Abstract
Objective: The literature concerning the association between coronary slow flow (CSF) and anxiety and depression is controversial. Furthermore; there is no existing data in the literature on the potential association between CSF and adverse childhood experiences or alexithymia. Methods: The participants underwent coronary angiography through femoral access. Coronary artery blood flow rate was evaluated quantitatively for each coronary artery according to the Thrombolysis in Myocardial Infarction frame count (TFC) method. CSF was diagnosed as a corrected TFC value >27 in at least one coronary artery during the imaging. Symptoms of anxiety and depression were assessed through the Hospital Anxiety and Depression Scale (HADS). Alexithymia and ACE were evaluated by the Twenty-item Toronto Alexithymia Scale (TAS-20) and the Childhood Trauma Questionnaire (CTQ). Results: The study participants were categorized into two groups: normal coronary flow (n = 58) and CSF (n = 18). Total HADS score; HADS anxiety subscale (HADS-A) score; and HADS depression subscale (HADS-D) score were determined as significant factors associated with CSF in univariate logistic regression analysis. However; the TAS-20 and CTQ scores showed no significant association with CSF. Multivariate regression analysis performed in separate models demonstrated that total HADS score (OR: 1.27; 95 CI%: 1.08–1.50; p = 0.003); HADS-A score (OR: 1.25; 95 CI%: 1.03–1.51; p = 0.019); and HADS-D score (OR: 1.36; 95 CI%: 1.06–1.74; p = 0.014) were independently associated with CSF in multivariate logistic regression analysis. Conclusions: Neither alexithymia nor ACE was associated with CSF. On the other hand; measures of both anxiety and depression assessed through HADS were independently associated with CSF. Future studies should address the major limitations of this study; such as the limited sample size; lack of structured diagnostic interview by a psychiatrist; and the lack of establishment of causality
1. Introduction
Coronary slow flow (CSF) is an angiographic and clinical entity characterized by delayed antegrade contrast opacification of distal terminal vessels of coronary arteries in the absence of significant obstruction. Adverse outcomes of CSF primarily include recurrent chest pain, unstable angina requiring hospitalization, increased ventricular arrhythmia risk, and even cardiovascular mortality [1,2]. CSF was defined by Tambe and colleagues decades ago, and several other terms have been used since that time, such as “syndrome Y”, “slow coronary flow”, and “primary or idiopathic CSF” [2,3,4]. The underlying pathophysiological mechanisms of CSF include endothelial dysfunction, small vessel disease, subclinical atherosclerosis, increased vasomotor tone, impairment in platelet functioning, inflammation, and anatomical and genetic factors, which result in myocardial ischemia and systolic and/or diastolic dysfunction. These pathological alterations ultimately lead to angina, acute coronary syndrome (ACS), ventricular arrhythmias, and sudden death [1,2,5].
There is a bidirectional relationship between cardiovascular diseases (CVDs) and psychological distress, including anxiety and depression [6,7,8,9]. Alexithymia, which simply reflects a disordered affect regulation, is a multidimensional construct characterized by difficulty identifying feelings (DIF), difficulty describing feelings (DDF), and externally oriented thinking (EOT), and is thought to increase vulnerability to various health problems, including CVDs [10]. Adverse childhood experiences (ACE), including trauma and maltreatment, are considered important modulators of cardiovascular health and factors related to CVD development [11]. There is a significant interplay and mediation between each of these psychosocial conditions, psychological distress, and CVD. For instance, the relationship between ACE and cardiometabolic outcomes is partly explained by mental health issues such as anxiety and depression [11]. Depression has been identified as the underlying mechanism linking early life adversity to diminished cardiovascular reactivity [12]. In addition, a previous study involving African Americans suggested that alexithymia might contribute to CVD through negative emotional factors including depression and anxiety [13].
Previous studies exploring the association between CSF and psychiatric disorders have primarily focused on anxiety and depression, yielding controversial results. [14,15,16,17]. Moreover, to the best of our knowledge, no data currently exist in the literature regarding the potential association between CSF and ACE or alexithymia. Thus, we sought to investigate the relationship between CSF and aforementioned psychosocial conditions, including anxiety, depression, alexithymia, and ACE.
2. Methods
2.1. Study Design and Population
The present study was designed in a cross-sectional and observational manner and conducted prospectively at a single center. The patients who underwent diagnostic elective coronary angiography (CAG) between April 2021 and April 2022 were enrolled in the study prospectively. The indication for CAG was either the presence of typical angina or angina-equivalent symptoms and/or proven myocardial ischemia through noninvasive diagnostic tests. The study protocol was approved by the Yozgat Bozok University Clinical Research Ethics Committee (2017-KAEK-189_2021.03.31_14). Recommendations of the Good Clinical Practices Guidelines and the Declaration of Helsinki were followed during the study period and all participants provided written informed consent in order to participate in the study.
