Abstract
Recent years have seen a rapid expansion in research examining the relationship between homocysteine and stroke. In this study, we conducted a comprehensive bibliometric analysis of 233 articles related to homocysteine and stroke, published over the past 30 years in the Web of Science Core Collection. Our findings reveal a significant global increase in research on homocysteine and stroke, with China emerging as a leader, representing 39.9% of the total publications. Employing advanced methodologies such as co-citation analysis, bibliographic coupling, keyword co-occurrence, and citation burst analysis, we identified key research themes and emerging trends within the field. Notably, the results indicate a shift in focus from viewing homocysteine solely as a biomarker to recognizing its potential role in stroke prevention and management. These insights provide a valuable roadmap for future research directions and clinical strategies aimed at enhancing stroke prevention and improving patient outcomes.
1. Introduction
Homocysteine is a sulfur-containing amino acid structurally related to cysteine and methionine. Unlike these amino acids, which are directly involved in protein synthesis, homocysteine primarily functions in one-carbon metabolism, a crucial process for cellular function. It undergoes processing via three main pathways: (i) remethylation to methionine, (ii) conversion to cysteine through the trans-sulfuration pathway, and (iii) the formation of homocysteine thiolactone. These pathways depend on essential vitamins such as B6, B12, and B9 (folate) for their proper function [1]. Deficiencies in these vitamins or genetic mutations, particularly in the methylenetetrahydrofolate reductase (MTHFR) enzyme, can impair homocysteine metabolism. This impairment can result in elevated homocysteine levels, which have been linked to vascular damage and other health complications [2].
In circulation, homocysteine predominantly exists in a protein-bound state, with a smaller proportion present as low-molecular-weight disulfides or free thiols. Normal fasting plasma homocysteine concentrations range from 5 to 15 μmol/L, typically increasing with age and exhibiting higher baseline levels in males. Hyperhomocysteinemia is defined as plasma homocysteine levels exceeding 15 μmol/L and is recognized as an independent risk factor for cardiovascular and cerebrovascular diseases. Elevated homocysteine contributes to vascular pathology through mechanisms such as endothelial dysfunction, oxidative stress, and inflammation, all of which play critical roles in stroke pathogenesis [3]. Additionally, homocysteine interacts synergistically with other major risk factors, exacerbating their deleterious effects. For example, homocysteine-induced endothelial dysfunction aggravates hypertension, while oxidative stress accelerates atherogenesis in smokers. Furthermore, impaired renal clearance of homocysteine in kidney dysfunction leads to elevated plasma concentrations, further increasing stroke susceptibility. These interconnected pathophysiological pathways highlight the need for targeted interventions to regulate homocysteine levels as part of a comprehensive strategy for stroke prevention [1].
Despite the well-established association between hyperhomocysteinemia and stroke, significant research gaps persist, particularly concerning novel therapeutic strategies and emerging mechanistic insights. A bibliometric analysis provides a systematic approach to evaluating the scientific literature, identifying key research trajectories, and predicting future developments in this field [4]. This study employs bibliometric methods to analyze research published over the past three decades (1995–2024), offering a comprehensive assessment of the evolving relationship between homocysteine and stroke. Specifically, it aims to (i) characterize major research trends by quantifying publication patterns and evaluating their impact; (ii) identify emerging areas of investigation through keyword co-occurrence networks and thematic clustering, highlighting shifts in research focus; and (iii) assess the scope and limitations of existing research by analyzing publication productivity, author collaborations, and journal influence, thereby uncovering research gaps and future directions. To ensure a focused yet comprehensive assessment, this study employs bibliometric analysis rather than a scoping review, selecting publications that feature “homocysteine” and “stroke” in their titles. This data-driven approach facilitates an objective exploration of the field’s intellectual landscape, distinguishing it from traditional evidence synthesis methods while providing a quantitative foundation for understanding research trajectories.
2. Materials and Methods
2.1. Ethical Statement
No ethical approval was required, as this study did not involve human participants or animals.
2.2. Data Source and Search Strategy
All data used in this study were retrieved from the publicly accessible Web of Science Core Collection (WoSCC) database and are fully detailed in this article and Supplementary Materials. No new datasets were generated as part of this research. Data are available upon request for further inquiry. A comprehensive literature search was conducted in the WoSCC using the title field (TI) with the keywords “homocysteine” AND “stroke”. As of 23 September 2024, this search identified 419 relevant studies. To align with the study’s objective of examining recent advancements in homocysteine-related stroke research, the analysis was restricted to publications from the past 30 years (1995–2024). This time frame was selected to ensure a focused evaluation of evolving research trends and emerging insights within the field. To ensure a consistent dataset and avoid potential bias due to ongoing updates in the database, all records were extracted on 23 September 2024. The search results focused on English-language publications and excluded certain document types, including meeting abstracts (n = 118), review articles (n = 24), letters (n = 17), proceedings papers (n = 10), editorial materials (n = 8), corrections (n = 6), book chapters (n = 1), and retracted publications (n = 2). After applying these selection criteria, a final dataset comprising 233 articles published between 1995 and 2024 was included in the bibliometric analysis (Supplemental Figure S1).
2.3. Bibliometric Analyses
The Bibliometrix R package was employed to conduct a comprehensive bibliometric analysis, integrating both quantitative trends and qualitative impact to systematically evaluate the research landscape of homocysteine-related stroke studies. Beyond assessing publication output and citation patterns, the analysis incorporated key indicators of research quality, including journal impact factors, citation influence, and the identification of seminal studies that have significantly shaped the field. To objectively categorize journal productivity while prioritizing research significance over sheer publication volume, Bradford’s Law was applied to identify core journals contributing the most influential literature. Similarly, Lotka’s Law was utilized to analyze long-term research output trends, identifying authors with sustained scholarly impact over the past three decades. To further elucidate the intellectual structure of the field, advanced bibliometric mapping techniques were implemented using VOSviewer (version 1.6.20) and CiteSpace (version 5.8). Co-citation analysis was conducted to identify foundational studies, while burst detection highlighted emerging influential research. Bibliographic coupling was employed to delineate thematic linkages among high-impact contributions, providing insights into evolving research directions. Additionally, the systematic tracking of keyword frequencies, research hotspots, and thematic clustering enabled a comprehensive, data-driven assessment of both research volume and impact.
