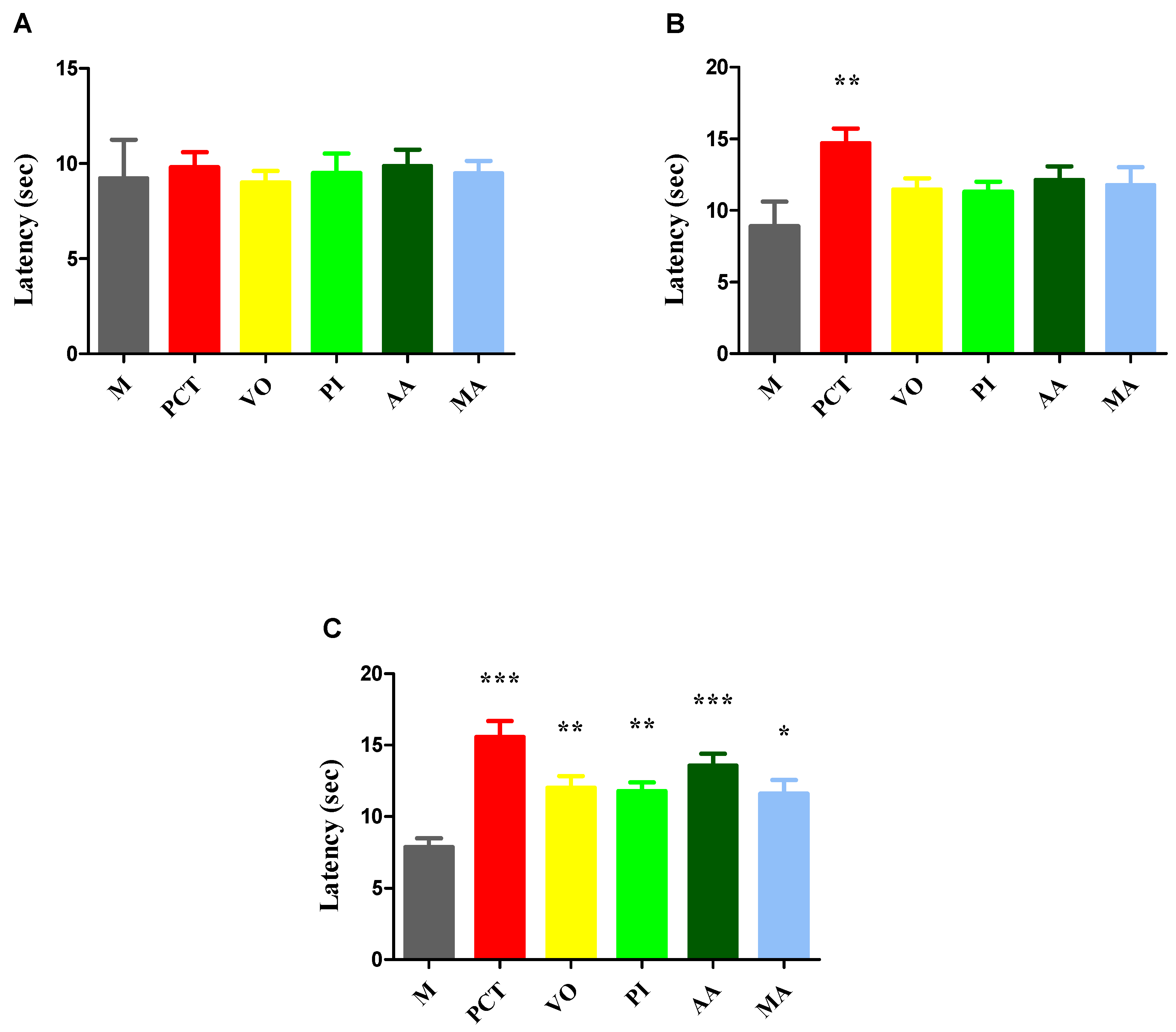
3.3.1. Hot-Plate Test
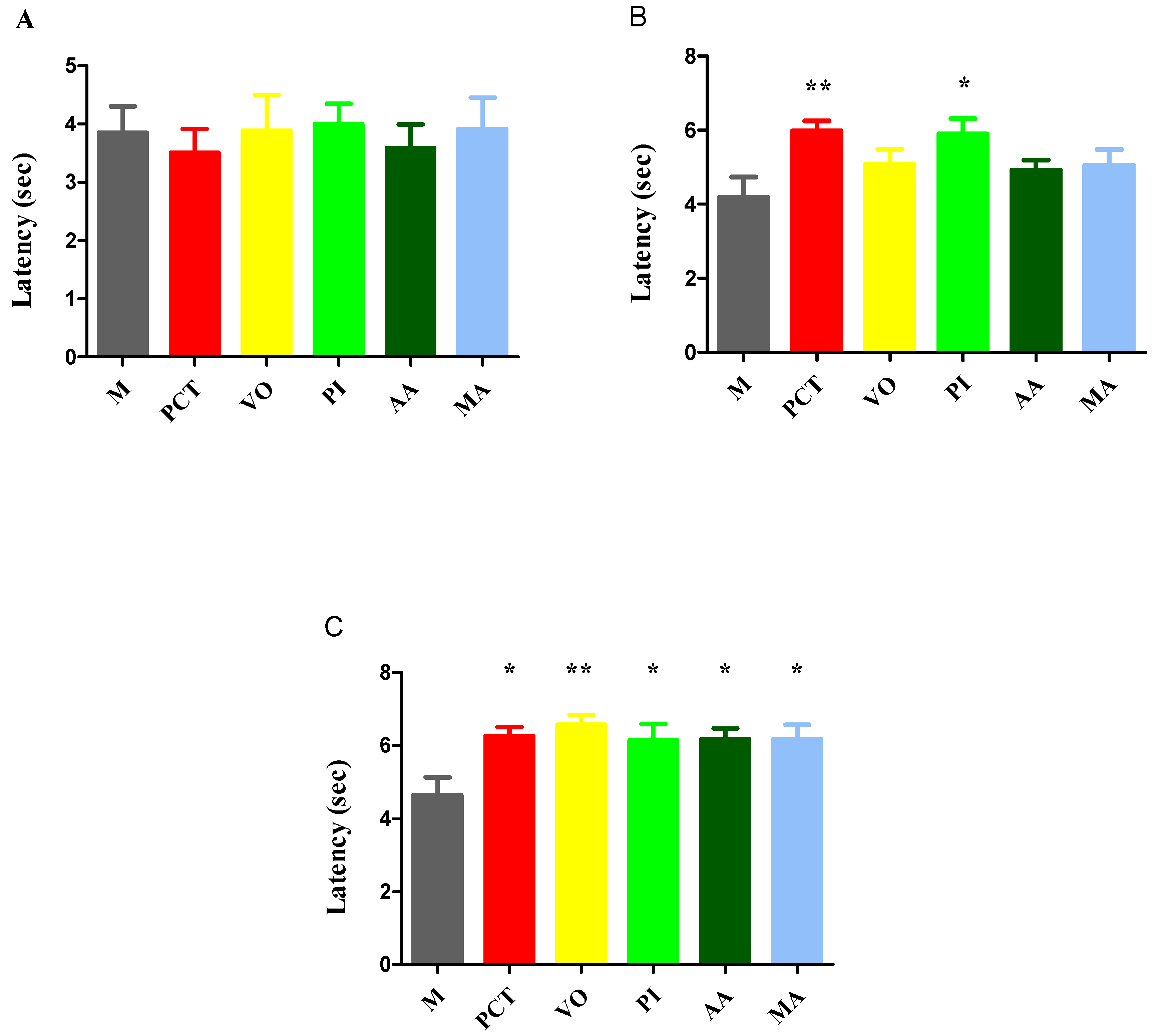
3.3.2. Tail-Flick Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Nagulapalli Venkata, K.C.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.; Pittalà, V.; et al. The Analgesic Potential of Glycosides Derived from Medicinal Plants. DARU J. Pharm. Sci. 2020, 28, 387–401. [Google Scholar] [CrossRef]
- Silva-Correa, C.R.; Campos-Reyna, J.L.; Villarreal-La Torre, V.E.; Calderon-Pena, A.A.; Gonzalez Blas, M.V.; Aspajo-Villalaz, C.L.; Cruzado-Razco, J.L.; Sagastegui-Guarniz, W.A.; Guerrero-Espino, L.M.; Hilario-Vargas, J. Potential Activity of Medicinal Plants as Pain Modulators: A Review. Pharmacogn. J. 2021, 13, 248–263. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Q. Global Research Trends of Herbal Medicine for Pain in Three Decades (1990–2019): A Bibliometric Analysis. J. Pain Res. 2021, 14, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Pușcașu, C.; Andrei, C.; Olaru, O.T.; Zanfirescu, A. Metabolite-Sensing Receptors: Emerging Targets for Modulating Chronic Pain Pathways. Curr. Issues Mol. Biol. 2025, 47, 63. [Google Scholar] [CrossRef] [PubMed]
- Tedore, T.; Weinberg, R.; Witkin, L.; Giambrone, G.P.; Faggiani, S.L.; Fleischut, P.M. Acute Pain Management/Regional Anesthesia. Anesthesiol. Clin. 2015, 33, 739–751. [Google Scholar] [CrossRef]
- Argoff, C.E. Recent Management Advances in Acute Postoperative Pain. Pain Pract. 2014, 14, 477–487. [Google Scholar] [CrossRef]
- Odoma, S.; Zezi, A.U.; Danjuma, N.M.; Ahmed, A.; Magaji, M.G. Elucidation of the Possible Mechanism of Analgesic Actions of Butanol Leaf Fraction of Olax Subscorpioidea Oliv. J. Ethnopharmacol. 2017, 199, 323–327. [Google Scholar] [CrossRef]
- Rauf, A.; Ali, J.; Khan, H.; Mubarak, M.S.; Patel, S. Emerging CAM Ziziphus Nummularia with in Vivo Sedative-Hypnotic, Antipyretic and Analgesic Attributes. 3 Biotech 2016, 6, 11. [Google Scholar] [CrossRef]
- Rauf, A.; Khan, R.; Raza, M.; Khan, H.; Pervez, S.; De Feo, V.; Maione, F.; Mascolo, N. Suppression of Inflammatory Response by Chrysin, a Flavone Isolated from Potentilla Evestita Th. Wolf. In Silico Predictive Study on Its Mechanistic Effect. Fitoterapia 2015, 103, 129–135. [Google Scholar] [CrossRef]
- Shchegol’kov, E.V.; Shchur, I.V.; Burgart, Y.V.; Saloutin, V.I.; Trefilova, A.N.; Ljushina, G.A.; Solodnikov, S.Y.; Markova, L.N.; Maslova, V.V.; Krasnykh, O.P.; et al. Polyfluorinated Salicylic Acid Derivatives as Analogs of Known Drugs: Synthesis, Molecular Docking and Biological Evaluation. Bioorg. Med. Chem. 2017, 25, 91–99. [Google Scholar] [CrossRef]
- Qadir, M.I.; Abbas, K.; Hamayun, R.; Ali, M. Analgesic, Anti-Inflammatory and Anti-Pyretic Activities of Aqueous Ethanolic Extract of Tamarix aphylla L. (Saltcedar) in Mice. Pak. J. Pharm. Sci. 2014, 27, 1985–1988. [Google Scholar]
