Abstract
Diuretics are a class of pharmacological agents that promote the renal excretion of water and electrolytes, increasing urine output and reducing fluid retention. They play a critical role in the management of edematous syndromes, irrespective of their etiology (cardiac, renal, or hepatic), as well as in the treatment of hypertension (HTA). The mechanism of action of diuretics can be classified as either renal, as seen with saluretic diuretics that inhibit sodium and water reabsorption at various segments of the nephron, or extrarenal, involving alterations in the glomerular filtration pressure or osmotic mechanisms. Based on their site of action and mechanism, diuretics are categorized into multiple classes, including loop diuretics, thiazide and thiazide-like diuretics, potassium-sparing diuretics, carbonic anhydrase inhibitors, and osmotic diuretics. These agents are frequently used in combination with other antihypertensive or heart failure medications to optimize therapeutic efficacy. By reducing the blood volume and peripheral vascular resistance, diuretics improve cardiac function, lower blood pressure, and enhance exercise tolerance. Additionally, they are employed in managing chronic kidney disease (CKD), electrolyte imbalances, and specific metabolic disorders. Given the potential for adverse effects such as electrolyte disturbances and renal dysfunction, diuretic therapy should be individualized, with the careful monitoring of the dosage, patient response, and comorbid conditions. Patient education on adherence, lifestyle modifications, and the recognition of side effects is essential for optimizing the therapeutic outcomes and minimizing the risks associated with diuretic therapy.
1. Introduction
Diuretics are widely used as first-line therapy for hypertension, as they effectively lower blood volume and normalize blood pressure, even in small doses. In cases of refractory hypertension, they are often combined with other antihypertensive agents to enhance the therapeutic efficacy [1].
Beyond hypertension, diuretics play a crucial role in the management of edematous syndromes associated with heart failure (HF), liver cirrhosis, nephrotic syndrome, and other renal pathologies. Their primary mechanism of action involves reducing blood volume and decreasing vascular resistance, which leads to improved cardiac function, lower blood pressure, and an enhanced exercise tolerance. The most commonly used diuretics include thiazide and thiazide-like diuretics, often in combination with potassium-sparing diuretics or loop diuretics for optimal fluid and electrolyte balance [2,3].
Diuretics are also employed in the treatment of renal failure (RF) and electrolyte imbalances. Certain diuretics help lower urinary calcium excretion, preventing kidney stone formation, while others increase calcium excretion to manage hypercalcemia. Additionally, osmotic diuretics and loop diuretics are used to maintain urinary flow and manage conditions associated with impaired kidney function [4,5].
Apart from their primary cardiovascular and renal applications, thiazide diuretics have also been explored in the treatment of osteoporosis in postmenopausal women, although this use is not Food and Drug Administration (FDA)-approved. They may be administered alone or in combination with calcium or estrogen supplementation. Furthermore, hydrochlorothiazide has been shown to be beneficial in pituitary and nephrogenic diabetes insipidus [6].
The stimulation of diuresis is essential in conditions involving fluid overload, where excess water is abnormally retained in the body, leading to edema. Heart failure represents the quintessential edematous syndrome, as impaired cardiac pump function activates the renin–angiotensin–aldosterone system (RAAS) and causes prolonged venous stasis, ultimately resulting in fluid extravasation into the interstitial space. These mechanisms contribute to symptoms such as weight gain, dyspnea, and peripheral or splanchnic edema [1,2,3,4,5].
2. Classification of Diuretics
Diuretics are classified based on their mechanism of action and the site of their effect within the nephron. The major classes include the following (Figure 1):
- Carbonic anhydrase inhibitors (acetazolamide, metazolamide): these diuretics act on the proximal convoluted tubule, inhibiting the enzyme carbonic anhydrase, which decreases the formation of hydrogen ions (H+). This results in reduced H+ secretion and the decreased reabsorption of sodium (Na+) and bicarbonate (HCO3−), leading to mild diuresis.
- Loop diuretics (furosemide, bumetanide, piretanide, ethacrynic acid, indacrinone): these potent diuretics act on the ascending limb of the Henle loop, where they inhibit sodium (Na+) reabsorption. Due to their high efficacy, they remain effective even in cases of low glomerular filtration rates (GFRs) and are often used in conditions requiring aggressive diuresis [7,8,9].
- Thiazide and thiazide-like diuretics (hydrochlorothiazide, butizide, cyclopenthiazide, methiclothiazide, cyclothiazide, polythiazide, clopamide, chlorthalidone, xipamide, indapamide): these diuretics act on the distal convoluted tubule by inhibiting sodium (Na+) reabsorption, primarily at the cortical segment of the Henle loop. They are widely used for the long-term management of hypertension and edema [10].
- Potassium-sparing diuretics: these drugs inhibit sodium (Na+) reabsorption in the distal convoluted tubule while preventing potassium (K+) loss. They work through two mechanisms:
- (a)
- Competitive aldosterone antagonists (spironolactone, canrenone);
- (b)
- Effect antagonists of aldosterone (triamteren, amiloride).
- Osmotic Diuretics (mannitol, sorbitol): these act on the proximal tubule by inhibiting water reabsorption. Unlike other diuretics, they do not interfere with electrolyte reabsorption. Because human cells lack transport mechanisms for these substances, they are not absorbed via the gastrointestinal tract and must be administered intravenously (i.v.). Their primary mechanism involves creating an osmotic gradient, drawing water into the urine, and promoting diuresis [2,3,4].
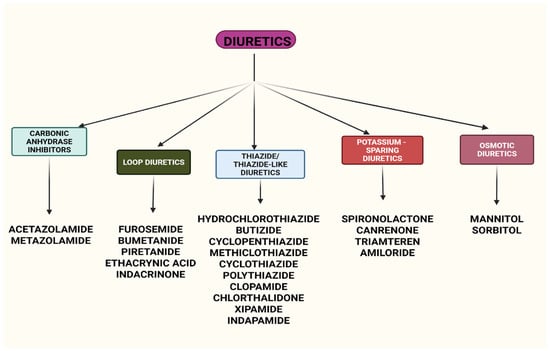
Figure 1.