The patients with the following conditions were excluded from the study: age <18 or ≥65 year old, coronary artery stenosis ≥40% in any of the coronary artery, ACS including unstable angina, previous coronary revascularization or history of myocardial infarction, peripheral vascular disease including transient ischemic attack, stroke, intermittent claudication, peripheral revascularization or amputation, uncontrolled hypertension and/or left ventricular hypertrophy, severe valvular disease, atrial fibrillation, significant life-threatening arrhythmias, conduction defects including bundle branch blocks or pacemaker rhythm, left ventricular ejection fraction <50%, clinically overt heart failure, cardiomyopathy, severe respiratory failure, active malignancy, thyroid function tests abnormality, severe renal and/or hepatic failure, active hematological disease including anemia, active infectious, inflammatory or rheumatological disease, being illiterate, active psychiatric disease including anxiety and depressive disorders, neurocognitive disorder, coronary aneurysm or ectasia, suspected coronary artery spasm that required further evaluation, myocardial bridge, coronary anomalies, suboptimal coronary imaging, any complication during or after CAG such as bradycardia and/or hypotension due to vagal reaction, air embolism and groin hematoma. Furthermore, we excluded patients who underwent CAG through radial access due to the need for premedication to prevent radial artery spasm and/or occlusion [18].
The patients’ clinical data and medications were obtained during study inclusion period and recorded. BMI was calculated by division of weight in kilograms to height in meters squared [19]. BSA was calculated according to Mosteller formula [20]. The patients who reported cigarette smoking in the past six months were described as smokers. A previous diagnosis of hypertension with or without treatment or mean office blood pressure measurements ≥140/90 mmHg at multiple visits was defined as hypertension. DM was designated as fasting glucose level ≥126 mg/dL in multiple measurements or glucose level ≥200 mg/dL, regardless of fasting status, or receiving any kind of antidiabetic treatment. Hyperlipidemia was designated as total cholesterol ≥200 mg/dL and/or low-density lipoprotein cholesterol ≥130 mg/dL, and/or receiving any kind of hypolipidemic agent.
2.2. Coronary Angiography
All patients underwent diagnostic CAG in a supine position according to the standard Judkins technique via the cannulation of femoral artery with a 6 French arterial sheath using the Seldinger technique. No pharmacological agent such as heparin and/or nitrate was applied during the procedure. Imaging of coronary arteries was performed by one experienced cardiologist selectively with diagnostic 6 French left and right Judkins catheters from the cranial and caudal angles in right and left oblique positions, as standardized (Allura Xper FD10, Philips Healthcare, Andover, MA, USA the Netherlands). The contrast media used during imaging was Iohexol (Omnipaque 300 mg/100 mL, Opakim, Istanbul, Turkey) and 5–8 mL contrast agent was injected during each image shot. The images were obtained and recorded at 15 frames per second. The anatomical severity of coronary lesions was determined qualitatively and quantitatively by at least two cardiologists.
Coronary artery blood flow was calculated for each artery according to the Thrombolysis in Myocardial Infarction (TIMI) frame count (TFC) method [21]. TFC was determined as the number of cine frames required for contrast dye to reach predefined distal landmarks. The first frame used for TFC calculation is the frame in which a column of dye extends across the entire width of coronary artery and touches both borders with antegrade motion. The last frame counted is the frame that contrast dye enters the distal landmark branch. These distal landmarks are the distal bifurcation known as the “whale’s tail” for the left anterior descending artery (LAD), the distal bifurcation of the longest segment dyed by contrast material for the circumflex artery (Cx), and the first branch of the posterolateral artery for the right coronary artery (RCA). The TFC of LAD and Cx were obtained from right anterior oblique projection with caudal angulation, and the TFC of RCA was obtained from left anterior oblique projection with cranial angulation. A conversion factor of 2 was used to convert the frame rate of each artery in order to adjust for the 30 frames per second acquisition speed. Because the TFC of LAD is 1.7 times longer than the other coronary arteries, the TFC values obtained for LAD were divided by 1.7 in order to provide the corrected TFC value of LAD [21,22]. Afterwards, the mean TFC value was obtained for each patient by dividing the sum of the corrected TFC values of LAD, Cx, and RCA by three [23]. CSF was determined as a corrected TFC value >27 in at least one coronary artery [2,21]. TFC calculations were made by the same cardiologist, blinded to the patients’ clinical data.
2.3. Psychometric Measures
All patients were subjected to psychometric evaluation through the Hospital Anxiety and Depression Scale (HADS), the Twenty-item Toronto Alexithymia Scale (TAS-20), and the Childhood Trauma Questionnaire (CTQ) before discharge from the hospital. The psychiatrist who computed the scale scores was unaware of the CAG results of the patients.
The HADS was originally developed by Zigmond et al. to identify anxiety and depression in people with a physical illness [24]. It is a self-report questionnaire that consists of seven items each for anxiety and depression subscales. Each item is scored between a scale of 0 (no impairment) to 3 (severe impairment), and the maximum score is 21 for the anxiety subscale (HADS-A), 21 for the depression subscale (HADS-D), and 42 for the total HADS score. Scores per scale are obtained by summing responses for each of the two subscales and the total HADS score is calculated through sum of the score of two subscales. A total subscale score between 0 and 7 indicates normal test result, 8–10 mild disease, 11–15 moderate disease, and 16–21 indicates severe anxiety or depression. Higher scores indicate higher levels of anxiety or depression, and the total HADS score reflects a global measure of psychological distress. The questionnaire can be completed within 2–5 min. The HADS was translated into the Turkish language and validated in the Turkish population by Aydemir et al. in 1997 [25].