- Ali, M.; Rauf, A.; Hadda, T.; Bawazeer, S.; Abu-Izneid, T.; Khan, H.; Raza, M.; Khan, S.; Shah, S.; Pervez, S.; et al. Mechanisms Underlying Anti-Hyperalgesic Properties of Kaempferol-3,7- Di-O-α-L-Rhamnopyranoside Isolated from Dryopteris Cycadina. Curr. Top. Med. Chem. 2016, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.H.; Gögenur, I.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Werner, M.U. Analgesic and Antihyperalgesic Effects of Melatonin in a Human Inflammatory Pain Model. Pain 2015, 156, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Reis-Pina, P.; Lawlor, P.G.; Barbosa, A. Cancer-Related Pain Management and the Optimal Use of Opioids. Acta Med. Port. 2015, 28, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Jayakar, S.; Shim, J.; Jo, S.; Bean, B.P.; Singeç, I.; Woolf, C.J. Developing Nociceptor-Selective Treatments for Acute and Chronic Pain. Sci. Transl. Med. 2021, 13, eabj9837. [Google Scholar] [CrossRef]
- Khan, H.; Rengasamy, K.R.R.; Pervaiz, A.; Nabavi, S.M.; Atanasov, A.G.; Kamal, M.A. Plant-Derived MPGES-1 Inhibitors or Suppressors: A New Emerging Trend in the Search for Small Molecules to Combat Inflammation. Eur. J. Med. Chem. 2018, 153, 2–28. [Google Scholar] [CrossRef]
- Khan, H.; Nabavi, S.M.; Sureda, A.; Mehterov, N.; Gulei, D.; Berindan-Neagoe, I.; Taniguchi, H.; Atanasov, A.G. Therapeutic Potential of Songorine, a Diterpenoid Alkaloid of the Genus Aconitum. Eur. J. Med. Chem. 2018, 153, 29–33. [Google Scholar] [CrossRef]
- Khan, H.; Amin, S.; Patel, S. Targeting BDNF Modulation by Plant Glycosides as a Novel Therapeutic Strategy in the Treatment of Depression. Life Sci. 2018, 196, 18–27. [Google Scholar] [CrossRef]
- Arai, Y.-C.; Makino, I.; Ikemoto, T.; Saisu, H.; Terajima, Y.; Owari, K. Kampo for the Treatment of Pain in Japan: A Review. Pain Ther. 2020, 9, 161–170. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.-Z.; Sawadogo, R.; Tan, T.; Yuan, C.-S. Effects of Herbal Medicines on Pain Management. Am. J. Chin. Med. 2020, 48, 1–16. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Liu, Y.; Li, J.; He, Q.; Zhang, C.; Wei, B.; Zhang, Y.; Wang, J. Extraction, Purification and Anti-Fatigue Activity of γ-Aminobutyric Acid from Mulberry (Morus alba L.) Leaves. Sci. Rep. 2016, 6, 18933. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Dua, K.; Kazmi, I.; Anwar, F. Anticonvulsant Activity of Morusin Isolated from Morus alba: Modulation of GABA Receptor. Biomed. Aging Pathol. 2014, 4, 29–32. [Google Scholar] [CrossRef]