Classification of the major classes of diuretics with their renal mechanisms. Created with BioRender (web application, accessed on 8 April 2025; BioRender Inc., Toronto, ON, Canada; www.biorender.com).
- Classification of diuretic drugs by their main mechanism of action:
- (a)
- Diuretics with an extrarenal mechanism—substances with an osmotic mechanism or that modify glomerular filtration pressure: drugs that increase the colloid osmotic pressure of the blood when it is reduced: dextrins, gelatin, albumin; drugs that cause the vasodilation of renal arterioles and increase renal blood flow: theophylline, theobromine, caffeine; and positive inotropic drugs (increase renal blood flow in heart failure): digoxin.
- (b)
- Diuretics with a renal mechanism, also called saluretic diuretics—act on the tubes and inhibit the reabsorption of Na+ and H2O.
- Classification of saluretic diuretics by the duration and intensity of their diuretic effect:
- (a)
- Diuretics with a long duration (12–24 h) and a moderate diuretic effect: spironolactone, eplerenone, clopamide, chlorthalidone, indapamide, xipamide;
- (b)
- Diuretics with a medium duration (6–12 h): hydrochlorothiazide (moderate diuretic effect), triamteren, amiloride (weak diuretic effect);
- (c)
- Short-term diuretics (4–6 h): furosemide, bumetanide, ethacrinic acid, torasemide (intense-effect diuretic).
- Classification of saluretic diuretics by their effect on the K+ ion:
- (a)
- Diuretics that remove the K+ ion:
- Those with an intense effect: thiazide diuretics and loop diuretics (hydrochlorothiazide, furosemide, bumetanide, ethacrinic acid, torasemide);
- Those with little effect: thiazide-related substances and carbonic anhydrase inhibitors (clopamide, chlorthalidone, indapamide, xipamide, acetazolamide) [11];
- (b)
- Diuretics that retain the K+ ion: algosterone antagonists (spironolactone, eplerenone, triamterene, amiloride).
The action of diuretics is influenced by several factors: marked hydrosaline retention increases the effectiveness of diuretics; hypoproteinemia reduces the GFR and decreases the effectiveness of diuretics; high blood pressure reduces renal blood flow due to the vasoconstriction of the arteries and decreases the intensity of the diuretic effect; and electrolyte imbalances reduce the effects of diuretics and the self-limitation of the diuretic effect occurs [1,12,13,14].
3. Mechanisms of Action of Diuretics
Diuretics exert their effects by targeting different segments of the nephron, altering the sodium, water, and electrolyte balance to promote diuresis. Each class of diuretics has a distinct mechanism of action:
3.1. Carbonic Anhydrase Inhibitors
Carbonic anhydrase inhibitors, such as acetazolamide and metazolamide, act on the proximal convoluted tubule, inhibiting the enzyme carbonic anhydrase. This leads to reduced sodium (Na+) and bicarbonate (HCO3−) reabsorption, resulting in the increased urinary excretion of these ions along with water. The loss of bicarbonate causes metabolic acidosis, which the body compensates for by lowering CO2 levels, inducing respiratory alkalosis through hyperventilation. A notable side effect of this class is potassium loss due to an altered electrolyte balance. Additionally, by lowering the blood volume, carbonic anhydrase inhibitors contribute to a reduction in blood pressure, intracranial pressure, and intraocular pressure [8,9].
3.2. Loop Diuretics
Loop diuretics, including furosemide, bumetanide, ethacrynic acid, indacrinone, and torasemide, act on the ascending limb of the Henle loop by inhibiting the Na+/K+/2Cl− cotransporter, thereby preventing sodium reabsorption and promoting water excretion. These are the most potent diuretics, effective even in conditions of a low GFR [11]. However, acute tolerance may develop shortly after the treatment initiation, leading to the ‘braking phenomenon,’ where sodium retention occurs due to compensatory mechanisms. This can often be managed by adjusting the dosage, frequency, or dietary sodium intake, or by combining loop diuretics with thiazide-related diuretics to achieve a sequential nephron blockade. Additionally, the combination of acetazolamide and loop diuretics has been shown to help overcome diuretic resistance by reducing sodium reabsorption in the proximal tubule [8,9].
3.3. Thiazide and Thiazide-like Diuretics
Thiazide diuretics, such as hydrochlorothiazide, clopamide, chlorthalidone, indapamide, and xipamide, act primarily on the distal convoluted tubule, where they inhibit sodium reabsorption, leading to increased sodium and water excretion. Thiazide-related diuretics differ slightly from thiazides in chemical structure, but share a similar mechanism of action. These agents are commonly used in the treatment of hypertension and edema, as they provide a moderate diuretic efficacy with fewer metabolic and electrolyte disturbances compared to loop diuretics [2,3,4].
3.4. Potassium-Sparing Diuretics
Potassium-sparing diuretics act on the distal convoluted tubule and collecting ducts, where they inhibit sodium reabsorption while preventing potassium excretion. They function via two mechanisms:
- Aldosterone antagonists (spironolactone, canrenone, canrenoic acid, eplerenone) block aldosterone receptors, reducing sodium retention and potassium loss.
- Sodium channel blockers (triamterene, amiloride) directly inhibit sodium channels in the distal tubule, reducing sodium reabsorption and preserving potassium.
These agents are particularly useful in conditions of excess aldosterone, such as hyperaldosteronism and heart failure. Amiloride and triamterene are commonly used to counteract potassium loss caused by other diuretics. Additionally, spironolactone has anti-androgenic properties, making it useful in the treatment of conditions such as hirsutism [2,3,4].