The TAS-20 was developed by Bagby et al. to evaluate alexithymia through self-administrated twenty items [26,27]. Each item is scored at five intervals: 1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, 5 = strongly agree. Some items are reverse-scored, and the total score is calculated as a sum of the items. Higher scores indicate greater impairment. The TAS-20 evaluates alexithymia in three subscales: DIF, DDF, and EOT. The validity and reliability of the TAS-20 in Turkish population was studied by Güleç et al. [28].
The CTQ is a self-report questionnaire that seeks to identify and classify different types of childhood trauma in a retrospective manner. The scale measures childhood trauma through five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. The original CTQ, developed by Bernstein et al., included 70 questions [29]. It was later shortened to 28 questions, which included 25 items with 5 subdimensions and 3 items for validation. The score of every question varies from 1 (never true) to 5 (very often true), and positive expressions are reverse-scored except for validation items. Therefore, the subdimension scores range from 5 (no neglect and abuse history) to 25 (very serious neglect and abuse history), and the sum of the five subscales yields the total CTQ score [30]. The short version of CTQ was applied in this study, and validity and reliability of this version in Turkish population was studied by Şar et al. in 2012 [31].
2.4. Statistical Analysis
The categorical variables were presented as frequencies and percentages and compared between groups by the chi-square test or Fisher’s exact test. The distribution pattern of continuous variables was tested through the Kolmogorov–Smirnov test. Afterwards, normally distributed parameters were presented as mean ± standard deviation and compared by using the Independent-Samples T test. On the other hand, skewed data were presented as median with 25th and 75th percentiles, and comparisons were performed by applying Mann–Whitney-U test. The correlation among psychometric test results and mean TFC values were examined by the Spearman’s correlation test.
The association between psychometric tests and CSF were tested by logistic regression analysis. Variables that reached statistical significance in group comparisons and could be confounders for CSF such as gender, height, hyperlipidemia and antiplatelet usage were included in the regression model. The psychometric tests which were determined as p < 0.100 in univariate analysis were included in multivariate analysis. In order to avoid multicollinearity, the psychometric tests that reached statistical significance in univariate analysis were included in multivariate logistic regression analysis separately. The goodness-of-fit assumption of each model was examined by the Hosmer–Lemeshow method and satisfied when p value was above 0.05. The results of logistic regression analysis were reported with 95% confidence interval (CI) and odds ratio (OR). Afterward, receiver-operating characteristics (ROC) curve analysis was conducted in order to identify the cut-off value of the total HADS score, and its sensitivity and specificity to predict CSF, and then the area under the ROC curve (AUC) was calculated. A two-sided alpha value <0.05 was determined as statistically significant for all tests, and all statistical analyses were carried out by using SPSS 22.0 (Statistical Product and Service Solutions for Windows, Version 22.0, IBM Corp., Armonk, New York, NY, USA, 2013).
3. Results
The final study cohort comprised 76 patients with a mean age of 55 ± 9 years and 39.5% (n = 30) male gender. The mean BMI of the study population was 31.3 ± 4.8, indicating a moderately obese patient population, and 38.2% (n = 29) of the patients were current smokers. Hypertension, DM, and hyperlipidemia were present in 55.3% (n = 42), 27.6% (n = 21), and 55.3% (n = 42) of the final study population, respectively. The patients were categorized into two groups, namely normal coronary flow (NCF) (n = 58) and CSF (n = 18), according to coronary flow patterns (Table 1).

Table 1.
Basal characteristics, medications, and coronary flow pattern of the study participants.
The baseline clinical parameters, including age, weight, BMI, and BSA, were similar between groups, whereas the male gender was more prevalent in the CSF group compared to the NCF group (77.8% vs. 27.6%; p < 0.001). In addition, CSF group patients were taller than NCF group patients (p = 0.021). CVD risk factors such as smoking, hypertension, and DM were comparable between the two groups. However, patients suffering from hyperlipidemia significantly differed between the NCF and CSF groups (62.1% vs. 33.3%; p = 0.032). Baseline medications of the groups were similar except antiplatelet therapy usage, which was significantly higher in CSF group compared to NCF group (41.2% vs. 15.8%; p = 0.026). TFC measurements of LAD, Cx, and RCA, and the mean TFC calculation of three vessels were all significantly higher in the CSF group than the NCF group, as expected (p < 0.001 for all) (Table 1).
The total HADS score, HADS-A score, and HADS-D score were all significantly higher in CSF group patients than NCF group patients (20.1 ± 4.1 vs. 15.3 ± 6.8; p = 0.001, 9.8 ± 2.9 vs. 7.5 ± 4.4; p = 0.038, 10.7 ± 3.7 vs. 7.9 ± 3.8; p = 0.010, respectively). The total TAS-20 score and its subfactor scores of DIF, DDF, and EOT were comparable between two groups. Similarly, the CTQ score and its subfactor scores of physical abuse, emotional abuse, sexual abuse, physical negligence, and emotional negligence did not differ among the NCF and CSF groups (Table 2).

Table 2.
Psychometric evaluations of the study participants.