- Zaugg, J.; Eickmeier, E.; Rueda, D.C.; Hering, S.; Hamburger, M. HPLC-Based Activity Profiling of Angelica Pubescens Roots for New Positive GABAA Receptor Modulators in Xenopus Oocytes. Fitoterapia 2011, 82, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Elsas, S.-M.; Rossi, D.J.; Raber, J.; White, G.; Seeley, C.-A.; Gregory, W.L.; Mohr, C.; Pfankuch, T.; Soumyanath, A. Passiflora incarnata L. (Passionflower) Extracts Elicit GABA Currents in Hippocampal Neurons in Vitro, and Show Anxiogenic and Anticonvulsant Effects in Vivo, Varying with Extraction Method. Phytomedicine 2010, 17, 940–949. [Google Scholar] [CrossRef]
- Yuan, C.-S.; Mehendale, S.; Xiao, Y.; Aung, H.H.; Xie, J.-T.; Ang-Lee, M.K. The Gamma-Aminobutyric Acidergic Effects of Valerian and Valerenic Acid on Rat Brainstem Neuronal Activity. Anesth. Analg. 2004, 98, 353–358. [Google Scholar] [CrossRef]
- Hovaneţ, M.; Viorel Ancuceanu, R.; Dinu, M.; Oprea, E.; Adriana Budura, E.; Negreş, S.; Ştefan Velescu, B.; Elena Duţu, L.; Adriana Anghel, I.; Ancu, I.; et al. Toxicity and Anti-Inflammatory Activity of Ziziphus jujuba Mill. Leaves. Farmacia 2016, 64, 5. [Google Scholar]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons: Chichester, UK, 2017; pp. 107–115. [Google Scholar]
- Gird, C.E.; Dutu, L.E.; Costea, T.; Nencu, I.; Popescu, M.; Balaci, T.O.O. Research Regarding Obtaining Herbal Extracts with Antitumour Activity. Note Ii. Phytochemical Analysis, Antioxidant Activity and Cytotoxic Effects of Chelidonium majus L. Medicago sativa L. and Berberis vulgaris L. Dry Extracts. Farmacia 2017, 65, 703–708. [Google Scholar]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of Five Bacterial Strains Producing Siderophores with Ability to Chelate Iron under Alkaline Conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.-A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–288. [Google Scholar]
- Zhan, Y. Protective Effect of Dexmedetomidine Combined with Ulinastatin on Myocardium in Patients Undergoing Valve Replacement. Farmacia 2019, 67, 437–441. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different Immune Cells Mediate Mechanical Pain Hypersensitivity in Male and Female Mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Vermeirsch, H.; Meert, T.F. Morphine-Induced Analgesia in the Hot-Plate Test: Comparison between NMRI Nu/Nu and NMRI Mice. Basic Clin. Pharmacol. Toxicol. 2004, 94, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Alijanpour, S.; Jafaripour, S.; Ghasemzadeh, Z.; Khakpai, F.; Zarrindast, M.-R. Harmaline Potentiates Morphine-Induced Antinociception via Affecting the Ventral Hippocampal GABA-A Receptors in Mice. Eur. J. Pharmacol. 2021, 893, 173806. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, N.; Bhatti, M.S.; Bhatti, R. Optimization of Extraction Conditions of Angelica archangelica Extract and Activity Evaluation in Experimental Fibromyalgia. J. Food Sci. 2020, 85, 3700–3710. [Google Scholar] [CrossRef]