3. Results
3.1. Overview of Research Landscape
This study conducts a comprehensive bibliometric analysis of the research landscape on homocysteine and stroke, examining 233 articles published across 137 journals between 1995 and 2024. Although the association between homocysteine and stroke was established earlier, this analysis focuses on the past three decades to capture recent advancements, emerging trends, influential studies, and key research themes that have shaped the field. The analysis reveals a steady annual growth rate of 5.32% in publications, reflecting increasing interest in this research area over nearly three decades. The average age of the documents analyzed was 11.4 years, with many foundational studies retaining relevance. This study involved contributions from approximately 1500 authors, highlighting a collaborative approach in this field. Notably, only four of these authors contributed solo publications, emphasizing the importance of teamwork in advancing research on homocysteine and stroke. About 17% of the papers included in the dataset involved international collaborations, with an average of seven co-authors per paper, suggesting a global effort to address this important health issue (Supplemental Figure S2A). The research output peaked in 2017, with 16 publications in that year alone, marking a high point in scholarly attention to the topic (Supplemental Figure S2B). Despite this peak, citation trends have fluctuated over time. On average, each article received 39.94 citations, reflecting moderate academic influence and impact within the scientific community (Supplemental Figure S2A,C).
3.2. Scholarly Journal Influence and Collaboration Networks
The analysis of the most-cited journals in homocysteine and stroke research indicates that Stroke journal exhibits the highest m-index (0.67) among the top-ranked journals, highlighting its substantial influence in the field. As a Q1 journal according to the Journal Citation Reports (2023), Stroke serves as a key high-impact publication venue. Moreover, all ten of the most-cited journals fall within Zone 1 of Bradford’s Law, suggesting their critical role in disseminating foundational research. This finding indicates that research impact is not solely driven by publication volume but also by the prominence and citation frequency of studies published in high-quality, widely recognized journals (Supplemental Table S1). The University of Western Australia leads in institutional output with 34 articles, and its most prolific author, Hankey, holds the highest h-index (10) and an m-index of 0.42 (Supplemental Table S2A,B). According to Lotka’s Law, Hankey is also the most published author, while 88.5% of contributors authored only a single paper (Supplemental Table S2C). Hankey has maintained consistent publication activity over the past 30 years (Supplemental Figure S3). Collaboration networks, as illustrated in Supplemental Figure S4A, highlight significant partnerships, with Australia collaborating with Canada on nine publications and China working with the United States (USA) on nine articles. These partnerships are among the most prolific in international research efforts. China leads in total publication volume with 331 articles but does not hold the top spot in citation impact, which belongs to the United Kingdom (UK), averaging 224.30 citations per article (Supplemental Table S2D). Additionally, China exhibits a higher frequency of intra-country collaborations among corresponding authors compared to international partnerships, while Singapore and Australia demonstrate exclusively intra-country collaborations (Supplemental Figure S4B). In terms of citation impact, the article by Toole et al. [5] has the highest Normalized Global Citations (NGCs) at 5.73 and Normalized Local Citations (NLCs) at 3.91, though its NGC/NLC ratio is 1.28 (Table 1). In contrast, Perry et al. [6] holds the highest NGC/NLC ratio at 1.54, suggesting that its relative impact, based on global citations, is stronger compared to its local citation count (Table 1).

Table 1.
Impact measures for the most cited documents.
3.3. Bibliographic Coupling
Bibliographic coupling, a method that connects two publications based on their citation of a common third source, was employed to identify overlapping research areas and concurrent themes in homocysteine studies. Unlike total citation counts, this approach delineates relationships between documents by analyzing shared references and highlights the interconnections between different facets of homocysteine research, particularly the foundational studies that continue to inform current investigations. Analyzing 233 documents, 175 items were found to meet a citation threshold of at least five citations, forming a major cluster composed of six sub-clusters. This network contained 7418 connections and achieved a Total Link Strength (TLS) of 15,448, providing a comprehensive view of the research landscape (Figure 1). In this network, the size of each node reflects its relevance, while the proximity of nodes indicates similarities in research focus. The lines connecting nodes represent the relationships between these topics.
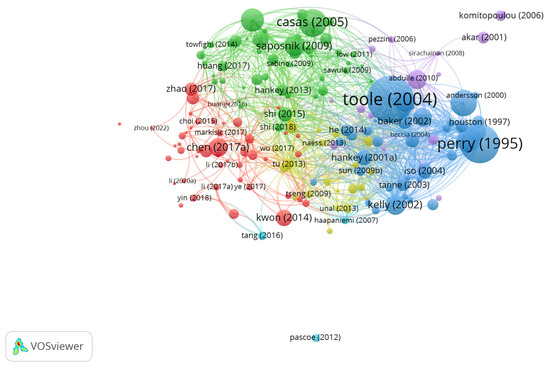
Figure 1.
Bibliographic coupling analysis with network visualization.
A key advantage of this bibliographic mapping approach is its ability to identify documents with shared characteristics, often linked by common authors or research themes. A prominent example is the work by Toole et al. [5], which represents the largest node, centrally positioned in Figure 1 and colored blue. It is closely surrounded by related studies, such as those by Perry et al. [6] and Casas et al. [15]. Toole et al. [5] holds the highest citation count, with 1037 citations, highlighting its central role in the Vitamin Intervention for Stroke Prevention (VISP) trial. This pivotal study investigated whether high doses of folic acid, vitamin B6, and B12 could reduce homocysteine levels and thereby lower the risk of recurrent stroke among 3680 patients with a history of cerebral infarction. Perry et al. [6] follows with 725 citations, further illustrating its influence within the field. This clustering of influential studies demonstrates the importance of bibliographic coupling in identifying key contributors and emerging research trends in homocysteine-related stroke research.
3.4. Co-Cited References
Co-citation analysis, which evaluates how often authors and publications are cited together, was employed to identify influential researchers and foundational papers in the field of homocysteine and stroke research. The frequency of co-citations highlights the importance of certain works in shaping the field, helping pinpoint key studies and leading experts. Among the 4507 cited references that met the minimum threshold of 20 citations, 26 articles were identified, forming three clusters interconnected by 308 links and a TLS of 1907 (Figure 2). The ten most co-cited articles in homocysteine research are listed in Table 2, with eight of these being observational studies and two meta-analyses, each garnering over 25 citations.