Correlation analyses of the mean TFC calculation and the total HADS score (r = 0.47, p < 0.001), HADS-A score (r = 0.36, p = 0.001), and HADS-D score (r = 0.40, p < 0.001) revealed positive and moderate correlation (Figure 1). On the other hand, there was no correlation among the mean TFC calculation and the total TAS-20 and CTQ scores (p > 0.050 for both).
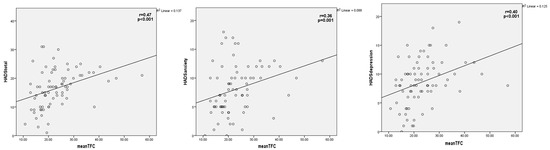
Figure 1.
Correlation between mean TIMI frame count calculation and the total HADS score, HADS anxiety score, and HADS depression score. HADS = Hospital anxiety depression scale, TIMI = Thrombolysis in myocardial infarction.
The total HADS score, HADS-A score, and HADS-D score were determined as significant factors associated with CSF in univariate logistic regression analysis (OR: 1.13, 95% CI: 1.02–1.24, p = 0.012; OR: 1.14, 95% CI: 1.00–1.30, p = 0.043; OR: 1.21, 95% CI: 1.03–1.41, p = 0.015, respectively). However, the TAS-20 total score and the CTQ score did not associate with CSF (p = 0.844, p = 0.460, respectively). Multivariate analysis performed in separate models demonstrated that the total HADS score (OR: 1.27, 95 CI%: 1.08–1.50, p = 0.003), HADS-A score (OR: 1.25, 95 CI%: 1.03–1.51, p = 0.019), and HADS-D score (OR: 1.36, 95 CI%: 1.06–1.74, p = 0.014) are independently associated with CSF in multivariate logistic regression analysis (Table 3). The ROC curve analysis for the total HADS score to predict CSF demonstrated an AUC value 0.740 (95% CI: 0.620–0.860, p = 0.002). The cut-off value of the total HADS score (18.5) was associated with 72.2% sensitivity and 73.7% specificity (Figure 2).

Table 3.
Predictors of CSF by logistic regression analysis.
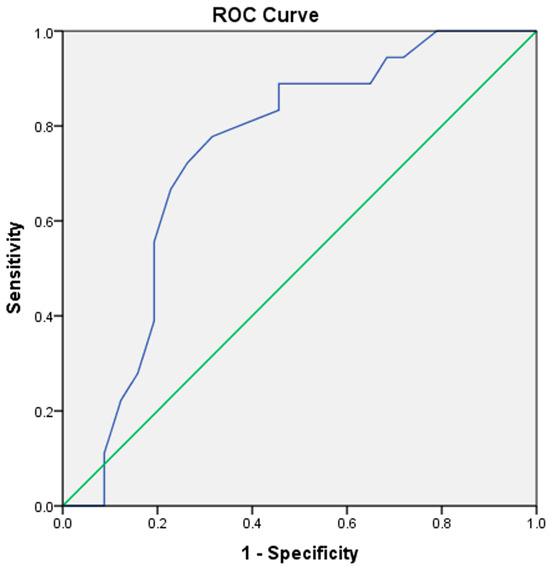
Figure 2.
ROC curve analysis of the total HADS score to predict CSF. The blue line indicates the ROC curve of the total HADS score. The green line serves as the reference line. HADS = Hospital anxiety depression scale, ROC = Receiver operating characteristic, CSF = Coronary slow flow.
4. Discussion
The results of the study do not support the hypothesis that alexithymia and ACE, evaluated by the TAS-20 and CTQ, are associated with CSF. However, both anxiety and depression scores assessed through the HADS are independently associated with CSF. To our knowledge, this is the first study in the literature that investigated the relationship between CSF and psychosocial conditions such as alexithymia and ACE.
Patients with CSF are usually diagnosed as having “normal coronary arteries” after CAG, yet they frequently experience recurrent chest pain, unstable angina, and frequent hospital admissions, which can lead to a diminished quality of life and increased psychological distress, including anxiety and depression [1,5]. However, studies yield conflicting results regarding the association between CSF and psychological distress. Yalvac et al. found that patients with CSF are in a more anxious and depressive situation compared to patients with NCF [14]. Durmaz et al. demonstrated that anxiety and depression scores are independent risk factors for CSF in their study [15]. Depression was independently linked with CSF and both anxiety and depression scores measured through the Beck Anxiety and Depression Inventory significantly correlated with TFC calculations in a recent study published by Elamragy and colleagues [16]. On the contrary, Yavuz et al. found lower depression scores in CSF patients than NCF patients in their study population evaluated through the Hamilton Depression Rating Scale [17]. In our study, we demonstrated that both anxiety and depression scores evaluated through subscales of the HADS and the total HADS score significantly correlate with mean TFC calculations. Moreover, all of these measures of mental health evaluated in separate logistic regression models are independently associated with CSF.