3.5. Osmotic Diuretics
Mannitol and sorbitol act primarily in the proximal tubule, inhibiting water reabsorption by creating an osmotic gradient. Mannitol increases the serum osmolarity, drawing water from tissues into the bloodstream and ultimately into the urine. Since mannitol does not cross the blood–brain barrier, it is particularly effective at reducing cerebral edema and intracranial pressure [15]. Osmotic diuretics are also used in the management of acute RF, glaucoma crises, and hypovolemic conditions.
3.6. Vasopressin Receptor Antagonists
Conivaptan and tolvaptan selectively antagonize vasopressin receptors, leading to increased water excretion without significant sodium loss. Conivaptan, available only in intravenous form, acts non-selectively on vasopressin receptors, while tolvaptan, an oral agent, is a selective vasopressin V2 receptor antagonist. These agents are primarily used in hyponatremia and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) [1].
3.7. Sodium–Glucose Cotransporter 2 (SGLT2) Inhibitors
SGLT2 inhibitors, a class of oral antidiabetic drugs, inhibit SGLT2 receptors in the proximal tubule, increasing glucose and sodium excretion. This results in an osmotic diuretic effect, reducing the extracellular fluid volume. However, this diuretic effect is transient, as the kidneys eventually compensate to restore fluid balance [2,3,4,5].
3.8. Angiotensin-Converting Enzyme (ACE) Inhibitors
While primarily used as antihypertensive agents, ACE inhibitors exhibit mild natriuretic and diuretic effects by reducing angiotensin II-mediated sodium retention. Although this secondary effect contributes to lowering blood pressure, it is not potent enough for primary diuretic therapy. Clinicians widely prescribe ACE inhibitors for hypertension, heart failure, and chronic kidney disease. The regular monitoring of renal function and electrolyte levels is essential.
Hyperkalemia may occur due to decreased aldosterone secretion, particularly in patients with renal impairment or those taking potassium-retaining drugs such as sulfamethoxazole-trimethoprim, aldosterone antagonists, or aliskiren. Renal function and potassium levels should be closely monitored.
A slight reduction in the GFR at therapy initiation may lead to increased bilirubin and creatinine levels. This effect is more pronounced in patients with heart failure or bilateral renal artery stenosis, where poor renal perfusion further reduces the GFR. Hypotension is more common in patients with elevated baseline renin levels or hypovolemia. Caution is advised in those with salt depletion or volume depletion.
It is recommended to discontinue diuretics 2 to 3 days before initiating ACE inhibitor therapy. If discontinuation is not possible, treatment should begin with the lowest ACE inhibitor dose [16,17].
3.9. Alcohol as a Diuretic
Alcoholic beverages with an alcohol content exceeding 13.5% can transiently inhibit the antidiuretic hormone (ADH), promoting water excretion without significant electrolyte loss. However, in dehydrated individuals, alcohol does not exhibit diuretic effects due to compensatory mechanisms that prevent further fluid loss [18].
4. Indications for Diuretics
According to the TRANSFORM-HF study and the guidelines of the New York Heart Association (NYHA) and the European Society of Cardiology (ESC), cardiogenic pulmonary edema is an indication for emergency diuretic therapy. Loop diuretics, known for their rapid onset and efficacy, are the preferred choice for symptomatic HF, with furosemide being the most commonly used agent. These diuretics are initially administered in low doses, with gradual dose escalation based on diuresis monitoring and a body weight assessment [1,6,19].
When loop diuretics alone are insufficient for HF management, the addition of thiazide diuretics (such as metolazone or hydrochlorothiazide) enhances their diuretic effect, further alleviating symptoms [2].
Additionally, aldosterone antagonists have been shown to reduce the mortality and morbidity in patients with advanced systolic HF and those with a left ventricular ejection fraction (LVEF) below 35% [5].
Diuretics, alongside dietary sodium restrictions, are also the first-line treatment for ascites in decompensated liver cirrhosis. In this setting, spironolactone is the preferred agent due to its targeted action on the underlying pathophysiology and its antiandrogenic properties. A loop diuretic may be added if spironolactone alone proves insufficient or can be included as part of a combination therapy from the outset. In both HF and liver cirrhosis, renal dysfunction exacerbates fluid retention through the activation of the renin-angiotensin–aldosterone system (RAAS) [1,6].
Hyperhydration, a common consequence of RF and various nephropathies, is associated with increased mortality. Loop diuretics are the preferred initial treatment, though in advanced CKD with refractory hyperhydration, extrarenal replacement therapy may be necessary for long-term management. In nephrotic syndrome—characterized by proteinuria exceeding 3.5 g/24 h, hypoalbuminemia leading to reduced oncotic pressure and secondary fluid extravasation, and hyperlipidemia—diuretic therapy is essential. The combination of albumin with furosemide or furosemide with triamterene has demonstrated efficacy in patients with renal disease and hypoalbuminemia [2,3,4,5].
Thiazide and thiazide-like diuretics are among the first-line treatments for hypertension. A Cochrane study, along with the European Society of Hypertension (ESH) guidelines, suggests switching to a loop diuretic when the estimated glomerular filtration rate (eGFR) falls between 30 and 45 mL/min. If the eGFR drops below 30 mL/min, chlorthalidone may be combined with a loop diuretic, as recommended by the American College of Cardiology (ACC) and the ESH 2024 guidelines [1,19].
Low-dose, long-acting chlorthalidone has demonstrated a significant reduction in the risk of cardiovascular events when compared to other antihypertensive medications. Indapamide, with fewer metabolic side effects than chlorthalidone, does not interfere with lipid or glucose metabolism, making it a safer choice for hypertensive patients with diabetes. Thiazide diuretics also have a direct vasodilatory effect that contributes to long-term blood pressure reduction. However, loop diuretics remain the preferred choice when hypertension coexists with CKD, especially when the GFR is ≤30 mL/min. Potassium-sparing diuretics are also utilized in hypertensive patients with potassium (K+) or magnesium (Mg2+) deficiencies [1].