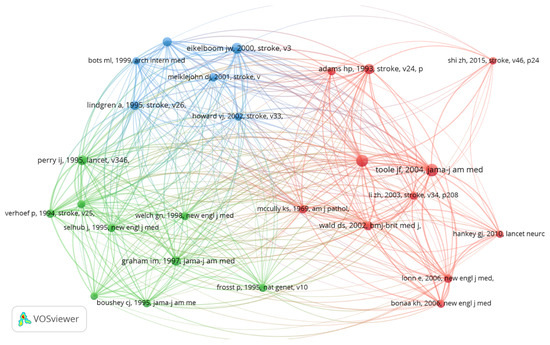
Figure 2.
Co-citation analysis of cited references with network visualization.

Table 2.
The top 10 co-cited references related to homocysteine and stroke.
The most co-cited article in this review is a 2004 clinical trial that demonstrated the efficacy of high doses of folic acid, vitamin B6, and vitamin B12 in significantly reducing homocysteine levels, thereby lowering the risk of recurrent stroke in patients with prior cerebral infarction. An intervention study conducted among patients in the USA, Canada, and Scotland demonstrated that a 3 µmol/L reduction in homocysteine levels through vitamin supplementation was associated with a 10% decrease in stroke risk, a 26% reduction in coronary heart disease events, and a 16% reduction in mortality in the low-dose group. However, the high-dose group exhibited minimal and statistically insignificant risk reductions, suggesting a potential plateau effect, wherein further homocysteine lowering does not confer additional benefits. Furthermore, moderate reductions in homocysteine showed no significant effect on vascular outcomes in patients with non-disabling cerebral infarction over a two-year follow-up period, indicating that the impact of homocysteine-lowering interventions may vary based on patient characteristics and baseline risk factors [5].
A follow-up meta-analysis of observational studies further strengthened the evidence linking elevated baseline homocysteine levels to an increased risk of ischemic heart disease (IHD) and stroke. The analysis revealed that individuals with lower baseline homocysteine levels exhibited a reduced risk of cardiovascular events. Specifically, a 25% reduction in homocysteine was associated with an 11% decrease in IHD risk and a 19% decrease in stroke risk [16]. Another pivotal study highlighted a strong correlation between elevated plasma homocysteine and ischemic stroke, particularly in both large-artery and small-vessel disease. Specifically, a 5 µmol/L increase in homocysteine was found to significantly raise the risk of stroke, highlighting the critical importance of monitoring and managing homocysteine levels, especially among high-risk populations in Australia [17].
Supporting the role of homocysteine as a stroke risk factor, a study of middle-aged British men identified significantly elevated homocysteine levels in stroke patients, independent of age and lifestyle factors [6]. A comprehensive meta-analysis of 72 genetic and 20 prospective studies further suggested that a 3 μmol/L reduction in homocysteine, achievable through folic acid supplementation, could lower the risk of IHD, deep vein thrombosis, and stroke [20]. However, findings from large-scale intervention trials remain inconclusive. The Cochrane systematic review by Martí-Carvajal et al. [21], which assessed homocysteine-lowering interventions in individuals with pre-existing cardiovascular disease across 15 randomized controlled trials (RCTs) involving 71,422 participants, reported mixed results. Although folic acid supplementation was associated with a modest reduction in stroke risk (RR 0.90, 95% CI 0.82–0.99), it did not significantly lower the risk of myocardial infarction (RR 1.02, 95% CI 0.95–1.10), all-cause mortality (RR 1.01, 95% CI 0.96–1.06), or serious adverse events (RR 1.07, 95% CI 1.00–1.14). These findings are consistent with large-scale trials such as NORVIT, SEARCH, WENBIT, WAFACS, VISP, HOPE-2, and HOST, which collectively demonstrated that although homocysteine levels were effectively reduced, the overall cardiovascular benefits of supplementation remained limited. Long-term epidemiological data from the Framingham Study further confirmed the association between elevated homocysteine levels and stroke risk, demonstrating that individuals in the highest quartile of non-fasting plasma homocysteine had a significantly greater incidence of stroke than those in the lowest quartile [8]. Additionally, research on homocysteine levels during the acute and convalescent phases of stroke in Caucasians revealed a significant increase in plasma homocysteine as stroke progressed, suggesting that elevated homocysteine may not only contribute to stroke risk but also result from stroke itself [7]. Collectively, these studies highlight the complexity of homocysteine-lowering interventions in stroke prevention. Although vitamin B supplementation may confer a modest reduction in stroke risk, large-scale clinical trials have failed to demonstrate substantial benefits in preventing myocardial infarction, reducing mortality, or improving overall cardiovascular outcomes. The effectiveness of supplementation appears to be influenced by baseline homocysteine levels, genetic variations, and concurrent antihypertensive therapy, making its preventive role less definitive. Moreover, uncertainties persist regarding the optimal dosage, timing of intervention, and long-term safety of high-dose supplementation, emphasizing the need for further research to clarify its clinical utility [21].
3.5. Thematic Structure, Thematic Evolution, Co-Word, and Burst Analysis
Figure 3A presents a thematic map identifying the 10 most frequently used keyword clusters in homocysteine and stroke research, offering a visual representation of the main topics driving inquiry in this area. Figure 3B tracks how these keywords have evolved over the past 30 years, illustrating shifts in research focus and the increasing prominence of certain themes, such as the role of homocysteine in stroke. Figure 3C shows a co-word analysis, confirming the interconnectedness of these prominent terms and demonstrating how the research field has matured by linking previously distinct concepts. These figures collectively emphasize the dynamic nature of homocysteine and stroke research, highlighting its growing complexity and the ongoing refinement of key concepts over time.