- Sultana, T.; Hossain, M.; Chowdhury, S. Evaluation of Analgesic and Neuropharmacological Activity of the Bark of Morus alba L. (Family: Moraceae). Jordan J. Pharm. Sci. 2020, 13, 11–18. [Google Scholar]
- Dhawan, K.; Kumar, S.; Sharma, A. Evaluation of Central Nervous System Effects of Passiflora incarnata in Experimental Animals. Pharm. Biol. 2003, 41, 87–91. [Google Scholar] [CrossRef]
- Shahidi, S.; Bathaei, A.; Pahlevani, P. Antinociceptive Effects of Valeriana Extract in Mice: Involvement of the Dopaminergic and Serotonergic Systems. Neurophysiology 2013, 45, 448–452. [Google Scholar] [CrossRef]
- Marder, M.; Paladini, A. GABA-A-Receptor Ligands of Flavonoid Structure. Curr. Top. Med. Chem. 2002, 2, 853–867. [Google Scholar] [CrossRef]
- Mohrland, J.S.; Johnson, E.E.; VonVoigtlander, P.F. An Ultrasound-Induced Tail-Flick Procedure: Evaluation of Nonsteroidal Antiinflammatory Analgesics. J. Pharmacol. Methods 1983, 9, 279–282. [Google Scholar] [CrossRef]
- Bianchi, M.; Panerai, A. The Dose-related Effects of Paracetamol on Hyperalgesia and Nociception in the Rat. Br. J. Pharmacol. 1996, 117, 130–132. [Google Scholar] [CrossRef][Green Version]
- Bannon, A.W.; Malmberg, A.B. Models of Nociception: Hot-Plate, Tail-Flick, and Formalin Tests in Rodents. Curr. Protoc. Neurosci. 2007, 41, 8–9. [Google Scholar] [CrossRef]
- Menéndez, L.; Lastra, A.; Hidalgo, A.; Baamonde, A. Unilateral Hot Plate Test: A Simple and Sensitive Method for Detecting Central and Peripheral Hyperalgesia in Mice. J. Neurosci. Methods 2002, 113, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Pușcașu, C.; Mihai, P.; Zbârcea, C.E.; Zanfirescu, A.; Chiriță, C.; Viorica Ghiță, C.I.; Negreş, S. Investigation of Antihyperalgesic Effects of Different Doses of Sildenafil and Metformin in Alloxan-Induced Diabetic Neuropathy in Mice. Farmacia 2023, 71, 3. [Google Scholar] [CrossRef]
- Blebea, N.M.; Mihai, D.P.; Andrei, C.; Stoica, D.M.; Chiriță, C.; Negreş, S. Evaluation of Therapeutic Potential of Cannabidiol-Based Products in Animal Models of Epileptic Seizures, Neuropathic Pain and Chronic Inflammation. Farmacia 2022, 70, 1185–1193. [Google Scholar] [CrossRef]
- Aburas, K.; Misbah, A.; Fehelbum, H.; Abukhdir, A. Analgesic Effect by Using Hot Plat and Tail Flick Test in Rats Models for Aqueuos Moringa oleifer Extract. Libyan J. Med. Res. 2023, 17, 107–119. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [CrossRef] [PubMed]