The primary objective of the study was not to investigate the relationship between mental health status and CSF. However, it is well established that alexithymia and ACE are frequently comorbid with mental health conditions such as depression and/or anxiety [32,33]. For this reason, we chose also to assess anxiety and depression using the HADS in addition to investigating alexithymia and ACE. We preferred HADS for a few reasons. First of all, the HADS is a brief, cost-effective, and practical self-report questionnaire that helps to evaluate anxiety and depression severity in approximately five minutes through fourteen easy to answer questions. Therefore, it is widely used and recommended as a screening tool. Furthermore, there is no need to apply multiple measures or complex instruments to patients. Second, the HADS does not include questions regarding somatic symptoms which makes it a useful tool to apply to patients with chronic conditions such as CVD [34]. Third, the usefulness of the HADS were validated in different nations of patients with cardiac disease [35,36]. The Turkish version of the HADS were shown to possess good internal consistency and reliability with a mean Cronbach’s alfa of 0.85 and 0.77 for anxiety and depression subscales, respectively [25].
CSF may be considered as a local manifestation of a systemic vascular abnormality. Impairment in endothelial functioning, subclinical atherosclerosis, platelet dysfunction, oxidative stress, and systemic inflammation are some of the suspected pathogenetic mechanisms that play role in CSF [1,2,37,38]. Many of these mechanisms also drive the pathogenesis of mental health issues including anxiety and depression [5,39,40]. On the other hand, both CSF and anxiety and depression impair echocardiographic parameters such as left atrium functions that further prone the heart to arrhythmia and heart failure [38,41,42]. Given the similarities of both conditions regarding pathophysiology and consequences, it is not surprising that there existed an independent association between CSF and measures of anxiety and depression in our study. However, the OR was higher for depression score than anxiety score which is consistent with the literature. For example, a previous study of 420 heart failure patients demonstrated that severity of depression but not anxiety was associated with heart failure symptom status [43]. Depression had the highest attributable risk associated with mortality compared to various CVD risk factors also including anxiety according to the results of the DenHeart survey [44]. In a similar vein, both depression and anxiety levels independently associated with 10-year CVD incidence, with depression distinctly increasing the CVD risk compared to anxiety in the ATTICA study [45]. It should be underlined that in clinical practice anxiety and depression frequently coexist and their symptoms overlap [46]. On the other hand, in order to avoid multicollinearity in our study, we evaluated the total HADS score, HADS-A score, and HADS-D score in separate logistic regression models.
Several studies in the literature have linked alexithymia to various health problems including CVDs [10]. Alexithymia was found to be associated with increased all-cause mortality risk in men, but not in women, according to a 10-year follow-up study [47]. However, Ossola et al. found that developing a first-ever depressive episode after an ACS, but not alexithymia, is associated with a recurrent cardiac event [48]. The same group also showed that alexithymia has no effect in predicting depression after the first ACS episode [49]. On the other hand, childhood adversity is known to be associated with cardiometabolic outcomes and CVD through behavioral, mental health and biological pathways [11]. A recent UK Biobank study found that child maltreatment is associated with incident CVD, and depressive symptoms mediate a significant part of this association [50]. In our study, neither alexithymia nor ACE showed an association with CSF.
The study has several limitations that need to be acknowledged. The observational and cross-sectional design of the study prohibits to draw causality between CSF and anxiety and depression. It remains unclear whether CSF leads to anxiety and depression or whether pre-existing psychological distress contributes to CSF. Therefore, longitudinal studies are needed to establish causality. The study establishes an association between CSF and mental health but does not explore the underlying biological mechanisms such as autonomic dysfunction, systemic inflammation, endothelial dysfunction, and/or platelet hyperreactivity. It should also be underlined that a biomarker-based study could provide more profound insights. For example, previous studies indicated that mental health and cognitive status in CVD patients are strongly linked to pathophysiological pathways such as inflammation and endothelial dysfunction [51,52]. The number of patients included in the study was small because of strict exclusion criteria of the study which increased the risk of type II error. For instance, we excluded many patients who underwent CAG through radial artery access. Premedication with nitrate and heparin is routinely suggested to prevent radial artery spasm and occlusion during or after CAG [18]. It is also known that intracoronary nitrate administration significantly affects TFC calculations [53]. Although premedication agents are applied through radial artery, we considered the possible systemic effects of these agents and aimed to standardize the CAG procedures for all patients. Therefore, we decided to exclude patients who underwent CAG through radial artery cannulation or needed further evaluation with pharmacologic agents for suspected coronary artery spasm. In addition, we excluded patients with coronary aneurysm or ectasia, coronary anomalies, myocardial bridge, suboptimal imaging, and complication during or after CAG procedure. The small sample size might have resulted in limited statistical power of the study. On the other hand, it should be mentioned that the study population consisted of middle-aged Turkish patients which limits the generalizability of the results to other populations. These factors may have caused selection bias. It would be better if we could use intracoronary imaging methods such as fractional flow reserve or intravascular ultrasonography which could also give us pathophysiological clues. Since CSF is a heterogeneous phenomenon that includes various pathophysiological mechanisms, more extensive and diverse CSF populations should be analyzed. Furthermore, there may be some residual confounding factors not evaluated in the study and could be associated with anxiety and depression such as socioeconomic level, marital status, income level, social support, personality traits, sleep disorders, stress level, dietary habits and/or education of the patients. All of the psychometric measures used in the study are self-report questionnaires that become limited if patients do not provide realistic answers which may introduce reporting bias. This might be one of the reasons why ACE and alexithymia were not associated with CSF in the study. For instance, the assessment of alexithymia by using TAS-20 can be limited due to the fact that alexithymia cannot be validly assessed by a self-report questionnaire because people with alexithymia cannot report their psychological state appropriately. For this purpose, it would be better if the study patients could be evaluated by the psychiatrist in a structured diagnostic interview in order to make definite psychiatric diagnoses of anxiety, depression, ACE, and alexithymia. On the other hand the HADS is a valid and reliable measure of anxiety and depression, and extensively used in research and clinical practice, but it only assesses symptom severity experienced in the last week [25,34]. It should also be underlined that retrospectively measured ACE may be influenced by the health status such as depression at the time of assessment. These limitations should be addressed in future studies. Future studies should also evaluate the clinical implications of the association between CSF and anxiety or depression.