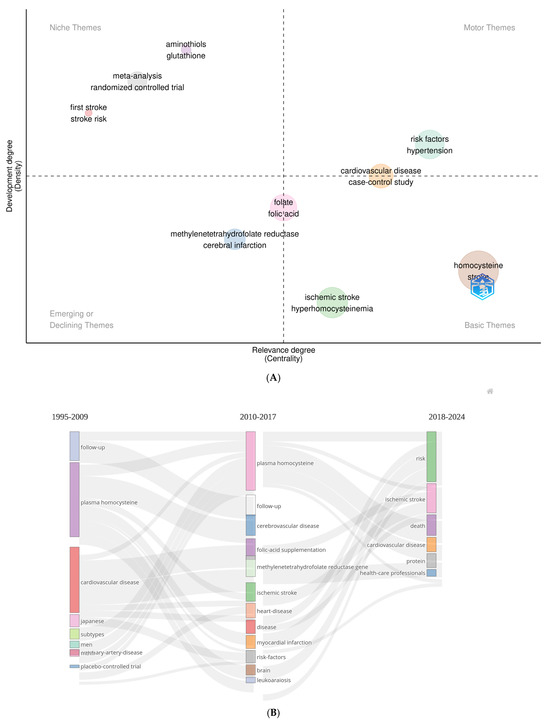
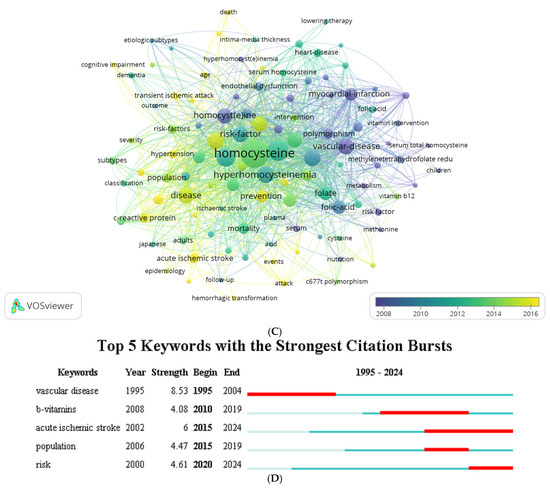
Figure 3.
Research hotspots and future trends. (A) Thematic analysis. (B) Thematic evolution analysis. (C) Co-word analysis. (D) Citation burst analysis.
For keyword analysis using a thematic map, the Spinglass clustering algorithm was implemented in Biblioshiny, selected for its robustness and reliability in network partitioning. In Figure 3A, keywords are classified based on co-occurrence relationships, with the x- and y-axes representing the dimensionality reduction output of the network. Keywords in close proximity indicate stronger associations in the literature, whereas those positioned farther apart reflect weaker connections. This method facilitates an objective, data-driven classification of research themes [4]. Figure 3A further categorizes keywords into four quadrants based on their density and centrality: niche, emerging or declining, basic, and motor themes. The niche quadrant highlights specialized but less frequently occurring topics, such as “aminothiols”, “glutathione”, and “meta-analysis”. While these topics remain relevant, they represent a relatively narrower focus within the broader landscape of homocysteine research. In contrast, terms such as “risk factor” and “hypertension” fall within the motor themes quadrant, indicating that they are not only central to the field but also have widespread relevance, particularly in stroke risk assessment and prevention strategies. However, keywords such as “cardiovascular disease” and “case–control study” show less centrality despite their relevance. The emerging themes quadrant reveals growing interest in terms such as “methylenetetrahydrofolate reductase” and “cerebral infarction”. Similarly, “ischemic stroke” and “hyperhomocysteinemia”, positioned in the lower-right quadrant, suggest areas that could evolve into key research directions with further exploration. Interestingly, terms such as “folate” and “folic acid” are positioned between emerging and basic themes, hinting at their transition into more foundational areas of study. Meanwhile, keyword emergence for terms such as “homocysteine” and “stroke” demonstrates their high centrality, as marked by a sudden increase in usage over a short period. By mapping these thematic clusters, this methodology enhances the understanding of research trends and supports the identification of future directions in the field.
Since 2018, there has been a notable shift in research focus toward keywords such as “risk”, “ischemic stroke”, “death”, “cardiovascular disease”, “protein”, and “health-care professional” (Figure 3B). This increasing prominence suggests a broader research emphasis on risk factors, clinical outcomes, and health-care interventions. Moreover, co-word analysis further identified 106 keywords that met the citation threshold, forming six clusters with 2443 links and a TLS of 6723 (Figure 3C). Node size reflects the frequency of keyword co-occurrences, with larger nodes signifying more frequent occurrences. The color of the node’s outer ring indicates the timing of these co-occurrences: cooler colors represent earlier years, while warmer colors signify more recent ones. Lines connecting the nodes depict the strength of the relationship. Among these nodes, the top five keywords, such as “homocysteine” (n = 157), “stroke” (n = 81), “plasma homocysteine” (n = 68), “hyperhomocysteinemia” (n = 54), and “risk” (n = 53), were highlighted as the most influential topics in the field (Figure 3C, Supplemental Table S3).
The keyword citation burst analysis using Citespace, with a five-year interval (Figure 3D), reveals key research hotspots and emerging trends in homocysteine studies. “Vascular disease” exhibited the highest burst strength (8.53), indicating its central importance in the research landscape, followed by “acute ischemic stroke” (6.00), “risk” (4.61), “population” (4.47), and “b-vitamins” (4.08). Notably, terms such as “vascular disease” and “b-vitamins” also maintained the longest burst durations, spanning up to 10 years, reflecting their sustained relevance in the field. In contrast, “population” was a focal point for a relatively shorter duration, from 2015 to 2019, suggesting more transient interest in demographic-related aspects. Likewise, emerging keywords such as “acute ischemic stroke” and “risk” have gained increasing attention. The prominence of these terms aligns with a growing emphasis on understanding stroke risk factors and interventions, suggesting that future studies may increasingly focus on the intersection of homocysteine levels, acute stroke management, and vascular disease prevention.
4. Discussion
4.1. Research Landscape and Evolution of Concepts
This study analyzes 233 research articles from the WoSCC database, published over the past three decades (1995–2024), to identify key trends, influential authors, and future directions in homocysteine–stroke research. Although early studies primarily focused on establishing the association between homocysteine and stroke, our analysis explores the evolution of the field, highlighting shifts in research priorities, methodological advancements, and emerging therapeutic approaches. The trajectory of homocysteine–stroke research reflects a distinct progression. Before 2004, relatively few studies were published, suggesting that the field was still in its early stages. However, since 2005, research activity expanded significantly, despite periodic fluctuations. Over the past four years, the field has experienced intensified interest, with at least 10 articles published annually, except in 2024, which has already recorded nine publications. This upward trend indicates the growing global interest in homocysteine’s role in stroke, with further expansion anticipated in the coming years. The evolution of research in this area can be categorized into three distinct phases. (i) The Vascular Hypothesis (1995–2005): During this period, homocysteine was primarily studied as an independent biomarker for stroke and cardiovascular disease. McCully’s work laid the foundation for understanding how hyperhomocysteinemia contributes to vascular dysfunction and atherosclerosis [22]. (ii) The B-Vitamin Intervention Era (2005–2015): Research during this phase focused on clinical trials, such as the VISP study, which examined whether B-vitamin supplementation could reduce stroke risk. However, inconsistent clinical outcomes revealed the complexity of homocysteine’s role in disease pathology [5,23]. (iii) The Molecular Pathways and Personalized Medicine Phase (2015–present): Recent studies have shifted toward exploring genetic polymorphisms, oxidative stress pathways, and personalized risk assessment. Increasing attention has been given to the interaction between homocysteine metabolism, inflammatory markers, and neurovascular damage, shaping novel treatment strategies [24,25].