Thiazide diuretics can be advantageous in patients with nephrolithiasis and hypercalciuria, as they promote calcium reabsorption. In contrast, loop diuretics like furosemide increase calciuria, which may be beneficial in patients with symptomatic hypercalciuria.
In diabetes insipidus, a condition marked by polyuria and the production of dilute urine with a low solute concentration, thiazide diuretics paradoxically help by promoting sodium (Na+) excretion at the proximal tubules, thereby reducing the kidneys’ maximal dilution capacity.
Acetazolamide, a sulfonamide lacking antibacterial activity, induces metabolic acidosis by promoting bicarbonate excretion. It is used prophylactically for high-altitude sickness to counteract hypoxia-induced respiratory alkalosis. Acetazolamide is also effective at reducing intraocular pressure and is commonly employed in the short-term treatment of open-angle glaucoma when other treatments are unsuitable. Additionally, it has been used in epilepsy management, as it can decrease seizure duration and severity [2,3,4].
Osmotic diuretics, such as mannitol, primarily enhance water excretion rather than sodium elimination, making them unsuitable for conditions associated with sodium retention. However, mannitol effectively reduces intracranial pressure within an hour, though an initial transient increase in pressure may occur. It is also used to stimulate diuresis in acute kidney injury (AKI) and to facilitate the excretion of toxic metabolic substances [1].
Diuretics are further employed in the elimination of toxins through forced diuresis, increasing the urinary output per unit time. Clinical pharmacists utilize loop diuretics and urinary alkalization in cases of poisoning with substances such as salicylates, phenobarbital, or lithium [1].
5. How to Administer Diuretics
Diuretics are typically administered orally, but in cases requiring maximum potency—such as advanced heart failure (HF), acute pulmonary edema, cerebral edema, or barbiturate intoxication—they are given intravenously in hospital settings or emergencies. In such scenarios, continuous infusion is preferred over bolus injections. The choice of diuretic, the dosage, and the route of administration should be carefully considered based on the patient’s comorbidities to anticipate and minimize adverse effects [1,2].
5.1. Loop Diuretics
Loop diuretics are rapidly absorbed, reaching peak concentrations within 30 min to 2 h. Their oral bioavailability varies, with bumetanide and torasemide exceeding 80%, while furosemide has the lowest bioavailability at 61%. These diuretics are available in both oral and i.v. forms. Furosemide is primarily excreted unchanged in urine (66%) or metabolized by the kidneys, whereas bumetanide and torasemide undergo hepatic metabolism. Torasemide has the longest half-life (3 to 4 h) and the longest duration of action (12 h), allowing for once-daily dosing. Unlike furosemide and bumetanide, torasemide’s absorption is not affected by food intake [1].
Since furosemide is the most commonly used loop diuretic, dose conversions between different agents should be approached with caution. Generally, for oral administration, the equivalent doses are 80 mg furosemide = 20 mg torsemide = 1 mg bumetanide. For intravenous administration, the conversion is 40 mg furosemide = 20 mg torsemide = 1 mg bumetanide [1,20].
The frequency of administration is crucial, as shorter-acting loop diuretics may lead to post-diuretic sodium retention once their effect wears off. For this reason, they are usually prescribed twice daily.
5.2. Thiazide Diuretics
Thiazide diuretics, which contain a sulfonamide structure, are well absorbed from the gastrointestinal (GI) tract when taken orally. Their bioavailability ranges from 50% to 95%, with hydrochlorothiazide and chlorthalidone having bioavailabilities of 71% and 65%, respectively. Hydrochlorothiazide is prescribed at doses of 25–100 mg (oral) and should be taken in the morning or at noon, but never in the evening [1].
Indapamide, a thiazide-like diuretic with a high bioavailability of 93%, is typically prescribed at a dose of 2.5 mg/day (or 1.5 mg/day in sustained-release form) for the treatment of essential hypertension. For edematous conditions, the recommended dosage ranges from 2.5 to 5 mg/day [2,3].
5.3. Potassium-Sparing Diuretics
Amiloride is not metabolized and is excreted unchanged, with approximately 50% eliminated through the urine and the remainder via feces. In contrast, triamterene is metabolized in the liver into active metabolites. Due to its lack of hepatic metabolism, amiloride is preferred in patients with liver disease. However, it should be avoided in cases of renal dysfunction because of the risk of drug accumulation and hyperkalemia. While amiloride allows for once-daily dosing, triamterene requires twice-daily administration. Both drugs have an oral bioavailability of approximately 50% [2]. Eplerenone undergoes hepatic metabolism, but does not produce any active metabolites. It has a serum protein binding level of approximately 50% to albumin. About 67% of the drug is excreted via the urine, while the remaining 32% is eliminated through the feces [9].
Food intake does not affect the absorption of these medications; in fact, taking them with food may help minimize gastric irritation and reduce related adverse effects [2,3]. Spironolactone, a synthetic compound and a structural analogue of aldosterone, has a low bioavailability p.o. (25%) due to the strong effect of the first hepatic passage, causing intense biotransformation in the liver that results in two active metabolites: canrenone and canrenone acid. The substance is eliminated slowly through the urine and has a weak diuretic effect and a long latency of over 24 h; it reaches maximum effect in 2–3 days and has a duration of 2–3 days after discontinuation of treatment. Eplerenone has an oral bioavailability of approximately 69% and undergoes hepatic metabolism without forming active metabolites. It binds to serum albumin at about 50%, with around 67% of the drug excreted in the urine and 32% eliminated via the feces. Food does not interfere with its absorption; in fact, taking eplerenone with food may help reduce gastric irritation and associated adverse effects [2,3]. Eplerenone is more selective than spironolactone, but it is also a weaker mineralocorticoid receptor antagonist and has a shorter half-life. Consequently, eplerenone must be administered twice daily to exert its full effect [21]. A newer class of mineralocorticoid receptor antagonists, which includes finerenone and hexaxenone, is now available, but these drugs are not used as diuretics. Finerenone, for example, is approved for reducing IR progression, HF, myocardial infarctions, and cardiovascular death in people with type 2 diabetes and CKD, but not for high blood pressure. Of note, although it is associated with a small, but significant, risk of hyperkalemia, hexaxerone is approved for the treatment of hypertension in Japan [22].