5. Conclusions
In conclusion, our findings demonstrate that neither alexithymia nor ACE is associated with CSF. On the other hand, measures of both anxiety and depression assessed through the HADS are independently associated with CSF.
Author Contributions
The results of the study were presented as an oral presentation in the 5th International Academy of Young Cardiologists Congress in September 2024. Conceptualization, H.M.O., I.H.I., M.C., Y.T., H.K. and S.O.; methodology, H.M.O., I.H.I., M.C., Y.T., H.K. and S.O.; software, H.M.O., I.H.I., M.C. and S.O.; validation, H.M.O., I.H.I., Y.T., H.K. and S.O.; formal analysis, H.M.O., I.H.I., M.C., Y.T., H.K. and S.O.; investigation, H.M.O., I.H.I., H.K. and S.O.; resources, H.M.O., I.H.I., M.C., Y.T. and S.O.; data curation, H.M.O., I.H.I., Y.T., H.K. and S.O.; writing—original draft preparation, H.M.O., I.H.I., H.K. and S.O.; writing—review and editing, H.M.O., I.H.I., M.C. and S.O.; visualization, H.M.O., I.H.I., M.C. and S.O.; supervision, M.C., Y.T. and S.O.; project administration, H.M.O., I.H.I., M.C., Y.T., H.K. and S.O.; funding acquisition, H.M.O., I.H.I., M.C. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards. The Yozgat Bozok University Clinical Research Ethics Committee approved the study design and protocol (2017-KAEK-189_2021.03.31_14).
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Zhu, Q.; Wang, S.; Huang, X.; Zhao, C.; Wang, Y.; Li, X.; Jia, D.; Ma, C. Understanding the pathogenesis of coronary slow flow: Recent advances. Trends Cardiovasc. Med. 2022, 34, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Chalikias, G.; Tziakas, D. Slow Coronary Flow: Pathophysiology, Clinical Implications, and Therapeutic Management. Angiology 2021, 72, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Tambe, A.A.; Demany, M.A.; Zimmerman, H.A.; Mascarenhas, E. Angina pectoris and slow flow velocity of dye in coronary arteries—A new angiographic finding. Am. Heart J. 1972, 84, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.C.; Gori, T.; Fineschi, M. The coronary slow flow phenomenon: A new cardiac “Y” syndrome? Clin. Hemorheol. Microcirc. 2008, 39, 185–190. [Google Scholar] [CrossRef]
- Aparicio, A.; Cuevas, J.; Moris, C.; Martin, M. Slow Coronary Blood Flow: Pathogenesis and Clinical Implications. Eur. Cardiol. 2022, 17, e08. [Google Scholar] [CrossRef]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e763–e783. [Google Scholar] [CrossRef]
- Murphy, B.; Le Grande, M.; Alvarenga, M.; Worcester, M.; Jackson, A. Anxiety and Depression After a Cardiac Event: Prevalence and Predictors. Front. Psychol. 2019, 10, 3010. [Google Scholar] [CrossRef]
- Greenage, M.; Kulaksizoglu, B.; Cilingiroglu, M.; Ali, R. The role of anxiety and emotional stress as a risk factor in treatment-resistant hypertension. Curr. Atheroscler. Rep. 2011, 13, 129–131. [Google Scholar] [CrossRef]
- Khan, F.M.; Kulaksizoglu, B.; Cilingiroglu, M. Depression and coronary heart disease. Curr. Atheroscler. Rep. 2010, 12, 105–109. [Google Scholar] [CrossRef]
- Kojima, M. Alexithymia as a prognostic risk factor for health problems: A brief review of epidemiological studies. Biopsychosoc. Med. 2012, 6, 21. [Google Scholar] [CrossRef]
- Suglia, S.F.; Koenen, K.C.; Boynton-Jarrett, R.; Chan, P.S.; Clark, C.J.; Danese, A.; Faith, M.S.; Goldstein, B.I.; Hayman, L.L.; Isasi, C.R.; et al. Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e15–e28. [Google Scholar] [CrossRef] [PubMed]
- Keogh, T.M.; Howard, S.; Gallagher, S. Early Life Adversity and Blunted Cardiovascular Reactivity to Acute Psychological Stress: The Role of Current Depressive Symptoms. Psychosom. Med. 2022, 84, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.M.; Lumley, M.A. Relationship of alexithymia to cardiovascular disease risk factors among African Americans. Compr. Psychiatry 2007, 48, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yalvac, D.; Ozturk, S.; Sivri, N.; Kilic, Y.; Bulut, E.; Celik, A.; Barlas, Y.; Tengiz, I.; Yetkin, E. Effects of patients anxiety and depression scores on coronary flow in patients with normal coronary arteries. Int. J. Cardiol. 2015, 180, 55–57. [Google Scholar] [CrossRef]