Geographically, China has emerged as the leading contributor to homocysteine–stroke research, accounting for 39.9% of global publications. This rapid growth over the past three decades reflects the country’s expanding research capacity, particularly in domestic publications. However, despite high publication volume, high-impact citations remain concentrated in Western institutions, including those in the USA, UK, Sweden, and Canada. This imbalance suggests a need for stronger international collaboration to enhance research quality, promote knowledge exchange, and elevate the global impact of findings in this field. Notably, Soochow University ranks eighth among the world’s most productive institutions, and three of the top ten most-cited authors are affiliated with China. However, most of the top ten co-cited papers were authored by researchers from the USA [5,8,16,18], UK [6,19,20], Canada [17], and Sweden [7]. This indicates that while China generates a high volume of research, there is potential to enhance its global influence and research quality. Strengthening international collaboration, particularly between Chinese and global researchers, could help raise the quality and impact of future studies.
In terms of journal impact, Stroke holds the highest m-index and h-index, placing it in Zone 1 according to Bradford’s Law, signifying its significant influence in homocysteine and stroke research. Current studies in this field are primarily published in journals focused on neurology, atherosclerosis, and multidisciplinary topics, with a strong emphasis on clinical trials [5,18] and observational studies [6,7,17]. However, there has been a relative lack of large-scale meta-analyses with comprehensive subgroup analyses of multi-populations in the past four years. Such studies could provide guidelines applicable to the general population, highlighting a critical gap and opportunity for further synthesis of existing evidence in this field.
From an authorial perspective, Hankey is the most prolific researcher, with the highest h-index, which reflect the broad impact of his work. His research primarily focuses on meta-analyses, randomized controlled trials, and risk factors for neurological diseases. Hankey and Eikelboom’s research in 1999 has laid a foundation for understanding the relationship between homocysteine and stroke [1]. Although Toole ranks lower in terms of productivity, his work has the highest normalized citation counts both globally and locally. Toole’s VISP trial, ranked first among the top ten co-cited papers, demonstrated that high doses of folic acid, vitamin B6, and B12 significantly reduce recurrent stroke risk by lowering homocysteine levels. However, the trial also revealed that moderate homocysteine reduction did not affect vascular outcomes after cerebral infarction [5]. Several other scholars have also made substantial contributions, advancing knowledge of homocysteine’s role in stroke and shaping future prevention and treatment strategies [9,20].
4.2. Research Trends and Key Hotspots
Research on homocysteine and stroke has predominantly focused on elucidating the association between elevated homocysteine levels and stroke risk, along with investigating the role of vitamin supplementation in lowering homocysteine. Seminal studies, such as Toole’s VISP trial, have laid the groundwork for this field [5], with high citation counts emphasizing the importance of randomized controlled trials and meta-analyses. Recently, the research focus has expanded beyond stroke risk reduction to include broader cardiovascular outcomes linked to homocysteine management. The sections below explore the key research themes that have emerged.
4.2.1. Evolution of Theories on Homocysteine and Vascular Disease
Research on homocysteine has long centered on its role as an independent risk factor for stroke and cardiovascular diseases, with vascular disease emerging as a key area of investigation. Since Kilmer McCully first implicated homocysteine in vascular disease in the 1970s, it has been recognized as a crucial biomarker for cardiovascular disease [22], stroke [2], and cognitive decline [26]. However, recent research has shifted from viewing homocysteine as an isolated risk factor to examining its interactions with other stroke determinants. Notably, homocysteine has been shown to interact with hypertension, accelerating cognitive impairment in stroke patients [27]. Additionally, polymorphisms in MTHFR genes have been identified as critical modifiers of stroke risk, particularly in Asian populations [28,29].
Hyperhomocysteinemia, or elevated homocysteine levels, is primarily attributed to B-vitamin deficiencies [2], oxidative stress, and disruptions in redox balance [30] rather than being a direct cause of disease. It disrupts essential metabolic pathways, particularly remethylation and trans-sulfuration, leading to oxidative stress, endothelial dysfunction, and chronic inflammation, which are key contributors to atherosclerosis, stroke, and neurodegeneration [26,31]. Beyond homocysteine levels alone, vascular dysfunction is exacerbated by mechanisms such as protein homocysteinylation [32], the over-activation of N-methyl-D-aspartate receptors [24], and reduced nitric oxide bioavailability [33]. These processes are particularly relevant in acute ischemic stroke and cerebral infarction, where hyperhomocysteinemia amplifies endothelial damage and vascular impairment [24,32].
Despite the well-established relationship between hyperhomocysteinemia and disease, clinical trials aimed at lowering homocysteine through B-vitamin supplementation have produced mixed results, highlighting the complexity of its role in disease prevention [5,23]. Although elevated homocysteine has been associated with increased all-cause mortality, particularly among individuals with vascular disease, genetic studies have questioned its direct causality in stroke recurrence, suggesting that its influence may be overstated [25].
Addressing these gaps is crucial for advancing precision medicine in stroke prevention. Future research must focus on unraveling the complex interplay between homocysteine, vascular disease, and neurodegeneration, while integrating genetic and environmental factors to develop targeted interventions for at-risk populations.