- Pușcașu, C.; Negreș, S.; Zbârcea, C.E.; Ungurianu, A.; Ștefănescu, E.; Blebea, N.M.; Chiriță, C. Evaluating the Antihyperalgesic Potential of Sildenafil–Metformin Combination and Its Impact on Biochemical Markers in Alloxan-Induced Diabetic Neuropathy in Rats. Pharmaceuticals 2024, 17, 783. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Al-Snafi, A.E.; Thuwaini, M.M.; Teibo, J.O.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; et al. Morus alba: A Comprehensive Phytochemical and Pharmacological Review. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 1399–1413. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Gui, X.; Chen, L.; Huang, B. Natural Flavonoids as Promising Analgesic Candidates: A Systematic Review. Chem. Biodivers. 2016, 13, 1427–1440. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, X.; Yu, L.; Yin, Y.; Zhang, X.-D.; Wang, L.; Li, J.-X.; Zhu, Q.; Luo, J.-L. Current Status of GABA Receptor Subtypes in Analgesia. Biomed. Pharmacother. 2023, 168, 115800. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. Nature’s Functional Tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, Q.; Gu, M.; Guan, H.; Yang, W.; Zhang, M.; Mao, J.; Lin, Z.; Liu, Q.; Liu, J. Comprehensive Overview of Different Medicinal Parts from Morus alba L.: Chemical Compositions and Pharmacological Activities. Front. Pharmacol. 2024, 15, 1364948. [Google Scholar] [CrossRef]
- Kusano, G.; Orihara, S.; Tsukamoto, D.; Shibano, M.; Coskun, M.; Guvenc, A.; Erdurak, C.S. Five New Nortropane Alkaloids and Six New Amino Acids from the Fruit of Morus alba LINNE Growing in Turkey. Chem. Pharm. Bull. 2002, 50, 185–192. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liu, L.-K.; Hsu, J.-D.; Huang, H.-P.; Yang, M.-Y.; Wang, C.-J. Mulberry Extract Inhibits the Development of Atherosclerosis in Cholesterol-Fed Rabbits. Food Chem. 2005, 91, 601–607. [Google Scholar] [CrossRef]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical Composition and Antioxidant Activity of Certain Morus Species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, M.; Tian, X.; Ye, J. Study on Capillary Electrophoresis–Amperometric Detection Profiles of Different Parts of Morus alba L. J. Chromatogr. A 2006, 1116, 286–290. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nihei, M.; Nagai, H.; Fukushi, H.; Tabata, K.; Suzuki, T.; Akihisa, T. Albanol A from the Root Bark of Morus alba L. Induces Apoptotic Cell Death in HL60 Human Leukemia Cell Line. Chem. Pharm. Bull. 2010, 58, 568–571. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Bodensieck, A.; Seger, C.; Ellmerer, E.P.; Bauer, R.; Langer, T.; Stuppner, H. Discovering COX-Inhibiting Constituents of Morus Root Bark: Activity-Guided versus Computer-Aided Methods. Planta Med. 2005, 71, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baburin, I.; Zaugg, J.; Ebrahimi, S.; Hering, S.; Hamburger, M. HPLC-Based Activity Profiling—Discovery of Sanggenons as GABA A Receptor Modulators in the Traditional Chinese Drug Sang Bai Pi (Morus alba Root Bark). Planta Med. 2012, 78, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, M. New Triterpenoids from Morus alba L. Stem Bark. Nat. Prod. Res. 2013, 27, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-G.; Matsuzaki, K.; Takamatsu, S.; Kitanaka, S. Inhibitory Effects of Constituents from Morus alba Var. Multicaulis on Differentiation of 3T3-L1 Cells and Nitric Oxide Production in RAW264.7 Cells. Molecules 2011, 16, 6010–6022. [Google Scholar] [CrossRef]