- Durmaz, T.; Keles, T.; Erdogan, K.E.; Ayhan, H.; Bilen, E.; Bayram, N.A.; Akcay, M.; Oz, O.; Albayrak, Y.; Ozdemir, N.; et al. Coronary Slow Flow is Associated with Depression and Anxiety. Acta Cardiol. Sin. 2014, 30, 197–203. [Google Scholar]
- Elamragy, A.A.; Abdelhalim, A.A.; Arafa, M.E.; Baghdady, Y.M. Anxiety and depression relationship with coronary slow flow. PLoS ONE 2019, 14, e0221918. [Google Scholar] [CrossRef]
- Yavuz, F.; Alici, H.; Alici, D.; Inanc, I.H.; Ercan, S.; Davutoglu, V. The controversy about the association between depression and coronary slow flow phenomenon. Int. J. Cardiol. 2015, 186, 109–110. [Google Scholar] [CrossRef]
- Schussler, J.M. Effectiveness and safety of transradial artery access for cardiac catheterization. Bayl. Univ. Med. Cent. Proc. 2011, 24, 205–209. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T., Jr.; Alexander, B., Jr.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Harrigan, C.; Zorkun, C.; Palmer, A.M.; Ogando, K.J.; Biller, L.H.; Lord, E.E.; Williams, S.P.; Lew, M.E.; Ciaglo, L.N.; et al. Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years. J. Thromb. Thrombolysis 2009, 27, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Çanga, A.; Kocaman, S.A.; Çetin, M.; Cicek, Y.; Emre, M. Relationship between leukocyte and subtype counts, low-grade inflammation and slow coronary flow phenomenon in patients with angiographically normal coronary arteries. Acta Cardiol. Sin. 2012, 28, 306–314. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Guvenir, T.; Kuey, L.; Kultur, S. Validity and reliability of Turkish version of hospital anxiety and depression scale. Turk. Psikiyatri Derg. 1997, 8, 280–287. [Google Scholar]
- Bagby, R.M.; Parker, J.D.; Taylor, G.J. The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Bagby, R.M.; Taylor, G.J.; Parker, J.D. The Twenty-item Toronto Alexithymia Scale—II. Convergent, discriminant, and concurrent validity. J. Psychosom. Res. 1994, 38, 33–40. [Google Scholar] [CrossRef]
- Güleç, H.; Köse, S.; Güleç, M.Y.; Çitak, S.; Evren, C.; Borckardt, J.; Sayar, K. Reliability and factorial validity of the Turkish version of the 20-item Toronto alexithymia scale (TAS-20). Psychiatry Clin. Psychopharmacol. 2009, 19, 214. [Google Scholar]
- Bernstein, D.P.; Fink, L.; Handelsman, L.; Foote, J.; Lovejoy, M.; Wenzel, K.; Sapareto, E.; Ruggiero, J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 1994, 151, 1132–1136. [Google Scholar]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child. Abuse Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Şar, V.; Öztürk, E.; İkikardeş, E. Validity and reliability of the Turkish version of Childhood Trauma Questionnaire. Turk. Klin. J. Med. Sci. 2012, 32, 1054–1063. [Google Scholar] [CrossRef]
- Honkalampi, K.; De Berardis, D.; Vellante, F.; Viinamäki, H. Relations between Alexithymia and Depressive and Anxiety Disorders and Personality. In Alexithymia: Advances in Research, Theory, and Clinical Practice; Taylor, G.J., Luminet, O., Bagby, R.M., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 142–157. [Google Scholar]
- McLaughlin, K.A. Future Directions in Childhood Adversity and Youth Psychopathology. J. Clin. Child. Adolesc. Psychol. 2016, 45, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Lemay, K.R.; Tulloch, H.E.; Pipe, A.L.; Reed, J.L. Establishing the Minimal Clinically Important Difference for the Hospital Anxiety and Depression Scale in Patients With Cardiovascular Disease. J. Cardiopulm. Rehabil. Prev. 2019, 39, E6–E11. [Google Scholar] [CrossRef]
- Christensen, A.V.; Dixon, J.K.; Juel, K.; Ekholm, O.; Rasmussen, T.B.; Borregaard, B.; Mols, R.E.; Thrysoe, L.; Thorup, C.B.; Berg, S.K. Psychometric properties of the Danish Hospital Anxiety and Depression Scale in patients with cardiac disease: Results from the DenHeart survey. Health Qual. Life Outcomes 2020, 18, 9. [Google Scholar] [CrossRef]
- Bambauer, K.Z.; Locke, S.E.; Aupont, O.; Mullan, M.G.; McLaughlin, T.J. Using the Hospital Anxiety and Depression Scale to screen for depression in cardiac patients. Gen. Hosp. Psychiatry 2005, 27, 275–284. [Google Scholar] [CrossRef]