4.2.2. Emerging Focus on Ischemic Stroke and Risk Factors
Recent keyword analyses highlight an increasing focus on the relationship between elevated homocysteine levels and ischemic stroke. Systematic reviews and meta-analyses have confirmed a dose–response relationship, with stroke risk rising significantly when homocysteine levels exceed 15 µmol/L. One prominent meta-analysis reported a 43% increase in stroke risk for every 5 µmol/L rise in homocysteine, indicating a nonlinear relationship that warrants further exploration to fully understand its clinical implications [27]. The interaction between hypertension and hyperhomocysteinemia is particularly critical in determining stroke risk. Patients with both conditions demonstrate a significantly higher risk of early cognitive impairment following their first ischemic stroke. Multivariate analyses have identified several independent predictors of cognitive decline, including elevated serum homocysteine levels, years of education, and Fazekas scale scores, underscoring the need for targeted interventions in these high-risk populations [34]. Elevated homocysteine levels have also been associated with poorer survival outcomes in acute ischemic stroke patients, with studies reporting significantly higher homocysteine levels in stroke patients compared to healthy controls [35]. In a large study from China involving over 3000 participants, elevated homocysteine levels were found across all subtypes of ischemic stroke, according to the Trial of Org 10172 in the Acute Stroke Treatment (TOAST) classification, reinforcing the role of homocysteine as a major risk factor [28]. Furthermore, a broader analysis of 51,426 participants confirmed that elevated plasma total homocysteine increases the risk of various stroke types, including ischemic, hemorrhagic, and recurrent strokes. These findings highlight the critical importance of routine homocysteine monitoring and early intervention to improve stroke outcomes [29]. Additionally, research has emphasized the need to address genetic predispositions and vitamin deficiencies, particularly in younger adults, as part of preventive strategies. Maintaining adequate folate levels has been shown to significantly reduce mortality and major disability in ischemic stroke patients [3,5], further reinforcing the significance of homocysteine management in stroke prevention.
4.2.3. Rethinking B-Vitamin Supplementation for Stroke Prevention
Folic acid and vitamins B6 and B12 play a key role in homocysteine metabolism, a pathway directly related to vascular health and the prevention of stroke recurrence. Specifically, folic acid serves as a donor of methyl groups for the conversion of homocysteine to methionine, a reaction requiring vitamin B12 as a cofactor. Simultaneously, vitamin B6 facilitates the breakdown of homocysteine into cysteine, contributing to antioxidant defense through the production of glutathione [1]. However, the initial hypothesis that B-vitamin supplementation universally benefits stroke patients has undergone substantial revision due to inconsistent findings from early clinical trials. This has led to a shift toward investigating population-specific effects. Recent meta-analyses suggest that folic acid is particularly effective in populations with low baseline folate levels, such as pre-fortification cohorts in China and India [3,36]. Moreover, the efficacy of B-vitamin therapy appear to be influenced by kidney function and follow-up duration, with greater reductions in stroke risk observed in individuals without chronic kidney disease and in trials with longer follow-up periods [37,38].
Despite these complexities, emerging evidence highlights the potential advantages of B-vitamin supplementation. Some meta-analyses indicate that combining folic acid with vitamins B6 and B12 not only reduces homocysteine levels but also lowers the risk of stroke recurrence and other vascular events [37,38,39]. Furthermore, B-vitamin supplementation has been associated with a reduction in stroke-related mortality. Nonetheless, while B-vitamin supplementation shows promise in stroke prevention, its impact on major adverse cardiovascular events and overall mortality remains unclear [40]. These findings challenge the simplistic assumption that all stroke patients benefit equally from B-vitamin therapy and highlight the need for personalized interventions tailored to individual risk profiles.
4.2.4. Homocysteine Dynamics in Acute Versus Chronic Stroke Phases
During the acute phase, elevated homocysteine levels are closely associated with increased oxidative stress, endothelial dysfunction, and the progression of lacunar stroke. High homocysteine concentrations correlate with larger infarct size and impaired reperfusion, emphasizing the urgency of early intervention [41,42]. The timely administration of folic acid or B-vitamins during this stage is essential to mitigate complications such as post-ischemic hyperperfusion, tissue damage, and delayed recovery, ultimately improving patient outcomes [41]. The accurate measurement of homocysteine levels in the acute phase can also serve as a predictive marker for key outcomes, including reperfusion degree and ischemic injury severity [7,41,42]. SPECT imaging studies further demonstrate that effective homocysteine management, when combined with timely recanalization, can significantly reduce infarct size and accelerate neurological recovery [42]. In contrast, the chronic phase presents distinct challenges. Persistent hyperhomocysteinemia exacerbates post-stroke inflammation, oxidative stress, and neurovascular damage, thereby increasing the risk of recurrent ischemic events and impairing long-term recovery [43,44]. Studies suggest that sustained elevations in homocysteine levels during this phase contribute to chronic vascular dysfunction, highlighting the importance of long-term homocysteine regulation as an integral component of stroke prevention and rehabilitation [45]. This evolving perspective represents a critical shift in stroke research, moving away from a generalized approach toward precision-based, phase-specific interventions. By recognizing the distinct mechanisms of homocysteine dysregulation in the acute and chronic phases, clinicians can develop targeted treatment strategies to optimize stroke recovery, reduce vascular complications, and minimize the risk of recurrence.
4.3. Limitations and Future Directions
Despite significant advancements, several unresolved challenges hinder both research and clinical translation in homocysteine–stroke studies. One of the most fundamental controversies is the lack of consensus on whether homocysteine is a direct contributor to stroke or merely a biomarker of metabolic dysfunction. Although elevated homocysteine levels are strongly correlated with stroke risk, genetic studies suggest that hyperhomocysteinemia may be a consequence rather than a cause of vascular pathology. Resolving this debate is essential for determining the true therapeutic potential of homocysteine-lowering strategies. Another major challenge is the substantial variability in homocysteine levels across individuals, influenced by diet, medications, lifestyle, and genetic predisposition. This inconsistency complicates efforts to establish a universal risk threshold and reduces the reliability of homocysteine as a predictive marker. Additionally, genetic and ethnic differences remain under-explored. Variations in MTHFR polymorphisms significantly affect homocysteine metabolism and stroke susceptibility, yet most studies lack stratification by genetic risk factors. Future research should integrate genetic profiling and population-based differences to refine risk assessment and optimize interventions. Heterogeneous clinical trial outcomes further complicate the field. Although some studies report modest stroke risk reductions with folate and B-vitamin supplementation, others show little to no benefit, raising concerns about the generalizability of these interventions. Identifying factors that influence treatment efficacy, such as genetic predisposition, baseline homocysteine levels, and comorbidities, is crucial for developing more targeted therapeutic approaches. Moreover, the optimal timing of intervention remains unclear. Although early homocysteine reduction may improve outcomes, the lack of standardized protocols limits clinical implementation. Longitudinal studies are needed to determine the most effective intervention windows and long-term effects on stroke recurrence and vascular health. Demographic factors such as age, sex, and ethnicity may also modulate homocysteine metabolism, yet their impact on stroke risk and treatment efficacy is poorly understood. Personalized medicine approaches should incorporate these variables to enhance intervention precision. Additionally, public health measures, such as folic acid fortification policies, have likely influenced homocysteine levels and stroke trends over time. The multifactorial nature of stroke risk further complicates homocysteine-targeted therapies. Although homocysteine is a recognized risk factor, it interacts with hypertension, diabetes, and hyperlipidemia, making it difficult to isolate its direct impact. A more integrative approach, combining homocysteine-lowering strategies with broader cardiovascular risk reduction, may yield better clinical outcomes. From a methodological standpoint, reliance on a single bibliometric database poses limitations. Although the WOSCC provides robust indexing, integrating Scopus, PubMed, and Google Scholar could improve the comprehensiveness of bibliometric analyses. Additionally, while bibliographic coupling effectively maps research interconnections, it may overlook seminal studies that lack shared references [46,47]. To mitigate this, our analysis incorporated co-citation analysis to evaluate influential works that may not directly reference each other.