- Khunakornvichaya, A.; Lekmeechai, S.; Pham, P.P.; Himakoun, W.; Pitaksuteepong, T.; Morales, N.P.; Hemstapat, W. Morus alba L. Stem Extract Attenuates Pain and Articular Cartilage Damage in the Anterior Cruciate Ligament Transection-Induced Rat Model of Osteoarthritis. Pharmacology 2016, 98, 209–216. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.-C.; Moore, B.; Jiao, P.; Hong, M.; Nam, J.-B.; Kim, M.-R.; Hyun, E.-J.; Chu, M.; Brownell, L.; et al. Analgesic and Anti-Inflammatory Effects of UP1304, a Botanical Composite Containing Standardized Extracts of Curcuma Longa and Morus alba. J. Integr. Med. 2016, 14, 60–68. [Google Scholar] [CrossRef]
- Park, J.-H.; Jung, Y.-J.; Jung, J.-W.; Shrestha, S.; Lim, D.W.; Han, D.; Baek, N.-I. A New Flavonoid Glycoside from the Root Bark of Morus alba L. Nat. Prod. Res. 2014, 28, 1859–1863. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.H.; Kim, E.J.; Kim, H.Y.; Kwon, T.O.; Kang, D.G.; Lee, H.S. Hypotensive, Hypolipidemic, and Vascular Protective Effects of Morus alba L. in Rats Fed an Atherogenic Diet. Am. J. Chin. Med. 2011, 39, 39–52. [Google Scholar] [CrossRef]
- Kaur, A.; Bhatti, R. Understanding the Phytochemistry and Molecular Insights to the Pharmacology of Angelica archangelica L. (Garden Angelica) and Its Bioactive Components. Phyther. Res. 2021, 35, 5961–5979. [Google Scholar] [CrossRef]
- Kumar, D.; Shah, M.; Bhat, Z. Angelica archangelica Linn. Is an Angel on Earth for the Treatment of Diseases. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011, 1, 36. [Google Scholar] [CrossRef]
- Maurya, A.; Verma, S.C.; Gupta, V.; Shankar, M.B. Angelica archangelica L.—A Phytochemical and Pharmacological Review. Asian J. Res. Chem. 2017, 10, 852. [Google Scholar] [CrossRef]
- Wszelaki, N.; Paradowska, K.; Jamróz, M.K.; Granica, S.; Kiss, A.K. Bioactivity-Guided Fractionation for the Butyrylcholinesterase Inhibitory Activity of Furanocoumarins from Angelica archangelica L. Roots and Fruits. J. Agric. Food Chem. 2011, 59, 9186–9193. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Garg, S.; Shiekh, B.A.; Singh, N.; Singh, P.; Bhatti, R. In Silico Studies and In Vivo MAO A Inhibitory Activity of Coumarins Isolated from Angelica archangelica Extract: An Approach toward Antidepressant Activity. ACS Omega 2020, 5, 15069–15076. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Jachak, S.M. Coumarins as Privileged Scaffold for Anti-Inflammatory Drug Development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Coumarins from Angelica archangelica Linn. and Their Effects on Anxiety-like Behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 180–186. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Andres-Mach, M.; Gleńsk, M.; Skalicka-Woźniak, K. Anticonvulsant Effects of Four Linear Furanocoumarins, Bergapten, Imperatorin, Oxypeucedanin, and Xanthotoxin, in the Mouse Maximal Electroshock-Induced Seizure Model: A Comparative Study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Angelica Root Oil (Angelica archangelica L.). Available online: https://www.chembk.com/en/chem/Angelica%20root%20oil (accessed on 17 June 2025).
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos Cuadrado, M.P. Updating the Biological Interest of “Valeriana Officinalis”. Mediterr. Bot. 2021, 42, e70280. [Google Scholar] [CrossRef]
- Felgentreff, F.; Becker, A.; Meier, B.; Brattström, A. Valerian Extract Characterized by High Valerenic Acid and Low Acetoxy Valerenic Acid Contents Demonstrates Anxiolytic Activity. Phytomedicine 2012, 19, 1216–1222. [Google Scholar] [CrossRef]
- Letchamo, W.; Ward, W.; Heard, B.; Heard, D. Essential Oil of Valeriana officinalis L. Cultivars and Their Antimicrobial Activity as Influenced by Harvesting Time under Commercial Organic Cultivation. J. Agric. Food Chem. 2004, 52, 3915–3919. [Google Scholar] [CrossRef]
- Pino, A.; Palumbo, D.R.; Samperi, S.; De Pasquale, R.; Sturlese, E.; Circosta, C.; Occhiuto, F. Relaxing Effects of Valeriana officinalis Extracts on Isolated Human Non-Pregnant Uterine Muscle. J. Pharm. Pharmacol. 2009, 61, 251–256. [Google Scholar] [CrossRef]
- Chen, H.-W.; Wei, B.-J.; He, X.-H.; Liu, Y.; Wang, J. Chemical Components and Cardiovascular Activities of Valeriana Spp. Evid. Based. Complement. Alternat. Med. 2015, 2015, 947619. [Google Scholar] [CrossRef]
- Taherianfard, M.; Taherianfard, M.; Karamifard, M. Evaluation of the GABA A Receptor on Pain Sensitivity in Male Rat Pretreated with Valeriana officinalis Extract Using Formalin Test. Physiol. Pharmacol. 2018, 22, 118–123. [Google Scholar]
- Zare, A.; Khaksar, Z.; Sobhani, Z.; Amini, M. Analgesic Effect of Valerian Root and Turnip Extracts. WORLD J. Plast. Surg. 2018, 7, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Pan, X.; Tao, Q.; Zhang, G.; Ding, W.; Li, G.; Peng, D.; Du, B.; Li, P. Safety Evaluation of Aqueous Extract from Valeriana officinalis L. Roots: Genotoxicity, Acute, Subchronic and Teratology Toxicity. J. Ethnopharmacol. 2024, 335, 118687. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on Valeriana officinalis L., Radix and Humulus lupulus L., Flos; European Medicines Agency: Amsterdam, The Netherlands, 2017.