5.4. Carbonic Anhydrase Inhibitors and Osmotic Diuretics
Acetazolamide, which is rarely used in clinical practice, is well absorbed by the GI tract, is not metabolized, and has a plasma protein binding level of 93%. It has an onset of action within 1 to 1.5 h, with nearly 100% oral bioavailability and a plasma half-life of 13 h. It should be taken twice daily. Approximately 90% of acetazolamide is excreted unchanged in the urine through tubular secretion. As a result, it is ineffective in patients with renal insufficiency [1].
Mannitol is poorly absorbed orally, and is therefore administered only via a slow i.v. infusion at 1–1.5 g/kg/day. Effective osmotic diuresis requires 0.5–2 L of 10% mannitol. Once in the bloodstream, it exerts an osmotic effect, drawing water from the extravascular space into the circulation and reducing blood viscosity. More than 90% of mannitol is excreted unchanged via glomerular filtration, with no tubular reabsorption [1]. Due to the risk of exacerbating cerebral edema, mannitol should be administered cautiously at intervals of every 6 to 8 h. Excessive dosing may overwhelm the heart and circulatory system, potentially resulting in hyperperfusion syndrome [2,3,4,5].
5.5. Drugs Used in Hyponatremia
Vasopressin antagonists, such as tolvaptan, satavaptan, and leachaptan, are used to treat hyponatremia caused by inappropriate antidiuretic hormone (ADH) secretion. Tolvaptan selectively blocks vasopressin V2 receptors, promoting water excretion while increasing sodium concentration and decreasing urine osmolality. It is indicated for adults with hyponatremia due to the syndrome of inappropriate ADH secretion (SIADH) and is administered in divided doses, typically starting at 45 mg + 15 mg, followed by 60 mg + 30 mg, and up to 90 mg + 30 mg, taken twice daily [23].
Diabetes insipidus can be classified into pituitary diabetes (90% of cases) primary/hereditary or secondary to other diseases; circulatory disorders, tumors, or inflammations; and nephrogenic diabetes (10% of cases) due to the presence of mutations in the structure of aquaporin 2 (AQP2), a protein involved in water reabsorption, or genetic disorders [24].
The active drugs used for diabetes insipidus can be classified into natural substances and synthetic analogues to which substances are added, and synthetic substances.
- Natural substances and synthetic analogues: vasopressin, desmopressin, lypressin;
- Synthetic substances: thiazide diuretics and substances structurally related to thiazides (hydrochlorothiazide; oral antidiabetics (chlorpropramide); antiepileptics (carbamazepine)).
Vasopressin is quickly absorbed by injection or through the nasal mucosa. It activates V2 receptors in the renal tubes (increases cAMP), which stimulates passive water reabsorption in the distal tubes, decreases diuresis, and concentrates urine; this increases the permeability of the renal tubes to water [25].
Its other actions include activating V1 receptors in smooth muscles, stimulating phospholipase C (PLC), increasing the intracellular concentration of Ca2+, and causing vasoconstriction, coronary constriction, bronchoconstriction, and contractions of the uterine muscle. Its side effects include HTA; angina pectoris attacks; abortion in pregnancy; and an increase in intraocular pressure. It is administered in the form of a nasal spray [26].
Desmopressin, an analogue of ADH, has a more intense and long-lasting antidiuretic effect (24 h). It is administered in the form of nasal instillations or p.o. [1,19].
6. Side Effects of Diuretics
The most common adverse effect of diuretics is mild hypovolemia, which can lead to transient dehydration and increased thirst. In cases of prolonged diuretic therapy, severe hypovolemia may occur, resulting in hypotension, dizziness, and syncope. The general side effects associated with diuretic use include headaches, frequent urination, restlessness, weakness, fatigue, and lethargy. Gastrointestinal disturbances—such as nausea, vomiting, constipation, diarrhea, anorexia, and abdominal pain—are more frequently observed with loop diuretics than with other classes of diuretics [2,3,4,5,6].
Electrolyte imbalances are common with all diuretic agents. Hypokalemia (serum potassium < 3.5 mmol/L) occurs with most diuretics except those acting on the convoluted nephron collecting tubule, which instead can cause hyperkalemia (serum potassium > 5.5 mmol/L). Acid–base disturbances often accompany electrolyte abnormalities due to their interrelated reabsorption mechanisms in the renal tubules [4,7]. Hyperkalemia and metabolic acidosis are particularly associated with aldosterone receptor antagonists (ARAs) such as triamterene, amiloride, spironolactone, and eplerenone. Notably, the combination of mineralocorticoid receptor antagonists (MRAs) with sodium–glucose cotransporter 2 (SGLT2) inhibitors has been suggested to reduce the risk of hyperkalemia [27].
Diuretics may also induce metabolic disturbances affecting serum glucose, uric acid, and lipid levels. Less-common side effects include impotence, hyperglycemia, skin reactions, aplastic anemia, thrombocytopenia, agranulocytosis, hemolytic anemia, muscle cramps, and myalgia. In general, adverse effects are dose-dependent and most pronounced with loop diuretics, which have the strongest diuretic effect. These imbalances can include hyponatremia, hypokalemia, metabolic alkalosis, hypocalcemia, hypomagnesemia, hyperuricemia, hyperglycemia, and elevations in blood urea nitrogen (BUN) and creatinine. However, controlled formulations with lower doses have substantially reduced these side effects, particularly with thiazide diuretics [1].
Sodium is essential for regulating extracellular fluid concentration and volume, maintaining osmotic balance, supporting acid–base homeostasis, facilitating transmembrane transport, and ensuring proper neuromuscular excitability. The daily sodium requirement for the body ranges from 150 to 250 mEq/day (equivalent to 9–15 g of NaCl), with dietary intake being the primary source. Sodium is actively absorbed in the intestines, a process enhanced by hormones such as aldosterone and deoxycorticosterone. The elimination of sodium occurs through sweat, digestive secretions, and urinary excretion [28].