5. Conclusions
In conclusion, this comprehensive bibliometric analysis of 233 research articles over the past 30 years highlights the evolution of homocysteine–stroke research, tracing its progression from vascular risk assessment to more intricate studies on molecular mechanisms and personalized medicine. The analysis identifies major research schools, shifts in scientific focus, and emerging directions, emphasizing the growing global attention to this field, particularly from China. Moreover, a major shift in research focus has been observed, transitioning from simplistic homocysteine-lowering strategies toward a more complex understanding of metabolic and genetic interactions. Acute-phase management is crucial, as early homocysteine reduction may mitigate neurovascular damage and improve functional recovery, while long-term regulation is essential for preventing post-stroke complications and reducing recurrence risk. Despite these advancements, significant research gaps remain. Large-scale meta-analyses are needed to elucidate the precise roles of homocysteine in stroke pathogenesis and treatment efficacy. Future research should prioritize the development of personalized interventions, incorporating genetic and metabolic risk profiling, optimizing timing and dosage of interventions, and further exploring the molecular pathways underlying homocysteine-mediated neurovascular damage.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jmms12010008/s1. Figure S1. Overview of bibliometric analysis. Figure S2. Overview of this study. Figure S3. Author productivity over 30-year period. Figure S4. Collaborative patterns among countries. Table S1. The most cited journals for homocysteine and stroke; Table S2. (A) Prominent institutions published in homocysteine; (B) The most cited authors listed based on h-index; (C) Author productivity verified using Lotka Law; and (D) The top 10 affiliated countries. Table S3. The top 5 bibliographic-coupled references ranked based on citations. Table S4. The top 5 co-words related to homocysteine and stroke.
Author Contributions
L.K.W. and L.R.G.—Conceptualization; L.K.W.—methodology, software, formal analysis, investigation, data curation, writing—original draft preparation; L.K.W., S.M., and L.R.G.—resources and validation; S.M. and L.R.G. writing—review and editing; L.R.G. supervision; L.K.W. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education (MoHE), through the Fundamental Research Grant Scheme [FRGS/1/2021/SKK0/UTAR/02/4]. The research was also supported by the Universiti Tunku Abdul Rahman Research Publication Scheme [UTARRPS 6251/L10] and the Universiti Tunku Abdul Rahman Research Fund [IPSR/RMC/UTARRF/2021-C1/L07].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed are included in this published article.
Conflicts of Interest
The authors declare that they have no conflicts of interest in this work.
References
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and stroke. Curr. Opin. Neurol. 2001, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.K.; Au, A.; Menon, S.; Gan, S.H.; Griffiths, L.R. Clinical relevance of MTHFR, eNOS, ACE, and ApoE gene polymorphisms and serum vitamin profile among Malay patients with ischemic stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 2017–2025. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.B.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef]
- Wei, L.K.; Sutherland, H.G.; Griffiths, L.R. Epigenetics and ischemic stroke: A bibliometric analysis from 2014 to 2024. Epigenet. Insights 2024, 17, e004. [Google Scholar]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Howard, G. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef]
- Perry, I.J.; Morris, R.W.; Ebrahim, S.B.; Shaper, A.G.; Refsum, H.; Ueland, P.M. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 1995, 346, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Brattström, L.; Norrving, B.; Hultberg, B.; Andersson, A.; Johansson, B.B. Plasma homocysteine in the acute and convalescent phases after stroke. Stroke 1995, 26, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Rosenberg, I.H.; Silbershatz, H.; Jacques, P.F.; Selhub, J.; D’Agostino, R.B.; Wilson, P.W. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: The Framingham Study. Ann. Intern. Med. 1999, 131, 352–355. [Google Scholar] [CrossRef]
- Meiklejohn, D.J.; Vickers, M.A.; Dijkhuisen, R.; Greaves, M. Plasma homocysteine concentrations in the acute and convalescent periods of atherothrombotic stroke. Stroke 2001, 32, 57–62. [Google Scholar] [CrossRef]
- Iso, H.; Moriyama, Y.; Sato, S.; Kitamura, A.; Tanigawa, T.; Yamagishi, K.; Konishi, M. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation 2004, 109, 2766–2772. [Google Scholar] [CrossRef]
- Li, Z.; Sun, L.; Zhang, H.; Liao, Y.; Wang, D.; Zhao, B.; Zhu, Z.; Zhao, J.; Ma, A.; Han, Y.; et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke. Stroke 2003, 34, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Launer, L.J.; Lindemans, J.; Hoes, A.W.; Hofman, A.; Witteman, J.C.M.; Koudstaal, P.J.; Grobbee, D.E. Homocysteine and short-term risk of myocardial infarction and stroke in the elderly. Arch. Intern. Med. 1999, 159, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Guan, Y.; Huo, Y.R.; Liu, S.; Zhang, M.; Lu, H.; Yue, W.; Wang, J.; Ji, Y. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke 2015, 46, 2419–2425. [Google Scholar] [CrossRef]
- Saposnik, G.; Ray, J.G.; Sheridan, P.; McQueen, M.; Lonn, E. Homocysteine-lowering therapy and stroke risk, severity, and disability. Stroke 2009, 40, 1365–1372. [Google Scholar] [CrossRef]
- Casas, J.P.; Bautista, L.E.; Smeeth, L.; Sharma, P.; Hingorani, A.D. Homocysteine and stroke: Evidence on a causal link from mendelian randomisation. Lancet 2005, 365, 224–232. [Google Scholar] [CrossRef]