- Wang, X.; Nie, S.P. The coronary slow flow phenomenon: Characteristics, mechanisms and implications. Cardiovasc. Diagn. Ther. 2011, 1, 37–43. [Google Scholar]
- Shui, Z.; Wang, Y.; Sun, M.; Gao, Y.; Liang, S.; Wang, Y.; Wang, X.; Yu, Q.; Zhang, S.; Liu, L. The effect of coronary slow flow on left atrial structure and function. Sci. Rep. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Ozturk, H.M.; Ozturk, S.; Yetkin, E. Linkage between cardiovascular diseases and major depression: Contribution of platelet cells. Psychiatry Res. 2020, 287, 111026. [Google Scholar] [CrossRef]
- Sara, J.D.S.; Ahmad, A.; Toya, T.; Suarez Pardo, L.; Lerman, L.O.; Lerman, A. Anxiety Disorders Are Associated With Coronary Endothelial Dysfunction in Women With Chest Pain and Nonobstructive Coronary Artery Disease. J. Am. Heart Assoc. 2021, 10, e021722. [Google Scholar] [CrossRef]
- Oksuz, F.; Yarlioglues, M.; Ozturk, S.; Kaya, F.D.; Oksuz, E.; Murat, S.N.; Turak, O.; Celik, I.E.; Kilic, A.; Kurtul, A. Atrial electromechanical delay analysed by tissue Doppler echocardiography is prolonged in patients with generalised anxiety disorders. Kardiol. Pol. 2017, 75, 581–588. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.H.; Lim, S.Y.; Cho, G.Y.; Baik, I.K.; Lim, H.E.; Na, J.O.; Han, S.W.; Ko, Y.H.; Shin, C. Relationship between depression and subclinical left ventricular changes in the general population. Heart 2012, 98, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Cay, S.; Sensoy, B.; Murat, S.; Oksuz, F.; Cankurt, T.; Ali Mendi, M. Heart Failure Functional Class Associated with Depression Severity But Not Anxiety Severity. Acta Cardiol. Sin. 2016, 32, 55–61. [Google Scholar] [PubMed]
- Berg, S.K.; Rasmussen, T.B.; Thrysoee, L.; Thorup, C.B.; Borregaard, B.; Christensen, A.V.; Mols, R.E.; Juel, K.; Ekholm, O. Mental health is a risk factor for poor outcomes in cardiac patients: Findings from the national DenHeart survey. J. Psychosom. Res. 2018, 112, 66–72. [Google Scholar] [CrossRef]
- Kyrou, I.; Kollia, N.; Panagiotakos, D.; Georgousopoulou, E.; Chrysohoou, C.; Tsigos, C.; Randeva, H.S.; Yannakoulia, M.; Stefanadis, C.; Papageorgiou, C.; et al. Association of depression and anxiety status with 10-year cardiovascular disease incidence among apparently healthy Greek adults: The ATTICA Study. Eur. J. Prev. Cardiol. 2017, 24, 145–152. [Google Scholar] [CrossRef]
- Cosci, F.; Fava, G.A. When Anxiety and Depression Coexist: The Role of Differential Diagnosis Using Clinimetric Criteria. Psychother. Psychosom. 2021, 90, 308–317. [Google Scholar] [CrossRef]
- Terock, J.; Klinger-Konig, J.; Janowitz, D.; Nauck, M.; Volzke, H.; Grabe, H.J. Alexithymia is associated with increased all-cause mortality risk in men, but not in women: A 10-year follow-up study. J. Psychosom. Res. 2021, 143, 110372. [Google Scholar] [CrossRef]
- Ossola, P.; Gerra, M.L.; Beltrani, M.; Marchesi, C. Alexithymia and Cardiac Outcome in Patients at First Acute Coronary Syndrome. Int. J. Behav. Med. 2019, 26, 673–679. [Google Scholar] [CrossRef]
- Marchesi, C.; Ossola, P.; Scagnelli, F.; Mellini, L.; Tonna, M.; Ardissino, D.; De Panfilis, C. The role of alexithymia in predicting incident depression in patients at first acute coronary syndrome. Compr. Psychiatry 2015, 62, 86–92. [Google Scholar] [CrossRef]
- Ho, F.K.; Celis-Morales, C.; Gray, S.R.; Petermann-Rocha, F.; Lyall, D.; Mackay, D.; Sattar, N.; Minnis, H.; Pell, J.P. Child maltreatment and cardiovascular disease: Quantifying mediation pathways using UK Biobank. BMC Med. 2020, 18, 143. [Google Scholar] [CrossRef]
- Kang, W.; Malvaso, A. Understanding cognitive deficits in people with coronary heart disease (CHD). J. Pers. Med. 2023, 13, 307. [Google Scholar] [CrossRef]
- Kang, W.; Malvaso, A. Mental health in coronary heart disease (CHD) patients: Findings from the UK Household Longitudinal Study (UKHLS). Healthcare 2023, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Oguzhan, A.; Eryol, N.K.; Ergin, A. Effect of potential confounding factors on the thrombolysis in myocardial infarction (TIMI) trial frame count and its reproducibility. Circulation 1999, 100, 2219–2223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).