- Pereira Leal, A.E.B.; de Lavor, É.M.; de Menezes Barbosa, J.; de Moura Fontes Araújo, M.T.; dos Santos Cerqueira Alves, C.; de Oliveira Júnior, R.G.; de Lima, Á.A.N.; da Silva Almeida, J.R.G. Pharmacological Activities of the Genus Passiflora (Passifloraceae): A Patent Review. Curr. Top. Med. Chem. 2022, 22, 2315–2328. [Google Scholar] [CrossRef] [PubMed]
- Zucolotto, S.M.; Fagundes, C.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Duque, C.; Schenkel, E.P. Analysis of C-glycosyl Flavonoids from South American Passiflora Species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012, 23, 232–239. [Google Scholar] [CrossRef]
- Marchart, E.; Krenn, L.; Kopp, B. Quantification of the Flavonoid Glycosides in Passiflora incarnata by Capillary Electrophoresis. Planta Med. 2003, 69, 452–456. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, Clinical Application, Safety and Evaluation of Clinical Trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef]
- Rutland Biodynamics Ltd. Passiflora Incarnata—Safety Data Sheet. Available online: www.rutlandbio.com (accessed on 17 June 2025).
- Shaheed, C.A.; Ferreira, G.E.; Dmitritchenko, A.; McLachlan, A.J.; Day, R.O.; Saragiotto, B.; Lin, C.; Langendyk, V.; Stanaway, F.; Latimer, J.; et al. The Efficacy and Safety of Paracetamol for Pain Relief: An Overview of Systematic Reviews. Med. J. Aust. 2021, 214, 324–331. [Google Scholar] [CrossRef]
- Ayoub, S.S. Paracetamol (Acetaminophen): A Familiar Drug with an Unexplained Mechanism of Action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef]
- Sharma, C.V.; Mehta, V. Paracetamol: Mechanisms and Updates. Contin. Educ. Anaesth. Crit. Care Pain 2014, 14, 153–158. [Google Scholar] [CrossRef]
| Plant Extract | TP (g/100 g Eq Expressed in Tannic Acid) | TF (g/100 g Eq Expressed in Rutin) | TPA (g/100 g Eq Expressed in Chlorogenic Acid) |
|---|---|---|---|
| AA | 4.27 ± 1.13 | nd | nd |
| MA | 11.50 ± 0.05 | 2.79 ± 0.52 | 5.61 ± 0.43 |
| PI | 6.36 ± 0.66 | 1.51 ± 0.06 | 0.85 ± 0.04 |
| VO | 5.95 ± 0.09 | 0.64 ± 0.06 | 1.29 ± 0.11 |
| AA | MA | PI | VO | Ascorbic Acid | |
|---|---|---|---|---|---|
| IC50 (mg/mL) | 0.2599 | 0.0695 | 0.1044 | 0.1272 | 0.0165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the JMMS. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, F.; Șeremet, O.C.; Ștefănescu, E.; Pușcașu, C.; Ghiță, C.I.V.; Gîrd, C.E.; Ancuceanu, R.V.; Negreș, S. Natural Alternatives for Pain Relief: A Study on Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata. J. Mind Med. Sci. 2025, 12, 39. https://doi.org/10.3390/jmms12020039
Suciu F, Șeremet OC, Ștefănescu E, Pușcașu C, Ghiță CIV, Gîrd CE, Ancuceanu RV, Negreș S. Natural Alternatives for Pain Relief: A Study on Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata. Journal of Mind and Medical Sciences. 2025; 12(2):39. https://doi.org/10.3390/jmms12020039
Chicago/Turabian StyleSuciu, Felicia, Oana Cristina Șeremet, Emil Ștefănescu, Ciprian Pușcașu, Cristina Isabel Viorica Ghiță, Cerasela Elena Gîrd, Robert Viorel Ancuceanu, and Simona Negreș. 2025. "Natural Alternatives for Pain Relief: A Study on Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata" Journal of Mind and Medical Sciences 12, no. 2: 39. https://doi.org/10.3390/jmms12020039
APA StyleSuciu, F., Șeremet, O. C., Ștefănescu, E., Pușcașu, C., Ghiță, C. I. V., Gîrd, C. E., Ancuceanu, R. V., & Negreș, S. (2025). Natural Alternatives for Pain Relief: A Study on Morus alba, Angelica archangelica, Valeriana officinalis, and Passiflora incarnata. Journal of Mind and Medical Sciences, 12(2), 39. https://doi.org/10.3390/jmms12020039