The symptoms of hyponatremia primarily affect the central nervous system (CNS), especially when sodium levels decrease significantly and rapidly. Neurological symptoms generally do not manifest until serum sodium falls below 120–125 mEq/L, while levels below 115 mEq/L can result in a coma and neurological dysfunction. Patients at high risk include children, premenopausal women, psychiatric patients with polydipsia, and elderly individuals, particularly those on thiazide diuretics or suffering from hypoxia. As the sodium concentration drops, intracellular water influx leads to increased intracranial pressure [29].
Hyponatremia is a potential complication of thiazide diuretics, steroidal MRAs, and finerenone. A 2024 study found that, in adults aged 40 and older, thiazide diuretics were associated with a significantly higher risk of hyponatremia over a two-year period compared to non-thiazide antihypertensive agents, with the highest risk occurring within the first 60 days of treatment [30]. The use of thiazide diuretics alongside spironolactone or loop diuretics more than doubles the risk of hospitalization due to hyponatremia [31].
Hypomagnesemia is a common complication of both loop and thiazide diuretics [32]. Loop diuretics, while useful in the management of severe hypercalcemia, require careful volume management to avoid dehydration. Initial hypercalcemia treatment involves saline replenishment, followed by loop diuretics to promote calciuresis. While some studies question their precise role in hypercalcemia management [33], the natriuretic and calciuretic effects of loop diuretics remain beneficial in specific cases [34].
Diuretic resistance is the failure to achieve adequate sodium excretion, despite an appropriate dose of a loop diuretic relative to renal function. Conditions such as edematous states (e.g., associated with dihydropyridine calcium channel blockers, hypothyroidism, lymphedema, or venous stasis) may mimic diuretic resistance, but typically do not respond to diuretic therapy.
The chronic use of loop diuretics can lead to diuretic resistance, as demonstrated in an experimental study in which rats implanted with infusion pumps delivering a continuous furosemide dose developed resistance over time [35]. In cases of diuretic resistance, optimizing therapy may involve doubling the dose, switching to a more bioavailable loop diuretic such as torsemide or bumetanide, or utilizing a continuous i.v. infusion instead of a bolus administration to enhance the pharmacokinetic efficacy.
Amiloride may serve as an adjunct therapy for hypomagnesemia. Studies in rodents treated with furosemide demonstrated that amiloride reduced urinary magnesium losses in a dose-dependent manner, likely through direct renal effects [35].
Loop diuretics can cause ototoxicity, which manifests as sensorineural hearing loss or tinnitus. This effect is particularly pronounced when loop diuretics are combined with aminoglycoside antibiotics, but the symptoms generally resolve upon discontinuation of the medication [2,3,4,5,6,36]. To minimize the risk, hearing tests should be conducted before initiating high-dose bolus or rapid infusions, allowing for an individual assessment of the loop diuretics’ impact on auditory function. Periodic monitoring of hearing function is also recommended during prolonged treatment. If adverse effects arise, switching to an alternative diuretic therapy should be considered to prevent permanent auditory damage. Ototoxicity is most frequently observed with the high-dose bolus administration of furosemide in AKI due to elevated serum drug concentrations. Among loop diuretics, ethacrynic acid is the most ototoxic [35,36,37,38,39].
Several diuretics, including hydrochlorothiazide, furosemide, spironolactone, and triamterene, have been associated with hepatotoxicity. In elderly patients, diuretic-induced renal impairment can be exacerbated by a reduced thirst sensitivity, leading to excessive dehydration [1].
Diuretics may also increase the risk of AKI under conditions where renal perfusion is compromised, such as congestive heart failure, liver cirrhosis, and nephrotic syndrome. Additionally, higher diuretic doses are linked to glomerular basement membrane damage, tubular epithelial cell degeneration, and an increased susceptibility to nephrotoxicity, particularly when combined with other nephrotoxic drugs. As a result, the use of diuretics in conjunction with nephrotoxic agents should be carefully evaluated or avoided when possible [1,34,35,36,37,38,39,40]. Figure 2 summarizes the main side effects of diuretics.
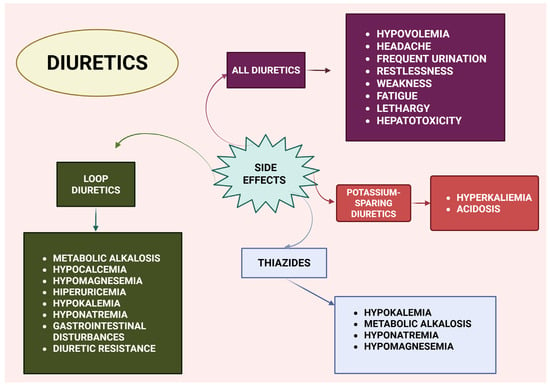
Figure 2.
Overview of diuretic side effects. Created with BioRender (web application, accessed on 8 April 2025; BioRender Inc., Toronto, ON, Canada; www.biorender.com).
Drug interactions can be distinct:
- (a)
- Useful interactions in therapy: an association between diuretics that eliminate the K+ ion and diuretics that retain K+ (furosemide + spironolactone; hydrochlorothiazide + amiloride/triamterene) or the combination of diuretics and other classes of antihypertensive drugs (ACE, BRA, BCC).
- (b)
- Unwanted interactions: non-steroidal anti-inflammatory drugs (NSAIDs) and steroidal anti-inflammatory drugs (AIS), which antagonize the effect of diuretics, as they cause hydrosaline retention. NSAIDs inhibit cyclooxygenase (COX) and decrease the synthesis of renal vasodilator prostaglandins, and AIS inhibits phospholipase A2 and decreases the release of arachidonic acid from membrane phospholipids (thereby reducing the synthesis of vasodilator renal prostaglandins). Thiazide diuretics antagonize the effect of hypoglycemic medicinal products and uricosuric drugs; they can cause thiazidic diabetes or severe hyperuricemia. Diuretics that eliminate potassium ions potentiate the toxicity of cardiotonic glycosides and lithium [41].