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Hankey, G.J.; Anand, S.S.; Lofthouse, E.; Staples, N.; Baker, R.I. Association between high homocyst(e)ine and ischemic stroke due to large- and small-artery disease but not other etiologic subtypes of ischemic stroke. Stroke 2000, 31, 1069–1075. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, G.E.E., 3rd. Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Graham, I.M.; Daly, L.E.; Refsum, H.M.; Robinson, K.; Brattström, L.E.; Ueland, P.M.; Palma-Reis, R.J.; Boers, G.H.; Sheahan, R.G.; O Israelsson, B.; et al. Plasma homocysteine as a risk factor for vascular disease. JAMA 1997, 277, 1775–1781. [Google Scholar] [CrossRef]
- Wald, D.S. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [PubMed]
- McCully, S.K. Homocysteine, vitamins, and vascular disease prevention. Am. J. Clin. Nutr. 2007, 86, 1563S–1568S. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, Z.; Duan, Y.; Tang, H.; Zhang, H.; Liu, H. MTHFR C677T, hyperhomocysteinemia, and their interactions with traditional risk factors in early neurological deterioration in Chinese patients with ischemic stroke. Heliyon 2024, 10, e31003. [Google Scholar] [CrossRef]
- Yang, Z.J.; Huang, S.Y.; Zhong, K.Y.; Huang, W.G.; Huang, Z.H.; He, T.T.; Yang, M.-T.; Wusiman, M.; Zhou, D.-D.; Chen, S.; et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m6A-YTHDF2-dependent manner. Redox Biol. 2024, 69, 103026. [Google Scholar] [CrossRef]
- Holmen, M.; Hvas, A.M.; Arendt, J.F.H. Hyperhomocysteinemia and ischemic stroke: A potential dose-response association—A systematic review and meta-analysis. TH Open 2021, 5, e420–e437. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Y.; Yang, T.; He, X.; Yang, Y.; Chen, J.; Han, L. Blood biomarkers for post-stroke cognitive impairment: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107632. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Li, J.; Li, X.L.; Ding, M.; Mao, C.J.; Zhu, X.Y.; Liu, C.-F. Hypertension with hyperhomocysteinemia increases the risk of early cognitive impairment after first-ever ischemic stroke. Eur. Neurol. 2019, 82, 75–85. [Google Scholar] [CrossRef]
- Li, H.; Shu, L.; Dai, Q.; Wu, T. Association between plasma total homocysteine (tHcy) and strokes: A meta-analysis. Pteridines 2022, 33, 58–68. [Google Scholar] [CrossRef]
- Ji, Y.; Tan, S.; Xu, Y.; Chandra, A.; Shi, C.; Song, B.; Qin, J.; Gao, Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease. Neurology 2013, 81, 1298–1307. [Google Scholar] [CrossRef]
- Wei, L.K.; Sutherland, H.; Au, A.; Camilleri, E.; Haupt, L.M.; Gan, S.H.; Griffiths, L.R. A potential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke. Biomed. Res. Int. 2015, 2015, 167976. [Google Scholar]
- Sikora, M.; Bretes, E.; Perła-Kaján, J.; Utyro, O.; Borowczyk, K.; Piechocka, J.; Głowacki, R.; Wojtasz, I.; Kaźmierski, R.; Jakubowski, H. Homocysteine thiolactone and other sulfur-containing amino acid metabolites are associated with fibrin clot properties and the risk of ischemic stroke. Sci. Rep. 2024, 14, 11222. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 deficiency and the nervous system: Beyond metabolic decompensation—Comparing biological models and gaining new insights into molecular and cellular mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Z.; Chi, X.; Fan, F.; Li, S.; Song, Y.; Zhang, Y.; Qin, X.; Sun, N.; Wang, X.; et al. Folic acid supplementation for stroke prevention: A systematic review and meta-analysis of 21 randomized clinical trials worldwide. Clin. Nutr. 2024, 43, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, J.; Tian, Y. The prognostic value of homocysteine in acute ischemic stroke patients: A systematic review and meta-analysis. Front. Syst. Neurosci. 2021, 14, 600582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, Y.; Zhang, S.; Tie, T.; Cheng, Y.; Su, X.; Man, Z.; Hou, J.; Sun, L.; Tian, M.; et al. The association between homocysteine and ischemic stroke subtypes in Chinese. Medicine 2020, 99, e19467. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Nath, M.; Misra, S.; Kumar, P. From A to E: Uniting vitamins against stroke risk—A systematic review and network meta-analysis. Eur. J. Clin. Investig. 2024, 54, e14165. [Google Scholar] [CrossRef]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Z.; Bai, X.; Song, Y.; Li, P.; Lu, X.; Huo, Y.; Zhou, Z. Dosage exploration of combined B-vitamin supplementation in stroke prevention: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2024, 119, 821–828. [Google Scholar] [CrossRef]
- Spence, J.D.; Hankey, G.J. Problem in the recent American Heart Association guideline on secondary stroke prevention: B vitamins to lower homocysteine do prevent stroke. Stroke 2022, 53, 2702–2708. [Google Scholar] [CrossRef]
- Dong, W.C.; Guo, J.L.; Xu, L.; Jiang, X.H.; Chang, C.H.; Jiang, Y.; Zhang, Y.-Z. Impact of homocysteine on acute ischemic stroke severity: Possible role of aminothiols redox status. BMC Neurol. 2024, 24, 175. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, R.; Liu, Y. Plasma homocysteine (Hcy) concentration functions as a predictive biomarker of SPECT-evaluated post-ischemic hyperperfusion in acute ischemic stroke. Pharmacogenom. Pers. Med. 2023, 16, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Žitňanová, I.; Šiarnik, P.; Kollár, B.; Chomová, M.; Pazderová, P.; Andrezálová, L.; Ježovičová, M.; Koňariková, K.; Laubertová, L.; Krivošíková, Z.; et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke. Oxidative Med. Cell. Longev. 2016, 2016, 9761697. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, M.Y.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status. Redox Rep. 2021, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.Z.; Lefter, R.; Jaber, H.; Balmus, I.M.; Ciobica, A.; Iordache, A.C. The role of potential oxidative biomarkers in the prognosis of acute ischemic stroke and the exploration of antioxidants as possible preventive and treatment options. Int. J. Mol. Sci. 2023, 24, 6389. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Liu, W. Caveats for the use of Web of Science Core Collection in old literature retrieval and historical bibliometric analysis. Technol. Forecast. Soc. Change 2021, 172, 121023. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).