Table 1 summarizes the diuretic classes, their effects on electrolytes, common adverse reactions, their mechanisms of action, and drug interactions.

Table 1.
Diuretic classes: effects and interactions.
7. Management of Diuretic Therapy
Diuretic therapy necessitates the close monitoring of extracellular fluid volume, urine output, plasma and urine electrolyte levels, body weight, serum glucose, and blood pressure. Regular evaluations are especially important for patients with cardiovascular, hepatic, renal, or metabolic conditions, as well as for the elderly. Hypovolemia caused by diuretics can result in extrarenal azotemia, highlighting the need to monitor the BUN and creatinine levels closely [1].
Ototoxicity is a known risk associated with loop diuretics, and appropriate precautions are essential. Baseline hearing assessments are recommended, particularly when high doses, bolus injections, or rapid infusions are planned. These tests help evaluate the potential impact of loop diuretics on auditory function and allow for individualized monitoring over the course of treatment [36,37,38,39]. The periodic, sequential monitoring of auditory function is also advised during loop diuretic therapy. If signs of ototoxicity or other adverse effects emerge, switching to an alternative diuretic regimen should be considered to minimize further risk and preserve hearing function [37,40].
In cardiac patients receiving diuretic therapy, the close evaluation of their hemodynamic status is crucial, as these individuals are at risk of heart failure due to sudden shifts in the fluid balance between compartments. If a diuretic fails to induce diuresis, the treatment should be discontinued, and a potential undiagnosed renal pathology should be investigated [36,37,38].
8. Conclusions
The effective management of diuretic therapy requires a thorough understanding of potential adverse effects, as well as necessary dietary and lifestyle modifications during treatment. Regular check-ups with a cardiologist and nephrologist are essential for monitoring disease progression and adjusting the treatment accordingly. In CKD, diuretic therapy should be individualized, with the careful selection of the type, dosage, and schedule to meet each patient’s specific needs. The close monitoring of electrolytes, renal function, and blood pressure is crucial to ensure both safety and efficacy [40,42]. Optimizing blood pressure control while minimizing diuretic-related side effects can be achieved through a combination of diuretics with other antihypertensive agents and dietary sodium restriction. In cases of diuretic resistance, combining loop and thiazide diuretics may be beneficial when monotherapy is insufficient [9,43]. Patients, particularly those receiving outpatient treatment, should be encouraged to report any adverse effects promptly. Strict adherence to treatment protocols and avoiding overdoses can significantly improve both the clinical outcomes and the effectiveness of diuretic therapy. In outpatient settings, pharmacists play a critical role in ensuring proper medication use, identifying potential drug interactions, and verifying the correct dosing of prescriptions. Their vigilance contributes to the overall safety and success of diuretic therapy.
Author Contributions
Conceptualization, N.-M.B.; methodology, A.M.S.; formal analysis, C.P. and N.-M.B.; data curation, A.M.S.; writing—original draft preparation, C.P. and N.-M.B.; writing—review and editing, C.P. and E.Ș.; visualization, A.M.S. and E.Ș. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arumugham, V.B.; Shahin, M.H. Therapeutic Uses Of Diuretic Agents. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cristea, A. General Pharmacology, 2nd ed.; Didactic and Pedagogical Publishing House: Bucharest, Romania, 2018. [Google Scholar]
- Kehrenberg, M.C.A.; Bachmann, H.S. Diuretics: A Contemporary Pharmacological Classification? Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lüllmann, H.; Mohr, K.; Hein, L.; Bieger, D. Color Atlas of Pharmacology; FarmaMedia: Targu Mures, Romania, 2011. [Google Scholar]
- Wells, B.G.; DiPiro, J.T.; Schwingammer, T.L.; DiPiro, C.V. Pharmacotherapy Manual, 10th ed.; Prior MediaGroup: Bucharest, Romania, 2019. [Google Scholar]
- Types of Diuretics Medications: Uses, Side Effects, Interactions, List of Drugs. Available online: https://www.rxlist.com/diuretics/drugs-condition.htm (accessed on 4 March 2025).
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolytes Blood Press. 2015, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H. Clinical Pharmacology in Diuretic Use. Clin. J. Am. Soc. Nephrol. 2019, 14, 1248–1257, Erratum in Clin. J. Am. Soc. Nephrol. 2019, 14, 1653–1654. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.D.; Cooper-DeHoff, R.M. Mechanisms for Blood Pressure Lowering and Metabolic Effects of Thiazide and Thiazide-like Diuretics. Expert Rev. Cardiovasc. Ther. 2010, 8, 793–802. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T. Diuretics with Carbonic Anhydrase Inhibitory Action: A Patent and Literature Review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef]
- Shanmuganathan, P.; Kumarappan, M. Evaluation of Diuretic, Saluretic and Natriuretic Activity of Hydrochlorothiazide in Combination with Misoprostol in Wistar Rats. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1226. [Google Scholar] [CrossRef]
- Diuretics—Classification | Pharmacological Actions, Pharmacokinetics, Uses, Adverse Effects, Interactions | Pharmacology. Available online: https://www.pharmacy180.com/article/diuretics-1197/ (accessed on 17 March 2025).
- Wile, D. Diuretics: A Review. Ann. Clin. Biochem. Int. J. Lab. Med. 2012, 49, 419–431. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Tang, S.-C.; Tsai, L.-K.; Yeh, S.-J.; Shen, L.-J.; Wu, F.-L.L.; Jeng, J.-S. Incidence and Risk Factors for Acute Kidney Injury Following Mannitol Infusion in Patients With Acute Stroke. Medicine 2015, 94, e2032. [Google Scholar] [CrossRef]
- Armstrong, C.; Joint National Committee. JNC8 Guidelines for the Management of Hypertension in Adults. Am. Fam. Physician 2014, 90, 503–504. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Polhuis, K.C.M.M.; Wijnen, A.H.C.; Sierksma, A.; Calame, W.; Tieland, M. The Diuretic Action of Weak and Strong Alcoholic Beverages in Elderly Men: A Randomized Diet-Controlled Crossover Trial. Nutrients 2017, 9, 660. [Google Scholar] [CrossRef]
- Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; de Pinho, R.M.; Albini, F.L.; Boivin, J.-M.; et al. 2024 European Society of Hypertension Clinical Practice Guidelines for the Management of Arterial Hypertension. Eur. J. Intern. Med. 2024, 126, 1–15. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H.W. Loop Diuretic Efficiency. Circ. Hear. Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef]
- Tromp, J.; Ouwerkerk, W.; van Veldhuisen, D.J.; Hillege, H.L.; Richards, A.M.; van der Meer, P.; Anand, I.S.; Lam, C.S.P.; Voors, A.A. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC Hear. Fail. 2022, 10, 73–84. [Google Scholar] [CrossRef]
- Parker, R.B.; Nappi, J.M.; Cavallari, L.H. Chronic Heart Failure. In Pharmacotherapy: A Pathophysiologic Approach; DiPiro, J.T., Yee, G.C.P.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Neuen, B.L.; Oshima, M.; Agarwal, R.; Arnott, C.; Cherney, D.Z.; Edwards, R.; Langkilde, A.M.; Mahaffey, K.W.; McGuire, D.K.; Neal, B.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation 2022, 145, 1460–1470. [Google Scholar] [CrossRef]
- Hui, C.; Khan, M.; Suheb, M.Z.K.; Radbel, J.M. Arginine Vasopressin Disorder (Diabetes Insipidus). Pract. Clin. Endocrinol. 2024, 89–98. [Google Scholar] [CrossRef]
- Boone, M.; Deen, P.M.T. Physiology and Pathophysiology of the Vasopressin-Regulated Renal Water Reabsorption. Pflügers Arch. Eur. J. Physiol. 2008, 456, 1005–1024. [Google Scholar] [CrossRef]
- Brunton LL, K.B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 14th ed; McGraw-Hill Education: New York, NY, USA, 2023. [Google Scholar]
- Blebea, N.M.; Stăniguţ, A.M. Therapeutic Use of Diuretics—An Overview. Farmacist.ro 2022, 3, 27. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Zietse, R. Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines. J. Am. Soc. Nephrol. 2017, 28, 1340–1349. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical Practice Guideline on Diagnosis and Treatment of Hyponatraemia. Nephrol. Dial. Transplant. 2014, 29, i1–i39. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Roniker, B.; Krause, S.L.; Weiss, R.J. Eplerenone, a Selective Aldosterone Blocker, in Mild-to-Moderate Hypertension. Am. J. Hypertens. 2002, 15, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Iijima, S. Antihypertensive Effects and Safety of Esaxerenone in Patients with Moderate Kidney Dysfunction. Hypertens. Res. 2021, 44, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Gittus, M.; Haley, H.; Harris, T.; Borrows, S.; Padmanabhan, N.; Gale, D.; Simms, R.; Williams, T.; Acquaye, A.; Wong, A.; et al. Commentary: Tolvaptan for Autosomal Dominant Polycystic Kidney Disease (ADPKD)—An Update. BMC Nephrol. 2025, 26, 79. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Wohlfahrt, J.; Feenstra, B.; Hviid, A.; Melbye, M.; Lund, M. Cumulative Incidence of Thiazide-Induced Hyponatremia. Ann. Intern. Med. 2024, 177, 1–11. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Lee, J.; Shin, J.; Kim, S.H.; Hwang, J.H. Thiazide-associated Hyponatremia in Arterial Hypertension Patients: A Nationwide Population-based Cohort Study. J. Evid. Based. Med. 2024, 17, 296–306. [Google Scholar] [CrossRef]
- Martin, K.J.; González, E.A.; Slatopolsky, E. Clinical Consequences and Management of Hypomagnesemia. J. Am. Soc. Nephrol. 2009, 20, 2291–2295. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Fuleihan, E.-H.G. Treatment of Hypercalcemia of Malignancy. Endocrinol. Metab. Clin. N. Am. 2021, 50, 781–792. [Google Scholar] [CrossRef]
- LeGrand, S.B.; Leskuski, D.; Zama, I. Narrative Review: Furosemide for Hypercalcemia: An Unproven yet Common Practice. Ann. Intern. Med. 2008, 149, 259–263. [Google Scholar] [CrossRef]
- Robey, R.B.; Lash, J.P.; Arruda, J.A.L. Does Furosemide Have a Role in the Management of Hypercalcemia? Ann. Intern. Med. 2009, 150, 146–147. [Google Scholar] [CrossRef]
- Agarwal, R.; Verma, A.; Georgianos, P.I. Diuretics in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2025, 21, 264–278. [Google Scholar] [CrossRef]
- Ganesan, P.; Schmiedge, J.; Manchaiah, V.; Swapna, S.; Dhandayutham, S.; Kothandaraman, P.P. Ototoxicity: A Challenge in Diagnosis and Treatment. J. Audiol. Otol. 2018, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chisholm-Burns, M.A.; Schwinghammer, T.L.; Malone, P.M.; Kolesar, J.M.; Bookstaver, P.B.; Lee, K.C. Pharmacotherapy: Principles & Practice, 6th ed.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Kapil, V. Hypertension. In Kumar & Clark Clinical Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1140–1143. [Google Scholar]
- Reddy, P.; Mooradian, A.D. Diuretics: An Update on the Pharmacology and Clinical Uses. Am. J. Ther. 2009, 16, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure With Reduced Ejection Fraction. JAMA 2020, 324, 488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).